Abstract
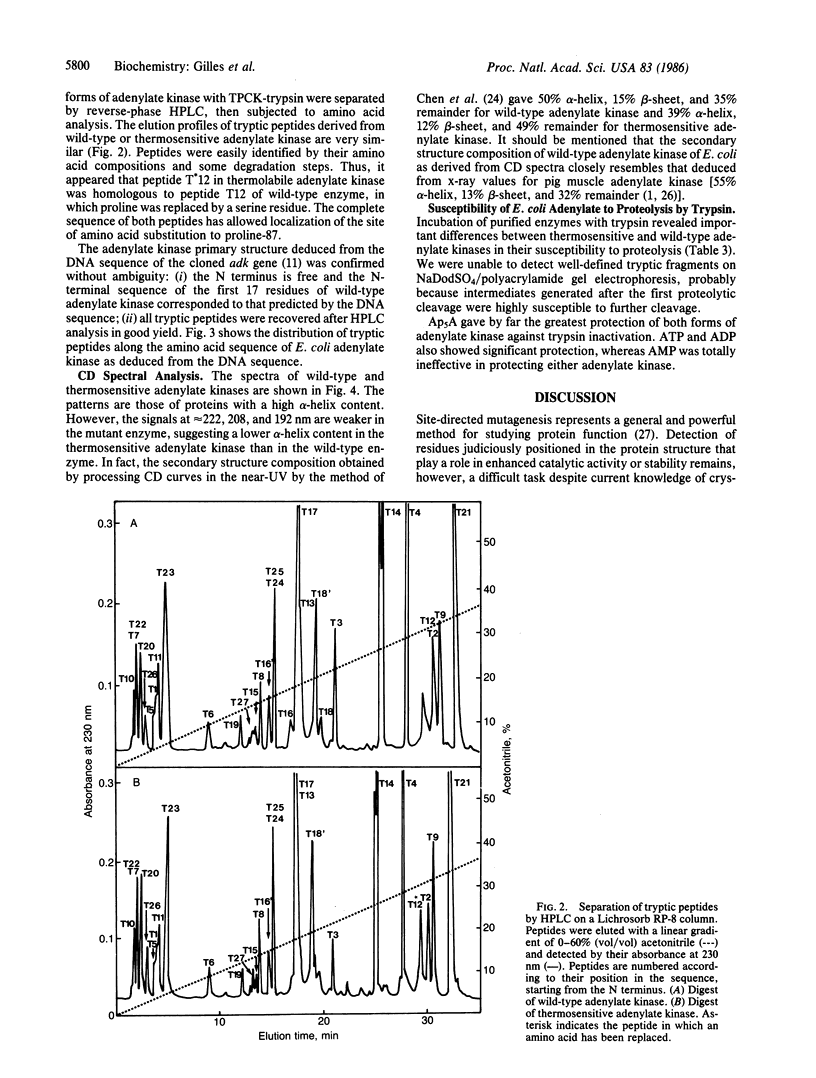
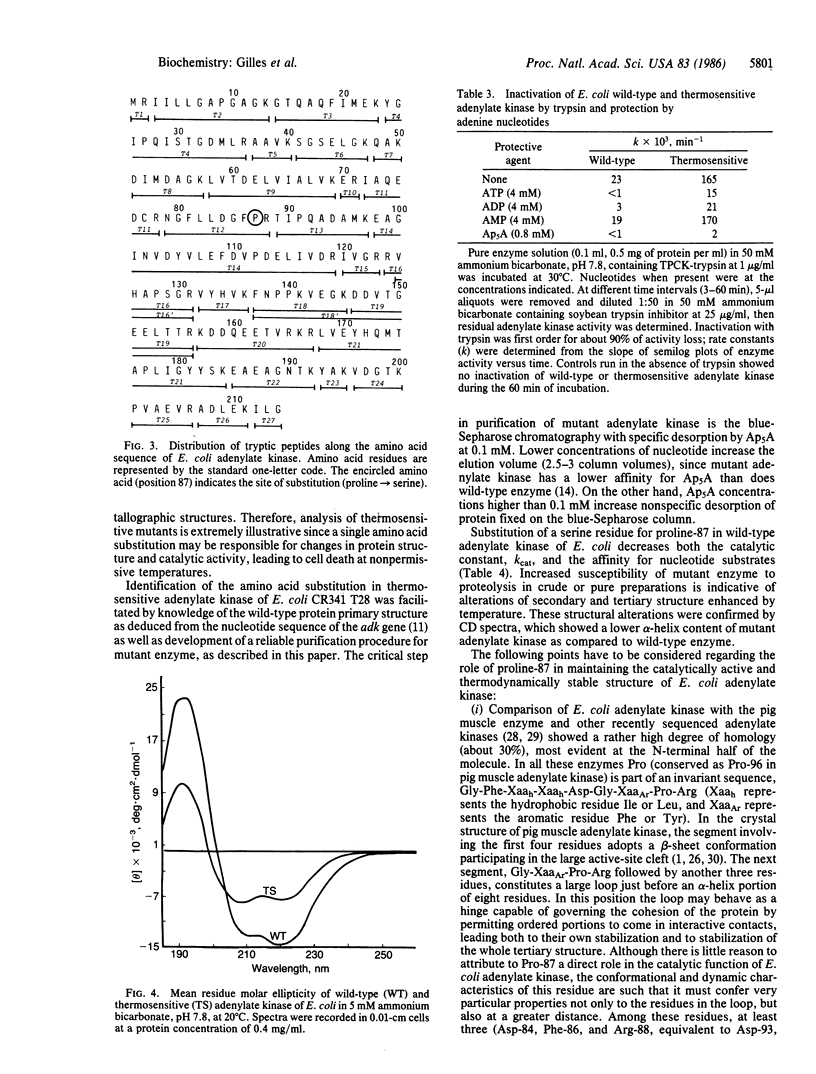
Amino acid analysis, HPLC separation of trypsin digests, and sequence analysis showed that the thermosensitivity of the adenylate kinase (EC 2.7.4.3) from Escherichia coli K-12 strain CR341 T28 results from substitution of a serine residue for proline-87 in the wild-type enzyme. This mutation is accompanied by decreased affinity for nucleotide substrates and decreased catalysis. Circular dichroism spectroscopy showed a significant change of the secondary structure. This mainly corresponds to a reduction in alpha-helix content (39%) of mutant protein as compared to wild-type adenylate kinase (50%). Altered conformation of thermosensitive adenylate kinase was also manifested by an increase in susceptibility to proteolysis by trypsin. Ap5A and ATP, known to induce important conformational changes in eukaryotic adenylate kinase(s), protected the mutant enzyme against inactivation by trypsin. This seems to indicate that the "loosening" of the three-dimensional structure of E. coli adenylate kinase by proline----serine substitution is largely compensated for when an enzyme X ATP or enzyme X Ap5A complex is formed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Shortle D. Strategies and applications of in vitro mutagenesis. Science. 1985 Sep 20;229(4719):1193–1201. doi: 10.1126/science.2994214. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brune M., Schumann R., Wittinghofer F. Cloning and sequencing of the adenylate kinase gene (adk) of Escherichia coli. Nucleic Acids Res. 1985 Oct 11;13(19):7139–7151. doi: 10.1093/nar/13.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bârzu O., Michelson S. Simple and fast purification of Escherichia coli adenylate kinase. FEBS Lett. 1983 Mar 21;153(2):280–284. doi: 10.1016/0014-5793(83)80624-3. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Cousin D., Buttin G. Mutants thermosensibles d'Escherichia coli K12. 3. Une mutation létale d'E. coli affectant l'activité de l'adénylate-kinase. Ann Inst Pasteur (Paris) 1969 Nov;117(5):612–630. [PubMed] [Google Scholar]
- Esmon B. E., Kensil C. R., Cheng C. H., Glaser M. Genetic analysis of Escherichia coli mutants defective in adenylate kinase and sn-glycerol 3-phosphate acyltransferase. J Bacteriol. 1980 Jan;141(1):405–408. doi: 10.1128/jb.141.1.405-408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R., Trosin M., Tomasselli A. G., Noda L., Krauth-Siegel R. L., Schirmer R. H. Mitochondrial adenylate kinase (AK2) from bovine heart. The complete primary structure. Eur J Biochem. 1986 Jan 2;154(1):205–211. doi: 10.1111/j.1432-1033.1986.tb09380.x. [DOI] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. NMR studies of the MgATP binding site of adenylate kinase and of a 45-residue peptide fragment of the enzyme. Biochemistry. 1985 Aug 13;24(17):4680–4694. doi: 10.1021/bi00338a030. [DOI] [PubMed] [Google Scholar]
- Glaser M., Nulty W., Vagelos P. R. Role of adenylate kinase in the regulation of macromolecular biosynthesis in a putative mutant of Escherichia coli defective in membrane phospholipid biosynthesis. J Bacteriol. 1975 Jul;123(1):128–136. doi: 10.1128/jb.123.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski C. C., Chapman A. G., Atkinson D. E. Adenylate energy charge in Escherichia coli CR341T28 and properties of heat-sensitive adenylate kinase. J Bacteriol. 1981 Mar;145(3):1374–1385. doi: 10.1128/jb.145.3.1374-1385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Cronan J. E., Jr Adenylate kinase of Escherichia coli: evidence for a functional interaction in phospholipid synthesis. Biochemistry. 1982 Jan 5;21(1):189–195. doi: 10.1021/bi00530a032. [DOI] [PubMed] [Google Scholar]
- Guiso N., Michelson S., Bârzu O. Inactivation and proteolysis of heat-sensitive adenylate kinase of Escherichia coli CR341 T28. J Biol Chem. 1984 Jul 25;259(14):8713–8717. [PubMed] [Google Scholar]
- Huss R. J., Glaser M. Identification and purification of an adenylate kinase-associated protein that influences the thermolability of adenylate kinase from a temperature-sensitive adk mutant of Escherichia coli. J Biol Chem. 1983 Nov 10;258(21):13370–13376. [PubMed] [Google Scholar]
- Kalbitzer H. R., Marquetant R., Rösch P., Schirmer R. H. The structural isomerisation of human-muscle adenylate kinase as studied by 1H-nuclear magnetic resonance. Eur J Biochem. 1982 Sep 1;126(3):531–536. doi: 10.1111/j.1432-1033.1982.tb06813.x. [DOI] [PubMed] [Google Scholar]
- Kuby S. A., Palmieri R. H., Frischat A., Fischer A. H., Wu L. H., Maland L., Manship M. Studies on adenosine triphosphate transphosphorylases. Amino acid sequence of rabbit muscle ATP-AMP transphosphorylase. Biochemistry. 1984 May 22;23(11):2393–2399. doi: 10.1021/bi00306a012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Suit J. L., Plate C. A. Initiation of transcription is temperature-dependent in an E. coli mutant with ts adenylate kinase. Biochem Biophys Res Commun. 1975 Nov 3;67(1):353–358. doi: 10.1016/0006-291x(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Nageswara Rao B. D., Cohn M. Asymmetric binding of the inhibitor di(adenosine-5') pentaphosphate (Ap5A) to adenylate kinase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5355–5357. doi: 10.1073/pnas.74.12.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Sachsenheimer W., Schulz G. E. Two conformations of crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):23–36. doi: 10.1016/0022-2836(77)90280-7. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Elzinga M., Marx F., Schrimer R. H. Three dimensional structure of adenyl kinase. Nature. 1974 Jul 12;250(462):120–123. doi: 10.1038/250120a0. [DOI] [PubMed] [Google Scholar]
- Theze J., Margarita D. Etude de l'adénylate kinase chez "E. coli" K 12 et chez un mutant thermosensible. Ann Inst Pasteur (Paris) 1972 Aug;123(2):157–169. [PubMed] [Google Scholar]
- Tomasselli A. G., Mast E., Janes W., Schiltz E. The complete amino acid sequence of adenylate kinase from baker's yeast. Eur J Biochem. 1986 Feb 17;155(1):111–119. doi: 10.1111/j.1432-1033.1986.tb09465.x. [DOI] [PubMed] [Google Scholar]
- Waxdal M. J., Konigsberg W. H., Henley W. L., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry. 1968 May;7(5):1959–1966. doi: 10.1021/bi00845a046. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Pauly E., Kratzin H., Hilschmann N. Chromatographie und Rechromatographie in der Hochdruckflüssigkeitschromatographie von Peptidgemischen. Die vollständige Primärstruktur einer Immunglobulin L-Kette vom kappa-Typ, Subgruppe I (Bence-Jones-Protein Den). Hoppe Seylers Z Physiol Chem. 1981 Aug;362(8):1131–1146. [PubMed] [Google Scholar]
- Yazawa M., Noda L. H. Studies on tyrosine residues in porcine muscle adenylate kinase. Circular dichroism spectra and chemical modification with tetranitromethane. J Biol Chem. 1976 May 25;251(10):3021–3026. [PubMed] [Google Scholar]