Abstract
Problem
Clostridium sordellii causes endometrial infections but little is known regarding host defenses against this pathogen.
Method of Study
We tested the hypothesis that the immunoregulatory lipid prostaglandin (PG) E2 suppresses human macrophage clearance of C. sordellii through receptor-induced increases in intracellular cAMP. The THP-1 macrophage cell line was used to quantify C. sordellii phagocytosis.
Results
PGE2 increased cAMP levels, activated protein kinase A (PKA), and inhibited the class A scavenger receptor-dependent phagocytosis of C. sordellii. Activation of the EP2 and EP4 receptors increased intracellular cAMP and inhibited phagocytosis, with evidence favoring a more important role for EP4 over EP2. This was supported by EP receptor expression data and the use of pharmacological receptor antagonists. In addition, the PKA isoform RI appeared to be more important than RII in mediating the suppression of ingestion of C. sordellii.
Conclusions
The endogenous lipid mediator PGE2 impairs human innate immune responses against C. sordellii.
Keywords: Abortion, endometritis, innate immunity, phagocytosis, pregnancy, toxic shock syndrome
Introduction
Clostridium sordellii is an anaerobic Gram positive bacillus that is found in the environment and is an uncommon cause of human infection. However, infections caused by toxigenic strains of C. sordellii can be severe due to the occurrence of a treatment-refractory toxic shock syndrome1. Women of reproductive age are at increased risk for C. sordellii infections (including endometritis) that complicate childbirth, abortion, and gynecological procedures2. Despite aggressive medical and surgical treatment, the mortality of C. sordellii infections has remained high1. The development of better therapeutic options for C. sordellii infection is limited by a lack of understanding of fundamental host-microbial interactions involved in the pathogenesis of infection.
Macrophages are important sentinels of innate immunity in the soft tissues and have been implicated as critical cellular participants in host defense against tissue-invasive clostridial infection3-5. It was recently reported that macrophage phagocytosis of vegetative C. sordellii was mediated by class A scavenger receptors, particularly the macrophage receptor with collagenous structure (MARCO)6. It was also demonstrated that misoprostol, a pharmacological analogue of E-series prostaglandins (PG), could impair the phagocytosis of C. sordellii by rodent macrophages7. This suggested that immune surveillance and clearance of C. sordellii were susceptible to negative regulation by endogenous PGs, which is potentially important because the female reproductive tract is replete with PGs during pregnancy8, 9.
The compound PGE2 is an arachidonic acid-derived lipid mediator generated in abundance at sites of infection and inflammation as a result of the rapid up-regulation of cyclooxygenase-2 and microsomal PGE synthase-1 enzymes10. It is also an important hormonal regulator of reproduction that is generated in the uterus where it is involved in early and late processes ranging from implantation of the fertilized egg to parturition11. PGE2 is a highly potent modulator of innate and adaptive immunity that influences cell behavior through the ligation of its four distinct G protein coupled E-prostanoid (EP) receptors, numbered EP1-412, 13. Both EP2 and EP4 are potent immunoregulatory receptors that share the capacity to increase intracellular concentrations of cyclic adenosine monophosphate (cAMP) within seconds to minutes of PGE2 binding13, 14. PGE2-dependent increases in cAMP have been shown to impair the phagocytic ability of different macrophage types against a range of pathogens15-18, and it can be suggested that such effects might have evolved to limit the extent of host inflammatory responses or trigger the resolution of inflammation. However, in clinical situations such as pregnancy and the puerperium, where local and systemic PGE2 levels are elevated for physiological reasons19-21, the immunosuppressive effects of PGE2 might be maladaptive, particularly when an opportunistic pathogen such as C. sordellii gains access to the normally uninfected uterus (or surrounding soft tissue).
The purpose of this study was to address the question of whether PGE2 and cAMP-signaling cascades could regulate the phagocytosis of C. sordellii by human macrophages and to determine the involvement and relative importance of EP2 and EP4 receptors in such regulation. A better understanding of endogenous regulators of innate immunity will enhance efforts to develop better preventive and therapeutic options against reproductive tract infections.
Materials and Methods
THP-1 cells
Phorbol-12-myristate-13-acetate (PMA)-differentiated THP-1 cells (a human macrophage-like cell line) were used in this study. These cells were obtained from the American Type Culture Collection (ATCC, TIB-202; Manassas, VA) and cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 1% antibiotic-antimycotic (Invitrogen) and 10% charcoal/dextran treated fetal bovine serum (FBS; HyClone, Waltham, MA), referred to as RPMI +/+. Cells were passaged every 2-4 days and were used through the 10th passage, at which time a new culture was started. THP-1 cells were matured into macrophages by culturing with 100 nM PMA (Sigma-Aldrich, St. Louis, MO) in RPMI +/+ for 24 hr at 37°C with 5% CO2. Cells were detached from the flask with non-enzymatic cell dissociation solution (Sigma-Aldrich) and gentle scraping. PMA-activated THP-1 cells were used for all experiments presented here, unless otherwise noted.
Bacteria
The lethal toxin-expressing C. sordellii strain 9714 was obtained from the ATCC and grown anaerobically for 48 hr at 37°C in reinforced clostridial medium (RCM; BD Biosciences, San Jose, CA). Bacterial concentrations were estimated from the optical density (OD) of bacterial cultures at 600 nm (OD600) and a standard curve of colony forming units (CFU) vs. OD600. Estimated bacterial concentrations were confirmed by serial 10-fold dilutions on solid RCM containing 1.5% agar, incubated overnight anaerobically. For phagocytosis experiments (below), heat-killed, vegetative C. sordellii were prepared by incubating at 65°C for 2 hr. Spore contamination was estimated by Schaeffer and Fulton Spore Stain (Sigma-Aldrich) to be < 10%. Heat-killed C. sordellii were then surfaced-labeled with either FITC, per our previously published protocol7, or [C15H16N3]+[Zn8S(SC6H5)15·H2O]− (abbr. JX90a) as previously published22. Although qualitative results using either fluorophore were similar, the fluorescent labeling was brighter with JX90a. Therefore it was used for many of the experiments in preference to FITC. Briefly, heat-killed C. sordellii were labeled overnight in NaHCO3 buffer (pH 9.2) with 100 μM of the bacterial dye JX90a. Bacteria were washed with PBS by centrifugation and stored at −80°C in single-use aliquots until each phagocytosis assay was performed. Herein, we refer to fluorescently labeled C. sordellii (using either FITC or JX90a) as FLUORC. sordellii.
Phagocytosis Assays
PMA-activated THP-1 cells were treated in RPMI +/− (lacking FBS) with compounds of interest and incubated for 15 or 30 min at 37°C as indicated, on 384-well tissue-culture treated plates. All conditions were performed in replicates of eight. Cells were inoculated with FITC- or JX90a-labeled C. sordellii (FLUORC. sordellii) at a multiple of infection (MOI) of 300 bacteria:1 cell and incubated for 3 hr at 37°C. Phagocytosis was quantified according to our published method of measuring intracellular fluorescence as a surrogate marker of bacterial ingestion by macrophages15. The fluorescence of intracellular FLUORC. sordellii was determined using a microplate fluorometer (485ex/535em FITC; 470ex/500em JX90a, SPECTRAMax GEMINI EM; Molecular Devices, Sunnyvale, CA) according to our previously published method15. Briefly, fluorescence was expressed in relative fluorescence units (RFU), which were converted into a phagocytic index (PI). The PI represents the fluorescence of intracellular (phagocytosed) bacteria (RFUi) and was calculated from the total fluorescence of the well (RFUtotal) by subtracting the fluorescence of extracellular bacteria (RFUex). The RFUex was determined by treating some cells with the phagocytosis inhibitor, cytochalasin D (20 μg/ml; EMD Chemicals, Billerica, MA), for 30 min prior to exposure to FLUORC. sordellii23. The mean RFUex determined from cytochalasin-treated wells was then subtracted from the RFUtotal. Therefore, the PI = RFUi = RFUtotal - RFUex15.
Treatments used in our phagocytosis assay included the phagocytosis inhibitor, cytochalasin D (30 min, 20μg/mL); prostaglandin E2 (PGE2; 15 min, 0.1, 1μM; Cayman Chemicals, Ann Arbor, MI); cAMP analogs adenosine 3′, 5′-cyclic monophosphate 8-bromo-sodium salt (8-Bromo-cAMP; dual activator of protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC-1)), adenosine 3′,5′-cyclic monophosphate N6–benzoyl sodium salt (6-Bnz-cAMP; PKA-specific), and adenosine 3′-5′-cyclic monophosphate 8-(4-Chlorophenylthio)-2′-O-methyl sodium salt (8-pCPT-cAMP; EPAC-1-specific) (each 30 min, 0.1, 0.2, 1, 2mM; EMD Chemicals); the EP2 agonist butaprost free acid (BFA; 15 min, 1, 10μM; Cayman Chemicals); the EP4 agonist L-902,688 (15 min, 1, 10μM; Cayman Chemicals); the EP2 antagonist AH6809 (15 min, 1μM; Cayman Chemicals); the EP4 antagonist ONO-AE1-208 (15 min, 1μM; gift from the Ono Pharmaceutical company in Osaka, Japan); the nonselective class A scavenger receptor antagonists fucoidan (30 min, 1mg/mL; Sigma-Aldrich) and dextran sulfate (30 min, 0.2mg/mL; MP Biomedicals, Solon, OH); and the negative control agent chondroitin sulfate (30min, 0.2mg/mL; Sigma-Aldrich); the PKA RI agonist 2-Cl-8-MA-cAMP and the PKA RII agonist 6-MBC-cAMP (both 30min, 500μM; Axxora, Farmingdale, NY).
Measurement of intracellular cAMP
PMA-activated THP-1 cells were cultured in 6-well tissue-culture treated plates at a concentration of 3 × 106 cells/well in RPMI +/−. Cells were incubated with PGE2, BFA, L-902,688, AH6809, or ONO-AE1-208 (1 or 10μM) for 15 min. Culture supernatants were removed and cells were lysed by incubation with 0.1M HCl for 10 min at room temperature followed by gentle scraping. Lysates were harvested by centrifugation and stored at −80°C. Intracellular cAMP levels were measured by EIA according to the manufacturer (Enzo/Assay Designs, Ann Arbor, MI), and all samples were assayed in triplicate.
Measurement of PKA activation
The activation of PKA was assessed by quantitative immunoblot of the PKA phosphorylation target vasodilator-stimulated phosphoprotein (VASP)24, 25. THP-1 cells were PMA-activated for 48 hr followed by an overnight rest period in RPMI +/+. PMA-activated THP-1 cells were then treated for 15 min with 1μM PGE2 in 100 mm2 tissue-culture treated dishes before lysis in Lysis Buffer #6 (R&D Systems, Minneapolis, MN). Protein samples (40μg) were resolved on 10% Tris-HCl polyacrylamide gels and transferred to a nitrocellulose membrane. Membranes were probed with phospho-(Ser157) VASP rabbit antibody (Cell Signaling Technology, Danvers, MA), followed by HRP-conjugated anti-rabbit secondary antibody and Pierce ECL detection reagents (Thermo Scientific, Rockford, IL). Quantification of the phospho-target was normalized to the housekeeping protein α-tubulin.
Immunoblot analysis of EP2 and EP4 receptors
Non-PMA treated THP-1 cells in suspension were centrifuged and lysed in Lysis Buffer #6. Protein samples (40μg) were resolved on 10% Tris-HCl polyacrylamide gels and transferred to a nitrocellulose membrane. Membranes were probed with the EP2 and EP4 receptor polyclonal antibodies (Cayman Chemicals), followed by HRP-conjugated anti-rabbit secondary antibody and Pierce ECL detection reagents. Quantification of each receptor was normalized to the housekeeping protein α-tubulin.
Quantitative real-time PCR analysis
PMA-activated THP-1 cells were stored in Trizol Reagent (Invitrogen) at −80°C until RNA was extracted and cDNA was generated per our previously published protocol6. Human primers and probes were designed using the Roche Universal Probe Library Assay Design Center. Primers were generated by Integrated DNA Technology and all probes were from Roche (Basel, Switzerland). Primers used are as follows: human EP2 forward 5′-GGA GGA GAC GGA CCA CCT-3′, EP2 reverse 5′- GTT TCA TTC ATA TAT GCA AAA ATC GT-3′ (Universal Probe Library #2); human EP4 forward 5′-CTC CCT GGT GGT GCT CAT-3′, EP4 reverse 5′-GGC TGA TAT AAC TGG TTG ACG A-3′ (Universal Probe Library # 58). The Universal Probe Library Gene Assay (Roche) for human GAPDH was also used (Universal Probe Library # 60). Samples were run on the Light Cycler 480 (Roche) with the following conditions: 95°C, 10 min (pre-incubation); 95°C 10 sec; 60°C, 30 sec; 72°C, 1 sec (amplification, 45 cycles); 95°C, 10 sec; 50°C, 30 sec; 70°C, 5 min (melting curve); 40°C, 30 sec (cooling). Analysis was performed using the Roche software and expression of each gene was referenced to the expression of the housekeeping gene GAPDH. Results were calculated using the 2−ΔΔCT method26.
Statistical analyses
Statistical analyses were carried out using GraphPad Prism 5.0 software for Windows (GraphPad Software, San Diego California USA). Unless otherwise stated, experimental data are presented as a percentage of the untreated control group (set at 100%). Error bars represent the standard error of the mean (SEM). All analyses were conducted on raw data prior to normalizing to the untreated control. Where appropriate, mean values were compared using a paired Student t-test or a repeated measured analysis of variance (ANOVA). A Dunnett post test was conducted for comparisons with the control value, or a Tukey test was performed for multiple comparisons. Differences were considered significant if P ≤ 0.05. Experiments were performed on at least three separate occasions.
Results
Prostaglandin E2 inhibits the class A scavenger receptor dependent phagocytosis of unopsonized C. sordellii by THP-1 cells
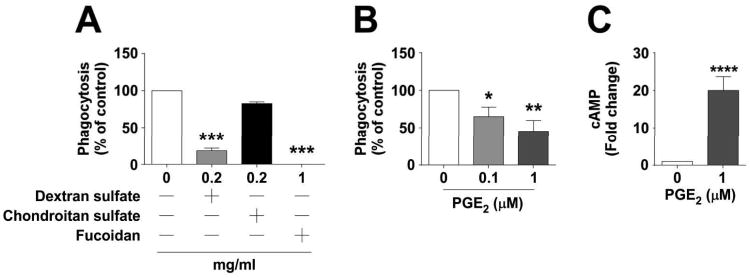
The PGE1 analogue misoprostol, which binds to the same four EP receptors as does PGE227, was previously found to inhibit the phagocytosis of vegetative C. sordellii by rodent macrophages7. The capacity for authentic PGE2 to regulate human phagocyte-clostridial interactions has not been examined. Human THP-1 macrophage-like cells were used to model the regulation of phagocytosis of unopsonized, vegetative C. sordellii. Although C. sordellii phagocytosis has been shown to be mediated by class A scavenger receptors (CASRs) in rodent macrophages and human decidual macrophages6, the dependence on CASRs for internalization of C. sordellii by THP-1 cells was unknown. Therefore, initial experiments were performed with the CASR-blocking compound fucoidan (1 mg/mL), which almost completely prevented the phagocytosis of FLOURC. sordellii by THP-1 cells (P < 0.001), confirming the importance of CASRs in this process (Fig. 1A). Additionally, when cells were treated with the standard, nonselective CASR blocking agent dextran sulfate at 0.2 mg/mL, there was an inhibition of 81.6 ± 3.5% of phagocytic activity (P < 0.001), while the negative control agent chondroitin sulfate had a minimal effect at the same dose (Fig. 1A). Exposure of THP-1 cells to exogenously-added PGE2 (0.1 or 1 μM) dose-dependently inhibited the phagocytosis of unopsonized FLUORC. sordellii (Fig. 1B), with an inhibition of 35 ±12.7% (P < 0.05) and 54.7 ± 14.5% (P < 0.01), respectively.
Figure 1. Prostaglandin E2 inhibits class A scavenger receptor-dependent phagocytosis of unopsonized C. sordellii by THP-1 cells.
Cells were treated with the class A scavenger receptor antagonists (A) fucoidan, dextran sulfate, or the structurally similar negative control chondroitin sulfate for 30 min (n=3-4), or (B) PGE2 (n=6) for 15 min prior to challenge with FLUORC. sordellii as described in Materials and Methods. Phagocytosis was quantified by fluorometry. Data are mean ± SEM. **, p<0.01; ***, p<0.001 by ANOVA. (C) Cells were treated with PGE2 for 15 min before lysis with 0.1M HCl and cAMP measurement by EIA (n=12). Data are mean ± SEM. ****, p<0.0001 by Student paired t-test compared to untreated controls.
Activation of EP2 and EP4 receptors increases cAMP in THP-1 cells and this effect is blocked with an EP4 antagonist
The Gαs coupled EP2 and EP4 receptors are important immunoregulatory receptors on macrophages15, 28-30 and THP-1 cells have been reported to express both EP2 and EP4 receptors31. We therefore verified that PGE2 could increase cAMP in THP-1 cells, finding a 20 ± 3.7-fold increase (P < 0.0001) with 1 μM PGE2 (Fig. 1C). That both EP2 and EP4 receptors were active in these cells was supported by an increase in cAMP observed when cells were incubated for 15 min with the selective EP2 or EP4 agonists BFA or L-902,688, respectively (Fig. 2A). The activation of the EP2 receptor evoked 1.8-fold and 3.3-fold increases in cAMP with BFA (1 and 10 μM, respectively), while EP4 stimulation with L-902,688 induced 7.1-fold (P < 0.001) and 5.7-fold (P < 0.05) increases in cAMP (1, 10 μM, respectively). To further explore EP2 and EP4 activation on THP-1 cell phagocytosis, cells were pretreated with L-902,688 or BFA for 15 min. It was found that L-902,688 (EP4 agonist) exposure suppressed the capacity of THP-1 cells to ingest unopsonized FLUORC. sordellii, while BFA was effective but not quite as potent (Fig. 2B). EP2 and EP4 antagonists were used to define the extent to which these receptors mediate the actions of PGE2 on THP-1 cells. As indicated in Fig. 2C, cAMP increases provoked by PGE2 were blocked by the EP4 antagonist ONO-AE1-208 but not by the EP2/DP1 antagonist AH6809 (1 μM each).
Figure 2. EP2 and EP4 receptors mediate PGE2-induced intracellular cAMP increase and phagocytosis inhibition in THP-1 cells.
Cells were treated for 15 min with (A) the EP2 agonist butaprost free acid (BFA) or the EP4 agonist L-902,688 (n=3-5). Cells were lysed and cAMP was measured by EIA. (B) Cells were treated for 15 min with BFA or L-902,688 followed by a challenge with FLUORC. sordellii (n=4). Phagocytosis was quantified by fluorometry. (C) Cells were pre-treated for 15 min with either the EP2 antagonist (AH6809) or the EP4 antagonist (ONO-AE1-208), then PGE2 was administered for an additional 15 min (n=3), cells were lysed and cAMP was measured by EIA. Data in (A-C) are represented as mean ± SEM. *, p<0.05; **, p<0.01; ****, p<0.0001 by ANOVA compared to the untreated control. Cells were lysed and immunoblot analysis was performed for (D) EP2 and (E) EP4 receptors (n=5 each; representative immunoblots). (F) qRT-PCR was performed for EP2 and EP4 on THP-1 cells (n=3). Data are represented as mean ± SEM of fold regulation. **, p<0.01 by unpaired Student t-test comparing EP2 to EP4.
EP4 receptors are expressed in greater abundance than EP2 receptors by THP-1 cells
To confirm EP2 and EP4 receptor expression by THP-1 cells, cells were lysed and subjected to immunoblot analysis for the detection of these receptors. A band at the expected molecular weight of ∼52 kDa was observed for the EP2 receptor but as evidenced in Fig. 2D several larger bands were also detected, which are of uncertain significance. A single band at the expected 65 kDa was detected for EP4 (Fig. 2E). Because the EP2 immunoblot result was inconclusive, experiments were conducted to determine mRNA expression levels of EP2 and EP4 using quantitative real-time PCR. RNA was isolated, cDNA was reverse transcribed, and real-time PCR was performed for EP2 and EP4. We found significantly higher expression of EP4 compared with EP2 by THP-1 cells (P < 0.01) (Fig. 2F).
PKA activation inhibits C. sordellii phagocytosis
Once cAMP is generated in a macrophage it can activate downstream signaling cascades by binding to effector proteins such as the Ser/Thr phosphorylating enzyme called protein kinase A (PKA) or the guanine-nucleotide exchange protein directly activated by cAMP (Epac-1)32. Experiments were conducted to determine whether cAMP itself could regulate phagocytosis of C. sordellii and, if so, through which effector proteins. Thus, cells were pretreated with the dual (non-selective) PKA/Epac-1 activator and cAMP analogue 8-Br-cAMP, which significantly reduced phagocytosis by 38.2 ± 7.4% (P < 0.01) at a concentration of 1 mM (data not shown). To determine whether the activation of either PKA or Epac-1 (or both) mediated the actions of cAMP on this process, cells were pretreated with the PKA or Epac-1-selective agonist's 6-Bnz-cAMP or 8-pCPT-2′-O-Me-cAMP, respectively. As illustrated (Fig. 3A&B), only PKA activation resulted in suppression of phagocytosis.
Figure 3. The PGE2-mediated inhibition of phagocytosis seen in THP-1 cells is PKA dependent and not EPAC-1 dependent.
Cells were treated for 30 min with (A) 6-Bnz-cAMP, a PKA-dependent cAMP analogue (n=3) or (B) 8-pCPT-cAMP, an EPAC-1 dependent cAMP analogue (n=3), or (D) the PKA RI agonist 2-Cl-8-MA-cAMP or the PKA RII agonist 6-MBC-cAMP for 30 min (n=5), followed by a challenge with FLUORC. sordellii. Phagocytosis was quantified by fluorometry. Data in A, B, and D panels are represented as mean ± SEM. **, p<0.01 by a 1 way ANOVA with a Dunnett's multiple comparison test compared to untreated control. (C) Cells were treated with 1 μM PGE2 for 15 min, lysed, and immunoblot analysis was performed for phospho-VASP, a marker of PKA activation (n=5). Data are shown as mean ± SEM. *, p<0.05 by a paired Wilcoxon matched-pairs signed rank test.
PKA is activated by PGE2 as seen by phosphorylation of VASP
The data above demonstrate that PGE2 both inhibited C. sordellii phagocytosis and enhanced cAMP in THP-1 macrophages, while the cAMP-dependent activation of PKA was sufficient to suppress phagocytosis. To determine whether PGE2 treatment can directly activate PKA, we measured the phosphorylation of a canonical protein target of PKA in response to treatment of cells with PGE2. VASP is a member of the Ena-VASP protein family that is phosphorylated by PKA and is a robust surrogate for that activity24, 25. THP-1 cells were exposed for 15 min with 1 μM PGE2 and immunoblot analysis was performed for phospho-VASP (Fig. 3C). As noted, PGE2 treatment resulted in an 11.2-fold (P < 0.05) increase in phosphorylation of VASP when compared to untreated control.
Activation of PKA isozyme type I, not type II, counter-regulates C. sordellii phagocytosis
The cAMP-dependent PKA exists in two major isoforms, defined by their regulatory (cAMP-binding) subunits: types RI and RII33. Emerging data suggest that cellular functions in macrophages are governed by distinct isoforms34. We examined the capacity for type RI and RII agonists (2-Cl-8-MA-cAMP and 6-MBC-cAMP, respectively) to regulate phagocytosis of C. sordellii and found that activation of PKA type RI resulted in an inhibition of 33.8 ± 9.4% (P < 0.01), while PKA type RII only inhibited phagocytosis by 7.2 ± 4.8% (Fig. 3D).
Discussion
Globally, more than 500,000 women die from complications of pregnancy and childbirth each year35, and nearly 1 in 8 maternal deaths is due to unsafe abortion36, 37. Sepsis is a principal cause of maternal death after childbirth38 or abortion37. Pregnancy itself is associated with major shifts in immune surveillance39 as the maternal immune system must be “detuned” to accommodate the immunologically-distinct fetus40. Despite this, a mother's immune system must be able to detect and respond to potentially pathogenic organisms. However, some pathogens have evolved mechanisms to evade host defense, apparently taking advantage of the immunological shifts associated with pregnancy. For example, certain Gram positive bacteria are adept at causing pregnancy-related infections, including Listeria monocytogenes, Streptococcus pneumoniae, Group A Streptococcus, Group B Streptococcus, and the clostridia41-43. Clostridium sordellii infections have increasingly been observed over the past decade in healthy women of reproductive age following childbirth or abortion2. In addition to C. sordellii, there is an unexplained association between C. difficile colitis and both pre- and postpartum women44, 45. The basis for the enhanced susceptibility of postpartum women to infection remains to be solved.
Major gaps in our understanding of immune surveillance and host defense against clostridial infections are apparent, in part because the field is understudied. Recent work in this area has focused on C. difficile and C. perfringens but has not explored reproductive tract immune defenses3, 5, 46, 47. Macrophages are important in defending the host against invasive clostridial infections such as C. perfringens3, 48 and are adept at recognizing clostridia as either spores or vegetative bacteria and targeting them for immune clearance5, 6, 49. Better understanding the host factors that regulate macrophage-clostridial interactions may reveal how such pathogens evade host defenses to establish infection.
Our experiments newly establish that macrophage phagocytosis of C. sordellii is subject to immunoregulation by the immunomodulatory lipid mediator PGE2. In the human THP-1 macrophage cell line this effect appeared to be primarily mediated by the EP4 receptor with additional involvement of the EP2 receptor. The evidence that EP4 might be more important than EP2 was based on pharmacological stimulation and/or antagonism of these receptors, as well as mRNA and Western immunoblot data. The latter immunoblot experiments identified a clear band of the appropriate size for the EP4 receptor but the EP2 antibody data were less conclusive. Further studies using receptor silencing or genetic knockout animals could provide additional evidence for the relative importance of these receptor isoforms in mediating PGE2's actions. Activation of adenylate cyclase by these receptors caused an acute burst of intracellular cAMP that activated the canonical target PKA. Further studies implicated the RI isoform of PKA as a regulatory signaling component governing PGE2/cAMP modulation of C. sordellii phagocytosis (summarized in Fig. 4). A key unanswered question requiring future study is how PKA activation reduces CASR-dependent phagocytosis. It has been reported that PGE2 suppresses macrophage expression of the class B scavenger receptor CD3650, 51, suggesting that CASR expression might be similarly reduced. However, the effects of PGE2 on phagocytosis are rapid (within 15 minutes of exposure), which would support actions unrelated to new protein expression.
Figure 4. A conceptual model of PGE2 inhibition of vegetative C. sordellii phagocytosis by THP-1 cells.
Vegetative C. sordellii bacteria are phagocytosed via class A scavenger receptors (CASR). PGE2 stimulated intracellular cAMP synthesis by adenylate cyclase (AC) following ligation of the Gαs-coupled EP2 and EP4 receptors. The cAMP burst in turn activated the type I isoform of protein kinase A (PKA-RI), which is involved in suppressing the internalization of C. sordellii. Pharmacological tools used in these experiments include the EP2 agonist butaprost free acid (BFA), the EP2 agonist L-902,688, the EP2 antagonist AH-6809, the EP4 antagonists ONO AE1-208, and the PKA RI agonist 2-Cl-8-MA-cAMP.
Our findings may have relevance to the pathogenesis of puerperal infections in addition to those caused by clostridia. Throughout gestation, PGE2 dampens maternal immune responses against fetal tissues52-55. At term, systemic and local PGE2 levels increase dramatically19, 21 to induce cervical softening and uterine smooth muscle contraction that aids in delivery9. It is this spike in PGE2 production at term that may pose a risk for puerperal sepsis. Relevant to this paradigm, a stable PGE2 analogue delivered into the maternal cervix postpartum in cows increased the incidence of puerperal endometritis56 while a mouse study reported that PGE2 facilitated the establishment of chlamydial uterine infections57. Thus, high PGE2 levels in the female reproductive tract at parturition might increase susceptibility to puerperal infection.
These investigations were limited by their in vitro design. Studies in women or animal models will be important to determine the extent to which PGE2 regulates clostridial pathogenesis in vivo. Preliminary experiments in our laboratory revealed increased mortality in mice exposed to PGE2 in utero during C. sordellii spore infection (data not shown) and this is a future direction for our lab. What is more, our work was largely conducted using a cell line. While the THP-1 cell is a standard human macrophage-like cell line58, these cells may not be an accurate model of primary reproductive tract macrophages. They were originally isolated from a child with leukemia [Tsuchiya, 1980 #3232]. We have previously found that THP-1 cells behave similarly to primary placental macrophages in their capacity to phagocytose S. pyogenes and to be regulated by lipid mediators59. Thus, understanding the mechanisms whereby PGE2 regulates THP-1-C. sordellii interactions may be relevant to reproductive tract innate immunity.
Another limitation of our work was the use of heat-killed bacteria. While advantageous for studying bacteria-receptor interactions without the confounding effects of bacterial products on host cell function, such effects might be relevant in vivo. Clostridium sordellii produces cytotoxins that could regulate macrophage function. Future studies will be needed to determine the extent to which C. sordellii toxins impact macrophage antibacterial defense functions. Lastly, we conducted these studies using vegetative forms of C. sordellii. As a spore-forming anaerobe, it may be equally relevant to define how macrophages recognize and attempt to clear spores of C. sordellii from the infected host. Future studies will be needed to address this issue.
In summary, these data reveal that the endogenous lipid molecule PGE2 can limit the capacity for THP-1 cells to phagocytose unopsonized C. sordellii and this occurs primarily via EP4-mediated activation of PKA signaling cascades. New preventive and therapeutic strategies against this and other reproductive tract bacterial infections may be identified by studying eicosanoid immunoregulation of immune defenses in the uterus.
Acknowledgments
This work was supported by National Institutes of Health grant HD057176 (D.M.A) and a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease award (D.M.A.). We thank Dr. Yibai Hao for assistance with immunoblots.
References
- 1.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43:1436–1446. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 2.Zane S, Guarner J. Gynecologic clostridial toxic shock in women of reproductive age. Curr Infect Dis Rep. 2011;13:561–570. doi: 10.1007/s11908-011-0207-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien DK, Therit BH, Woodman ME, Melville SB. The role of neutrophils and monocytic cells in controlling the initiation of Clostridium perfringens gas gangrene. FEMS Immunol Med Microbiol. 2007;50:86–93. doi: 10.1111/j.1574-695X.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 4.Paredes-Sabja D, Cofre-Araneda G, Brito-Silva C, Pizarro-Guajardo M, Sarker MR. Clostridium difficile Spore-Macrophage Interactions: Spore Survival. PloS one. 2012;7:e43635. doi: 10.1371/journal.pone.0043635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes-Sabja D, Sarker MR. Interactions between Clostridium perfringens spores and Raw 264.7 macrophages. Anaerobe. 2012;18:148–156. doi: 10.1016/j.anaerobe.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Thelen T, Hao Y, Medeiros AI, Curtis JL, Serezani CH, Kobzik L, Harris LH, Aronoff DM. The class A scavenger receptor, macrophage receptor with collagenous structure, is the major phagocytic receptor for Clostridium sordellii expressed by human decidual macrophages. J Immunol. 2010 doi: 10.4049/jimmunol.1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronoff DM, Hao Y, Chung J, Coleman N, Lewis C, Peres CM, Serezani CH, Chen GH, Flamand N, Brock TG, Peters-Golden M. Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii. Journal of Immunology. 2008;180:8220–8230. doi: 10.4049/jimmunol.180.12.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids. 2004;70:207–222. doi: 10.1016/j.plefa.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front Biosci. 2007;12:1329–1343. doi: 10.2741/2151. [DOI] [PubMed] [Google Scholar]
- 10.Koeberle A, Werz O. Inhibitors of the microsomal prostaglandin E(2) synthase-1 as alternative to non steroidal anti-inflammatory drugs (NSAIDs)--a critical review. Curr Med Chem. 2009;16:4274–4296. doi: 10.2174/092986709789578178. [DOI] [PubMed] [Google Scholar]
- 11.Fortier MA, Krishnaswamy K, Danyod G, Boucher-Kovalik S, Chapdalaine P. A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. J Physiol Pharmacol. 2008;59(1):65–89. [PubMed] [Google Scholar]
- 12.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 13.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E(2) and T cells: friends or foes? Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 16.Ballinger MN, Aronoff DM, McMillan TR, Cooke KR, Olkiewicz K, Toews GB, Peters-Golden M, Moore BB. Critical role of prostaglandin E2 overproduction in impaired pulmonary host response following bone marrow transplantation. Journal of Immunology. 2006;177:5499–5508. doi: 10.4049/jimmunol.177.8.5499. [DOI] [PubMed] [Google Scholar]
- 17.Goldmann O, Hertzen E, Hecht A, Schmidt H, Lehne S, Norrby-Teglund A, Medina E. Inducible Cyclooxygenase Released Prostaglandin E2 Modulates the Severity of Infection Caused by Streptococcus pyogenes. J Immunol. 2010 doi: 10.4049/jimmunol.1000838. [DOI] [PubMed] [Google Scholar]
- 18.Serezani CH, Kane S, Medeiros AI, Cornett AM, Kim SH, Marques MM, Lee SP, Lewis C, Bourdonnay E, Ballinger MN, White ES, Peters-Golden M. PTEN Directly Activates the Actin Depolymerization Factor Cofilin-1 During PGE2-Mediated Inhibition of Phagocytosis of Fungi. Sci Signal. 2012;5:ra12. doi: 10.1126/scisignal.2002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertelendy F, Woods R, Jaffe BM. Prostaglandin E levels in peripheral blood during labor. Prostaglandins. 1973;3:223–227. doi: 10.1016/0090-6980(73)90090-7. [DOI] [PubMed] [Google Scholar]
- 20.Kovatz S, Arber I, Korzets Z, Rathaus M, Ben Aderet N, Bernheim J. Urinary kallikrein in normal pregnancy, pregnancy with hypertension, and toxemia. Nephron. 1985;40:48–51. doi: 10.1159/000183426. [DOI] [PubMed] [Google Scholar]
- 21.Lee SE, Romero R, Park IS, Seong HS, Park CW, Yoon BH. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med. 2008;21:89–94. doi: 10.1080/14767050701830514. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Cao S, Good D, Wei M, Ren X. Combination of a fluorescent dye and a Zn-S cluster and its biological application as a stain for bacteria. Inorg Chem. 2010;49:1319–1321. doi: 10.1021/ic9023629. [DOI] [PubMed] [Google Scholar]
- 23.Wan CP, Park CS, Lau BH. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993;162:1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- 24.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002;16:583–585. doi: 10.1096/fj.01-0739fje. [DOI] [PubMed] [Google Scholar]
- 25.Benz PM, Feller SM, Sickmann A, Walter U, Renne T. Prostaglandin-induced VASP phosphorylation controls alpha II-spectrin breakdown in apoptotic cells. International immunopharmacology. 2008;8:319–324. doi: 10.1016/j.intimp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 28.Katsuyama M, Ikegami R, Karahashi H, Amano F, Sugimoto Y, Ichikawa A. Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem Biophys Res Commun. 1998;251:727–731. doi: 10.1006/bbrc.1998.9540. [DOI] [PubMed] [Google Scholar]
- 29.Ikegami R, Sugimoto Y, Segi E, Katsuyama M, Karahashi H, Amano F, Maruyama T, Yamane H, Tsuchiya S, Ichikawa A. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipopolysaccharide in C3H/HeN peritoneal macrophages. J Immunol. 2001;166:4689–4696. doi: 10.4049/jimmunol.166.7.4689. [DOI] [PubMed] [Google Scholar]
- 30.Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA, Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 31.Cheon H, Rho YH, Choi SJ, Lee YH, Song GG, Sohn J, Won NH, Ji JD. Prostaglandin E2 augments IL-10 signaling and function. Journal of immunology. 2006;177:1092–1100. doi: 10.4049/jimmunol.177.2.1092. [DOI] [PubMed] [Google Scholar]
- 32.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: Master Regulator of Innate Immune Cell Function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Serezani CH, Okunishi K, Zaslona Z, Aronoff DM, Peters-Golden M. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of Toll-like receptor signaling in alveolar macrophages. The Journal of biological chemistry. 2011;286:8875–8883. doi: 10.1074/jbc.M110.187815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. Maternal mortality in 2005: estimates developed by WHO, UNICEF, UNFPA and the World Bank. 2007 http://www.who.int/whosis/mme_2005.pdf.
- 36.Ahman E, Shah IH. New estimates and trends regarding unsafe abortion mortality. Int J Gynaecol Obstet. 2011;115:121–126. doi: 10.1016/j.ijgo.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Fawcus SR. Maternal mortality and unsafe abortion. Best practice & research Clinical obstetrics & gynaecology. 2008;22:533–548. doi: 10.1016/j.bpobgyn.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Seale AC, Mwaniki M, Newton CR, Berkley JA. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9:428–438. doi: 10.1016/S1473-3099(09)70172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. American journal of reproductive immunology. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason KL, Aronoff DM. Postpartum group A Streptococcus sepsis and maternal immunology. Am J Reprod Immunol. 2012;67:91–100. doi: 10.1111/j.1600-0897.2011.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deutscher M, Lewis M, Zell ER, Taylor TH, Jr, Van Beneden C, Schrag S. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis. 2011;53:114–123. doi: 10.1093/cid/cir325. [DOI] [PubMed] [Google Scholar]
- 42.Goulet V, Hebert M, Hedberg C, Laurent E, Vaillant V, De Valk H, Desenclos JC. Incidence of listeriosis and related mortality among groups at risk of acquiring listeriosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:652–660. doi: 10.1093/cid/cir902. [DOI] [PubMed] [Google Scholar]
- 43.Sriskandan S. Severe peripartum sepsis. J R Coll Physicians Edinb. 2011;41:339–346. doi: 10.4997/JRCPE.2011.411. [DOI] [PubMed] [Google Scholar]
- 44.Garey KW, Jiang ZD, Yadav Y, Mullins B, Wong K, Dupont HL. Peripartum Clostridium difficile infection: case series and review of the literature. American journal of obstetrics and gynecology. 2008;199:332–337. doi: 10.1016/j.ajog.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Rouphael NG, O'Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, Beekmann S, Guarner J, Killgore GE, Coffman B, Campbell J, Zaki SR, McDonald LC. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. American journal of obstetrics and gynecology. 2008;198:635, e631–636. doi: 10.1016/j.ajog.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa M, Kamada N, Jiao Y, Liu MZ, Nunez G, Inohara N. Protective Role of Commensals against Clostridium difficile Infection via an IL-1beta-Mediated Positive-Feedback Loop. Journal of immunology. 2012 doi: 10.4049/jimmunol.1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. Critical Role for MyD88-Mediated Neutrophil Recruitment during Clostridium difficile Colitis. Infection and immunity. 2012;80:2989–2996. doi: 10.1128/IAI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien DK, Melville SB. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect Immun. 2004;72:5204–5215. doi: 10.1128/IAI.72.9.5204-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Brien DK, Melville SB. Multiple effects on Clostridium perfringens binding, uptake and trafficking to lysosomes by inhibitors of macrophage phagocytosis receptors. Microbiology. 2003;149:1377–1386. doi: 10.1099/mic.0.26268-0. [DOI] [PubMed] [Google Scholar]
- 50.Vinals M, Bermudez I, Llaverias G, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Aspirin increases CD36, SR-BI, and ABCA1 expression in human THP-1 macrophages. Cardiovascular research. 2005;66:141–149. doi: 10.1016/j.cardiores.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y, Tsai SJ. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. The American journal of pathology. 2010;176:850–860. doi: 10.2353/ajpath.2010.090551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kvirkvelia N, Vojnovic I, Warner TD, Athie-Morales V, Free P, Rayment N, Chain BM, Rademacher TW, Lund T, Roitt IM, Delves PJ. Placentally derived prostaglandin E2 acts via the EP4 receptor to inhibit IL-2-dependent proliferation of CTLL-2 T cells. Clin Exp Immunol. 2002;127:263–269. doi: 10.1046/j.1365-2249.2002.01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parhar RS, Kennedy TG, Lala PK. Suppression of lymphocyte alloreactivity by early gestational human decidua. I. Characterization of suppressor cells and suppressor molecules. Cell Immunol. 1988;116:392–410. doi: 10.1016/0008-8749(88)90240-7. [DOI] [PubMed] [Google Scholar]
- 54.Scodras JM, Parhar RS, Kennedy TG, Lala PK. Prostaglandin-mediated inactivation of natural killer cells in the murine decidua. Cell Immunol. 1990;127:352–367. doi: 10.1016/0008-8749(90)90138-h. [DOI] [PubMed] [Google Scholar]
- 55.Tawfik OW, Hunt JS, Wood GW. Implication of prostaglandin E2 in soluble factor-mediated immune suppression by murine decidual cells. Am J Reprod Immunol Microbiol. 1986;12:111–117. doi: 10.1111/j.1600-0897.1986.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 56.Slama H, Vaillancourt D, Goff AK. Pathophysiology of the puerperal period: relationship between prostaglandin E2 (PGE2) and uterine involution in the cow. Theriogenology. 1991;36:1071–1090. [Google Scholar]
- 57.Liu W, Dubinett S, Patterson SL, Kelly KA. COX-2 inhibition affects growth rate of Chlamydia muridarum within epithelial cells. Microbes Infect. 2006;8:478–486. doi: 10.1016/j.micinf.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 59.Soares EM, Mason KL, Rogers LM, Serezani CH, Faccioli LH, Aronoff DM. Leukotriene B4 enhances innate immune defense against the puerperal sepsis agent Streptococcus pyogenes. J Immunol. 2013;190:1614–1622. doi: 10.4049/jimmunol.1202932. [DOI] [PMC free article] [PubMed] [Google Scholar]