Abstract
Serotonin (5-HT) has long been considered as a key transmitter in the neurocircuitry controlling aggression. Impaired regulation of each subtype of 5-HT receptor, 5-HT transporter, synthetic and metabolic enzymes has been linked particularly to impulsive aggression. The current summary focuses mostly on recent findings from pharmacological and genetic studies. The pharmacological treatments and genetic manipulations or polymorphisms of a specific target (e.g., 5-HT1A receptor) can often result in inconsistent results on aggression, due to “phasic” effects of pharmacological agents vs “trait”-like effects of genetic manipulations. Also, the local administration of a drug using the intracranial microinjection technique has shown that activation of specific subtypes of 5-HT receptors (5-HT1A and 5-HT1B) in mesocorticolimbic areas can reduce species-typical and other aggressive behaviors, but the same receptors in the medial prefrontal cortex or septal area promote escalated forms of aggression. Thus, there are receptor populations in specific brain regions that preferentially modulate specific types of aggression. Genetic studies have shown important gene × environment interactions; it is likely that the polymorphisms in the genes of 5-HT transporters (e.g., MAO A) or rate-limiting synthetic and metabolic enzymes of 5-HT determine the vulnerability to adverse environmental factors that escalate aggression. We also discuss the interaction between the 5-HT system and other systems. Modulation of 5-HT neurons in the dorsal raphe nucleus by GABA, glutamate, and CRF profoundly regulate aggressive behaviors. Also, interactions of the 5-HT system with other neuropeptides (arginine vasopressin, oxytocin, neuropeptide Y, opioid) have emerged as important neurobiological determinants of aggression. Studies of aggression in genetically modified mice identified several molecules that affect the 5-HT system directly (e.g., Tph2, 5-HT1B, 5-HT transporter, Pet1, MAOA) or indirectly (e.g. BDNF, nNOS, αCaMKII, Neuropeptide Y). The future agenda delineates specific receptor subpopulations for GABA, glutamate and neuropeptides as they modulate the canonical aminergic neurotransmitters in brainstem, limbic and cortical regions with the ultimate outcome of attenuating or escalating aggressive behavior.
1. Introduction
One of the oldest roots of the neurogenetic analysis of aggressive behavior can be traced to the domestication of feral animals, as illustrated experimentally in the elevated brain levels of serotonin in tame silver foxes (e.g., Popova et al. 1991). No other transmitter system has been more consistently implicated in the neurobiological mechanisms mediating impulsive aggressive behavior than serotonin (Miczek et al. 2002; Miczek et al. 2007; Takahashi et al. 2011). Serotonin emerged as the focus in the initial phase of “top-down” genetics of aggressive behavior, when the brains of individuals that were selected for high aggressive traits revealed lower levels of serotonin, its major acidic metabolite and some of the receptor or transporter molecules upon which this amine acts (Brown et al. 1979; Garattini et al. 1967; Linnoila et al. 1983). It continues to be the key transmitter system of interest in the more recent phase of “bottom-up” genetics, when the gene for a specific receptor or transporter was deleted and the behavioral phenotype indicated a higher than normal level of aggressive behavior (Bouwknecht et al. 2001a; Saudou et al. 1994).
The current understanding of serotonergic mechanisms in aggressive behavior has to accommodate two different but interacting sources of influence. Classically, serotonin has been studied for its role in the predisposition to engage in impulsive violent and anti-social behavior. The question of interest continues to be which features of serotonergic transmission – its rate of synthesis, uptake, metabolism, receptor and transporter expression – are characteristic of an aggressive heritable trait that runs in families (Brunner et al. 1993a). It has become clear that the seductively simple serotonin deficiency hypothesis that links low serotonin activity to the propensity for aggressive behavior is being replaced by more sophisticated and detailed schemes. Recent clinical and preclinical data point to important allelic differences in the genes for specific serotonin receptor subtypes, transporter molecules and metabolic enzymes in impulsively aggressive individuals (de Boer and Koolhaas 2005; Lesch and Merschdorf 2000; Miczek et al. 2002; Miczek et al. 2007; Nelson and Chiavegatto 2001; Takahashi et al. 2011).
A second role for serotonin is to modulate aggressive behavior by its phasic activity, particularly during the anticipation of aggressive and defensive acts and at the termination of an aggressive bout. Superimposed on the serotonergic tone are transient changes in release, impulse flow and receptor activation that are synchronized with the initiation and termination of bouts of aggressive and defensive acts (Ferrari et al. 2003). Phasic serotonergic activity appears to be linked to bouts of several species-normative consummatory behaviors ranging from feeding, drinking, and sexual acts to fighting, and to pathological excesses of these activities. Neuroplastic changes in serotonergic pathways from the dorsal raphé neurons to the neo- and paleocortical terminals result from repeated aggressive experiences as reflected in serotonin receptor regulation, firing rate and release. To further our understanding of the interaction between tonic and phasic serotonergic activities ideally requires real-time measurements over the course of a circadian cycle and arranging conditions that provide a challenge behaviorally and neurochemically.
During the last decade, epigenetic mechanisms for the expression of certain genes that encode for serotonergic metabolic enzymes, transporter proteins and receptors have been scrutinized for their role in impulsively violent individuals. Stressful experiences such as abusive maltreatment during a critical developmental period can lead young adults to engage in more impulsive and violent behavior, particularly in those individuals who are characterized by the expression of a certain allelic variety of Monoamine Oxidase A, an important metabolic enzyme for serotonin (Caspi et al. 2002).
The last decade has seen also a concerted effort to translate more readily between preclinical data and clinical observations, and vice versa. This is evident from both an increasing focus on escalated, atypical forms of aggressive behavior in animal models, and by efforts to define aggressive behaviors using operational and functional definitions and assessments in clinical settings (DSM V).
2. Definition and Measurement of Aggression
Most research on the neurogenetics of serotonin and aggression in both human and veterinary medicine seeks to understand and control pathological aggression (Volavka et al. 2005), while laboratory experiments using animal models typically deal with adaptive, species-typical aggressive behavior. When studying aggression in animals, it is important to consider the ethological significance of the behavior, including its phylogenetic and ontogenetic development and the functions it serves for individuals and the species. Aggressive signals, postures and acts are used to help an animal obtain specific goals, or to defend against threats actual attacks (Miczek et al. 2002). These behaviors occur when individuals compete for food, water and other resources necessary for survival and reproduction (resident-intruder aggression), when they defend their territory or offspring (territorial and maternal aggression), or in response to frustration or fear (Miczek et al. 2001). Engaging in aggressive behavior is often beneficial to the individual and the species. For example, dominance hierarchies are established and maintained through confrontations between rival males (Figure 1), and so-called isolation-induced aggression mimics many elements of the behavior used by resident males to exclude other breeding males from their home territory and their mates (Brain and Benton 1979; Miczek et al. 2001; Table 1). However, it should be noted that isolated housing results in social avoidance in a large proportion of a sample of mice, while aggressive behavior is seen in varying numbers of isolated animals depending on species and strain.
Figure 1. Mouse agonistic behavior.
Behaviors of resident and intruder mice engaged in an aggressive confrontation: (a) the resident leaps and bites the intruder as the intruder attempts to escape; (b) the resident (right) threatens as the intruder (left) holds a defensive upright posture; (c) the resident investigates the intruder’s anogenital region; (d) the resident pursues the fleeing intruder; (e) both resident and intruder engage in a mutual upright defensive posture. Reprinted with permission from Miczek and O’Donnell (1978).
Table 1.
Types of aggressive behavior in preclinical models.
| A. Species-typical aggressive behavior | |||
|---|---|---|---|
| Situational or Experimental variable |
Agonistic behavioral measurements |
References | |
| Dominant resident, mainly in primates and rats | In a stable colony where dominance hierarchy is to be established and maintained. | Frequency and duration of agonistic acts, postures, and displays including displacement, threat, pursuit, and fight. | Bennett et al. 2002 Bernstein et al. 1974 Fairbanks et al. 1999 Higley et al. 1996 Mehlman et al. 1994 Steiniger 1950 Vandenbergh 1967 |
| Territorial resident (resident intruder test), mainly in mice and hamsters | Requires an established territory. In the laboratory, home-cage of experimental male (resident) where it is pair-housed with a female. A male stimulus animal (intruder) that is group housed with other males is introduced into resident’s cage. | Frequency of attack bite, sideways threat, tail-rattle, pursuit, upright posture. Latency to the first bite. | Crawley et al. 1975 Eibl-Eibesfeldt 1950 Miczek and O’Donnell 1978 Van Oortmerssen and Bakker 1981 |
| Maternal aggression, mainly in rats, mice, and hamsters | Home-cage of lactating females from postpartum day 1 to 7. Either male or female of intruder is introduced into dam’s cage. | Frequency of attack bite (especially directed at the snout and the face), sideways threat, tail-rattle, pursuit, upright posture. Latency to the first bite | Haney et al. 1989 Hurst 1987 Lonstein and Gammie 2002 Noirot et al. 1975 Sgoifo et al. 1992 |
| Female aggression mainly in primates and rodents. | Dominance hierarchy among female monkeys. In the laboratory settings, female rodent pair-housed with a breeding male. Sexually mature female is introduced as an intruder. | Harrassing attacks by dominant female. Frequency of attack bite, sideways threat, tail-rattle, pursuit, upright posture. Latency to the first bite | DeBold and Miczek 1981 Palanza et al. 2005 Smuts 1986 Zitzman et al. 2005 |
| Isolation-induced aggression (similar to territorial aggression) | Male isolated for 24 h to 8 weeks prior to resident-intruder encounter. | Frequency of attack bite, sideways threat, tail-rattle, pursuit, upright posture. Latency to the first bite. | Cairns and Nakelski 1971 Malick 1979 Valzelli and Bernasconi 1979 Yen et al. 1959 |
| B. Escalated aggressive behavior. | |||
|---|---|---|---|
| Situational or Experimental variable | Agonistic behavioral measurements | References | |
| Alcohol heightened aggression, mainly in rats and mice | Ethanol (1.0 g/kg) is injected intraperitoneally or orally or self-administered before the resident-intruder encounter. | Frequency of attack bite, sideways threat, tail-rattle, pursuit, upright posture. Latency to the first bite Targets of attack bites (head, dorsal areas, ventral areas, appendages) |
Blanchard et al. 1987 Miczek and de Almeida 2001 Miczek et al 1992, 1998a Peeke and Figler 1981 |
| Social provocation (instigation), mainly in hamsters, mice, and rats | A resident male is exposed to another male behind a protective screen in his home-cage prior to the resident-intruder encounter. | Fish et al. 1999 Heiligenberg 1974 Potegal 1991 Potegal and Tenbrink 1984 | |
| Frustration-heightened aggression | A resident male is positively reinforced. The reinforcement is abruptly omitted before the resident-intruder encounter. | Berkowitz 1993 De Almeida and Miczek 2002 | |
| Aggression induced by low glucocorticoids | Resident rats are adrenalectomized and implanted with a low-dose corticosterone pellet | Haller et al. 2001 | |
| Affective defense (“rage”), mainly in Cats | Electrical stimulation (0.2–0.8 mA, 63 Hz, 1 ms per half cycle duration) delivered in medial hypothalamus or midbrain periaqueductal gray. | Hissing, arching of the back, retraction of the ears, piloerection, unsheathing of the claws, papillary dilatation and paw striking | Hess 1954 Leyhausen 1979 Siegel et al. 1999 |
Aggressive behavior can be classified as offensive or defensive based upon the distal and proximal conditions that precipitated it, the topography of the behavior, and its consequences (Blanchard and Blanchard 1977; Brain 1979). Defensive behaviors occur in response to threatening or fear-inducing stimuli and often result in escapes (Brain 1979). In male rodents, specific defensive behaviors include escape, freezing, defensive postures and threats, which occur in response to attacks by either predators or conspecifics (Blanchard et al. 2003; Pellis and Pellis 1988; Rasia-Filho et al. 2008). Postpartum female rodents engage in maternal aggression, which includes both defensive and offensive elements, to protect their newborn offspring from male intruders (Lucion and de Almeida 1996; Parmigiani et al. 1998).
“Violence” is a controversial concept among ethologists. The term has been used to describe escalated, pathological and abnormal forms of aggression characterized by prolonged and frequent attack bites and aggressive behavior with brief latencies (Miczek et al. 2002; Miczek et al. 2003). The differences are quantitative rather than qualitative, with violence marked by shorter attack latencies and higher frequencies and longer durations of fighting than adaptive aggression. Violence has been described to be qualitatively different from adaptive aggression. For instance, attack bites aimed at vulnerable parts of the opponent’s body are considered abnormally aggressive, when adaptive aggression consists of less-injurious bites directed at the intruder’s back and flanks (Haller et al. 2005). Additional qualitative distinctions that have been proposed include context-independent attacks, which are directed at an opponent regardless of its sex, responsiveness (free-living/anaesthetized/dead) or the test environment (home/neutral cage) (Koolhaas 1978), and lack of ritualistic behaviors, quantified as Attack/Threat (A/T) ratios (Haller et al. 2005). Therefore in principle “violence” could refer either to quantitatively escalated or hyper-aggression, or to qualitatively abnormal forms of aggression, or even rarely to aggression that is both escalated and abnormal (for review see Natarajan and Caramaschi 2010).
Human and non-human aggressive behaviors have some common features, but most animal aggression is less complex. Social norms establish boundaries for what is accepted as appropriate aggressive behavior, but the array of inappropriate interpersonal behaviors classified as violence is a serious social and mental health issue (Ferris et al. 2008). Numerous psychiatric disorders defined in the DSM-IV R and the new DSM-V (scheduled for publication in May 2013), including schizophrenia, brief reactive psychosis, anxiety disorder, adjustment disorder, impulse control disorder, antisocial personality disorder, attention deficit disorder, mania/depression, PTSD, autism, and substance abuse, specify aggressive behavior among their symptoms (Boles and Miotto 2003; Raine 2002; Rydén et al. 2009; Volavka et al. 2005).
It can be useful to classify human aggressive behavior as either defensive, premeditated, or impulsive-hostile in nature (Stoff and Vitiello 1996; Vitiello and Stoff 1997). The premeditated (e.g., predatory and instrumental) and impulsive forms of aggression are especially likely to be diagnosed as pathological and in need of treatment. Impulsive, but not premeditated, aggression is linked to biological and environmental causes, as well as pharmacological or psychological treatment response factors, by a growing body of empirical data (Coccaro et al. 2010).
Aggression as a behavioral state in humans has usually been measured by provoking subjects in competitive situations with fictitious opponents and then giving them opportunities to engage in quantifiable responses that are defined as aggressive (see Table 2, for review see Miczek et al. 2002). Human aggression as a trait is assessed using psychometric measures including inventories, questionnaires and scales. The role of 5-HT in human aggression has been successfully investigated using such laboratory techniques (Table 2), but relating laboratory indices of aggression to actual violence and aggression outside the laboratory remains a critical challenge for such approaches; identifying subtypes of human aggression with psychometric and laboratory methods is also difficult. The psychometric instruments used to differentiate between individuals with contrasting aggressive traits such as the impulsive – reactive – hostile – affective subtype versus the controlled – proactive – instrumental – predatory subtype (Stoff and Vitiello 1996) are summarized in Table 2.
Table 2.
Experimental protocols for assessing 5-HT effects on human aggressive behavior
| A. Experimental manipulations | |||
|---|---|---|---|
| Experimental Manipulation | Measurement | Trait/State | References |
| Aggressive responses toward a competitor are measured in the form of electric shock settings | Activate buttons at 5–10 settings, each corresponding to a different intensity or duration of electric shock | State | Buss 1961 Godlaski and Giancola 2009 |
| A fictitious instigator or competitor is the target of aggressive responses that are measured in the form of electric shock deliveries | Setting of electric shock level on a scale from 1–10 | State | Chermack and Giancola 1997 Taylor 1967 |
| The subjects are provoked by having points subtracted in a competitive task. The point losses are attributed to a fictitious opponent, but are actually random. Subjects responded by retaliation of point subtractions (= aggressive responses) | Number of point subtractions from a fictitious competitor | State | Cherek and Heistad 1971 Cherek and Lane 1999 Gowin et al. 2010 |
| Aggression was defined as delivery of electric shocks to a fictitious opponent | Uses a modified version of the Buss aggression machine. Setting of shock level on a scale from 1–5 | State | Giancola et al. 2009 Zeichner and Pihl 1979 |
| B. Psychometric inventories | |||
|---|---|---|---|
| Psychometric Assessment | Instrument | Trait/State | References |
| Aggression, impulsivity and hostility are measured by Minnesota Multiphasic Personality Inventory (MMPI) | Inventory | Trait | McKinley et al. 1948 Nagtegaal and Rassin 2004 |
| Buss-Durkee Hostility Inventory (BDHI), a self-rating scale of anger and hostility. 66 items with false/true answers; also contains 7 scales: assault, indirect aggression, irritability, negativism, resentment, suspicion and verbal aggression | Inventory | Trait | Buss and Durkee 1957 |
| Anger and anxiety are measured by State-Trait Anger Expression Inventory (STAXI) | Inventory | State/Trait | Kim et al. 2009b Spielberg et al. 1973 |
| Aggression is measured by Beck Anxiety Inventory and Beck Depression Inventory | Inventory | Trait | Beck et al. 1961 Lamar et al. 2009 |
3. Aggressive “trait” vs. “states”
A frequently reiterated theory, based on early clinical and preclinical studies, links impulsive, hostile, and violent behavior to a serotonin deficiency (Brown and Goodwin 1986; Goldman et al. 1992; Lesch and Merschdorf 2000; Linnoila and Virkkunen 1992; Mann 1999; Valzelli 1977). Individuals exhibiting such behavior may benefit from pharmacological treatments aimed at inhibiting 5-HT transporters (e.g., SSRIs such as fluoxetine or citalopram), activating 5-HT1A receptors (e.g., buspirone) or blocking 5-HT2A receptors (e.g., risperidone). Acute treatment with these drugs induces phasic changes in 5-HT function that are associated with their transient anti-aggressive effects. In vivo microdialysis allows transient changes in extracellular 5-HT levels to be monitored in anticipation of, during, and after aggressive encounters in rats. One study reported reduced 5-HT levels in the prefrontal cortex during and after the aggressive confrontation, while no changes were detected in the nucleus accumbens, another terminal region (Van Erp and Miczek 2000; Figure 2). By contrast, chronic treatment with these anti-aggressive compounds may promote as yet undefined neuroadaptive changes in 5-HT function such as autoreceptor desensitization, which may in turn be associated with the emergence of therapeutic effects.
Figure 2. Dopamine and serotonin during aggression.
Measurements of extracellular dopamine and serotonin via in vivo microdialysis in resident male rats before, during, and after a confrontation with an intruder. (a) In the nucleus accumbens (top panel), dopamine levels (gray circles) rise and remain elevated after the confrontation, while serotonin levels (black diamonds) do not significantly change. (b) In the prefrontal cortex (bottom panel), dopamine levels rise after the confrontation, while serotonin decline and remain lower after the confrontation. Samples were collected every 10 min and levels are expressed as mean percent of baseline ± SEM. Baseline was measured for 50 min before the fight. The vertical light gray bar indicates the occurrence of the 10-min fight. * and ** represent significant differences from baseline (dashed line) at the p < 0.05 and p < 0.01 levels, respectively. Reprinted with permission from Van Erp and Miczek (2000).
On the other hand, aggression as a “trait” is the focus of genetic studies. While these aggressive traits are clearly polygenic, it is remarkable that several studies have found an interaction between genotypes such as TPH2, MAO-A and 5-HTT polymorphisms and environmental triggers such as social stress underlying an increased likelihood of violent outbursts (see below). For example, a SNP in TPH2 gene (A2051C) has been linked to aggressive behavior in rhesus monkeys. Monkeys with the AA/AC genotype that were reared without their mother (peer-reared) showed increased aggressive acts compared to those with a CC genotype, but the difference disappeared when infants of both genotypes were reared by their mothers (Chen et al. 2010). This review will focus on the interaction between salient environmental events and genes and the subsequent effects on aggressive behaviors.
Gene-gene interactions are also of interest and need to be explored. For example, Passamonti et al. (Passamonti et al. 2008) showed interactions between 5-HTT and MAOA polymorphisms that affected the activity of the anterior cingulate cortex, a brain area that has been implicated in impulsivity, including impulsive aggression. Many other genes may have subtle effects on aggressive phenotypes, and stronger effects may emerge as a result of complex epistatic interactions between those genes (Miczek et al. 2001). In the past 15 years, most rodent studies on genetics and aggressive behavior have used conventional knockout techniques, in which the expression of a gene is completely deleted, affecting the whole body through all developmental stages and inducing compensatory changes in other genes (trait-like change; see Table 3). Newer, more subtle methods, including conditional knockout, viral vector microinfusion, and drug-inducible knockout techniques, can produce transient and local changes in gene expression, allowing the study of more “phasic” changes in gene expression and how they influence aggression. Such studies may explain some of the discrepancies in the results from previous genetic and pharmacological studies of 5-HT function and aggression.
Table 3.
Genes and aggressive behavior in mice
| Gene | Abb | Chr | Background strain (Generations of backcross) | Type of aggression | Effects | Serotonin function | References |
|---|---|---|---|---|---|---|---|
| Plasmacytoma expressed transcript 1 | Pet1 (Fev) | 1 | Mix C57BL/6 and 129Sv | Isolation-induced resident aggression | Increased | 90% reduction of brain 5-HT | Hendricks et al. 2003 |
| Arginine vasopressin receptor 1B | V1bR | 1 | Mix C57BL/6 and 129/SvJ | Isloation-induced aggression | Suppressed | Wersinger et al.2002 | |
| Neutral cage aggression | Suppressed | ||||||
| Aadenosine A1 receptor | A1AR | 1 | Mix C57BL and 129/OlaHsd | Isolation-induced resident aggression | Increased | Gimenez-Llort et al. 2002 | |
| Regulators of G protein signaling 2 | Rgs2 | 1 | C57BL/6J (N5) | Social dominance test | Suppressed | Oliveira-dos-Santos et al. 2000 | |
| v-abl Abelson murine leukemia viral oncogene homolog 2 (Abelson-related gene) | Arg | 1 | Mix C57BL/6 and 129/SvJ | Resident aggression | Suppressed | Koleske et al. 1998 | |
| Glutamic acid decarboxylase 2 | GAD65 | 2 | Mix C57BL/6 and CBA2 | Isolation-induced resident aggression | Suppressed | Stork et al. 2000 | |
| Calcium channel, voltage-dependent, N type, α 1B subunit | Cav2.2 | 2 | Mix C57BL/6J and 129S4/SvJae | Isolation-induced resident aggression | Increased | Increased hypothalamus 5-HT | Kim et al. 2009a |
| dopamine β-hydroxylase | Dbh | 2 | Mix C57BL/6J and 129/SvEv | Isolation-induced resident aggression | Suppressed | Marino et al. 2005 | |
| Brain-derived neurotrophic factor [+/−] | BDNF | 2 | C57BL/6J (>N10) | Isolation-induced resident aggression | Increased | Decreased brain 5-HT | Lyons et al. 1999 |
| β2-microglobulin | β2m | 2 | 129S2/SvPas (Mix C57BL/6J?) | Resident aggression | Suppressed | Loconto et al. 2003 | |
| Oxytocin | Oxt | 2 | Mix C57BL/6J and 129SvEv | Isolation-induced resident aggression | Increased | Winslow et al. 2000 | |
| Resident aggression | Increased | ||||||
| Suppressed | DeVries et al. 1997 | ||||||
| membrane metallo endopeptidase (enkephalinase) | NEP | 3 | Mix C57BL/6J and 129Sv | Resident aggression | Increased | Fischer et al. 2000 | |
| Cannabinoid receptor 1 | CB1 | 4 | CD1 (N15) | Isolation-induced resident aggression | Increased[1] | Martin et al. 2002 | |
| Preproenkephalin | Enk | 4 | Mix CD1 and 129 | Isolation-induced resident aggression | Increased[1] | Konig et al. 1996 | |
| endothelial nitric oxide synthase | eNOS | 5 | Mix C57BL/6 and 129Sv | Resident aggression | Suppressed | Increased 5-HT turnover | Demas et al. 1999 |
| Neutral cage aggression | Suppressed | ||||||
| Interleukin-6 | IL-6 | 5 | Mix C57BL/6, 129/SvEv | Isolation-induced resident aggression | Increased[2] | No difference in brain 5-HT concentration | Alleva et al. 1998 |
| Adrenergic receptor, α2c | Naα2C | 5 | C57BL/6J (N7) | Isolation-induced resident aggression | Increased | Sallinen et al. 1998 | |
| Neuronal nitric oxide synthase | nNOS | 5 | Mix C57BL/6J, 129Sv, DBA2 | Resident aggression | Increased | Reduced brain 5-HT turnover | Nelson et al. 1995 |
| Maternal aggression | Suppressed | Gammie and Nelson 1999 | |||||
| Acetylcholinesterase | AChE | 5 | 129S6/SvEvTac | Home-cage hierarchy | Suppressed | Duysen et al. 2002 | |
| Adenylate cyclase activating polypeptide 1 receptor 1 | PAC1 | 6 | Mix C57BL/6J and 129Sv | Resident aggression | Suppressed | Nicot et al. 2004 | |
| Tachykinin receptor 1 | NK-1r | 6 | Mix C57BL/6 and 129Sv | Isolation-induced resident aggression | Suppressed | De Felipe et al. 1998 | |
| A cluster of vomeronasal receptor genes | V1Ra/b | 6 | 129/SvEv | Maternal aggression | Suppressed | Del Punta et al. 2002 | |
| Oxytocin receptor | Oxtr | 6 | Mix C57BL/6 and 129Sv | Isloation-induced aggression | Increased | Takayanagi et al. 2005 | |
| Cage-mate injury | Increased | ||||||
| Histamine receptor H1 | H1 | 6 | Mix C57BL/6J and 129 | Isolation-induced resident aggression | Suppressed[2] | Increased 5-HT turnover | Yanai et al. 1998 |
| Amyloid β(A4) precursor protein-binding, family A, member 2 | X11L | 7 | C57BL/6 | Competitive feeding | Subordinate | Increased Hypothalamus 5-HT | Sano et al. 2009 |
| Transient receptor potential cation channel, subfamily C, member 2 | TRP2 | 7 | Mix C57BL/6J and 129Sv | Resident aggression | Suppressed | Stowers et al. 2002 | |
| Neuropeptide Y receptor Y1 | Y1 | 8 | Mix C57BL/6 and 129SvJ | Resident aggression | Increased | Reduced TPH mRNA expression in the raphe nuclei | Karl et al. 2004 |
| Prostaglandin E receptor 1 (Subtype EP1) | EP1 | 8 | C57BL/6 (N5) | Neutral cage aggression | Increased | No difference in 5-HT turnover | Matsuoka et al 2005 |
| Norepinephrine transporter | NET | 8 | Mix C57BL/6J and 129SvJ | Isolation-induced resident aggression | Increased[1] | Haller et al. 2002 | |
| Neural cell adhesion molecule 1 | NCAM1 | 9 | C57BL/6J (>N5) | Isolation-induced resident aggression | Increased | Stork et al. 1997 | |
| Aromatase (Cyp19a1) | Ar | 9 | Mix C57BL/6 and 129S/SvEv | Resident aggression | Suppressed | Matsumoto et al. 2003 | |
| Aggression toward female | Increased | ||||||
| 5-hydroxytryptamine (serotonin) receptor 1B | 5-HT1B | 9 | 129S2/SvPas | Resident aggression | Increased | Deleted 5-HT1B receptor expression | Saudou et al. 1994 |
| Maternal aggression | Increased | Brunner and Hen 1997 | |||||
| Estrogen Receptor-α | ERα | 10 | Mix C57BL/6J and 129 | Resident aggression | Suppressed | Ogawa et al. 1998b | |
| Female aggression | Increased | Ogawa et al. 1998a | |||||
| Fyn tryrosine kinase | Fyn | 10 | Mix C57BL/6 and CBA | Isolation-induced resident aggression | Suppressed | Miyakawa et al. 2001 | |
| nuclear receptor subfamily 2, group E, member 1 | Nr2e1 | 10 | C57BL/6J (>N6) | Resident aggression | Increased | Young et al. 2002 | |
| Adenosine A2a receptor | A2AR | 10 | CD1 (N4) | Isolation-induced resident aggression | Increased | Ledent et al. 1997 | |
| Tryptophan hydroxylase 2 | Tph2 | 10 | FVB/N (N7) | Home-cage injury | Increased | Reduced brain 5-HT | Alenina et al. 2009 |
| Glutamate receptor, ionotropic, AMPA1 (α1) | GluR-A | 11 | C57BL/6J (N5) | Isolation-induced aggression | Suppressed | No difference in brain 5-HT level | Vekovischeva et al. 2004 |
| Neutral cage aggression | Suppressed | ||||||
| 5-hydroxytryptamine (serotonin) transporter | SERT | 11 | C57BL/6J (N8) | Isolation-induced resident aggression | Suppressed | Increased extracelluler 5-HT | Holmes et al. 2002 |
| Estrogen Receptor-β | ERβ | 12 | Mix C57BL/6J and 129 | Resident aggression | Increased[1] | Ogawa et al. 1999 | |
| Dopamine transporter | DAT | 13 | Mix C57BL/6J and 129SvJ | Dyadic encounter aggression | Increased | Rodriguiz et al. 2004 | |
| 5-hydroxytryptamine (serotonin) receptor 1A | 5-HT1A | 13 | 129S1/Sv | Resident aggression | Suppressed | Deleted 5-HT1A receptor expression | Zhuang et al. 1999 |
| Catechol-O-methyltransferase 1 [+/−] | COMT | 16 | Mix C57BL/6J and 129SvJ | Neutral cage aggression | Increased | No difference in brain 5-HT level | Gogos et al. 1998 |
| Calcium/calmodulin-dependent protein kinase II alpha [+/−] | αCaMKII | 18 | Mix C57BL/6, 129/OU, BALB/c | Defensive aggression | Increased | Reduced 5-HT release in the dorsal raphe nucleus | Chen et al. 1994 |
| Melanocortin-5 receptor | MC5R | 18 | C57BL/6 (>N7) | Neutral cage aggression | Suppressed | Morgan et al. 2004 | |
| Monoamine oxidase A | MAOA | X | C3H/HeJ | Resident aggression | Increased | Increased brain 5-HT up to 9 fold | Cases et al. 1995 |
| Cage-mate injury | Increased | ||||||
| Cyclic nucleotide– gated channel a2 | Cnga2 | X | Mix C57BL/6J and 129SvEv | Resident aggression | Suppressed | Mandiyan et al. 2005 | |
| Guanosine diphosphate (GDP) dissociation inhibitor 1 | Gdi1 | X | C57BL/6J (N5) | Isolation-induced resident aggression | Suppressed | D'Adamo et al. 2002 | |
| Androgen receptor[3] | AR | X | C57BL/6 and 129SvEv | Isolation-induced resident aggression | Suppressed[2] | Raskin et al. 2009 |
[+/−]: Data obtained from heterozygote of knockout mouse
Behavioral change only at the first encounter
Behavior change after repeated exposure
Nestine-Cre-LoxP conditional knockout mice that selectively lack AR expression in the nervous system.
4. 5-HT receptors
Considerable evidence suggests that serotonin receptors regulate aggressive behaviors in various animal species. Among the multiple 5-HT receptor subtypes, it is mainly the first two families of serotonin receptors (5-HT1 and 5-HT2) which have been studied in regard to their role on aggression (Miczek et al. 2002; Olivier 2004). There is some, but less, information on the involvement of 5-HT3 receptors on aggressive behaviors (McKenzie-Quirk et al. 2005; Ricci et al. 2004; Rudissaar et al. 1999). Further development of adequate pharmacological tools that can activate or inhibit a certain subtype of 5-HT receptor specifically is required to study the role of other receptor subtypes (e.g., 5-HT5, 6, 7, 1e, 1f) on aggression.
4-1. 5-HT1 family
Pharmacological approach
The 5-HT1A receptor partial agonist buspirone has been used clinically to reduce general anxiety. It was shown that buspirone also reduces aggressive behavior in mentally retarded patients (Kavoussi et al. 1997; Ratey et al. 1991), and this compound has been used for the management of aggressive outbursts associated with neuropsychiatric disorders in adults and children (Connor and Steingard 1996; Pabis and Stanislav 1996). Similar findings have been reported in preclinical studies; systemic administration of 5-HT1A receptor agonists (e.g. 8-OH-DPAT, repinotan, alnespirone) dose-dependently decrease aggressive behaviors in a wide range of animal species, including fish, amphibians, birds, rodents, guinea pigs and non-human primates (Bell and Hobson 1994; Blanchard et al. 1988; Clotfelter et al. 2007; de Boer et al. 1999; de Boer and Koolhaas 2005; Dompert et al. 1985; Haug et al. 1990; Joppa et al. 1997; Lindgren and Kantak 1987; McMillen et al. 1988; Miczek et al. 1998b; Muehlenkamp et al. 1995; Nikulina et al. 1992; Olivier et al. 1992; Sanchez et al. 1993; Sperry et al. 2003; Ten Eyck 2008; Tompkins et al. 1980). One exception are fruit flies (Drosophila melanogaster), as they showed increased aggressive behavior after 8-OH-DPAT treatment (Johnson et al. 2009). Selective antagonists of 5-HT1A receptors such as WAY-100635 successfully reversed the effect of 5-HT1A agonists, while the administration of the antagonist by itself did not show any effect on aggression (de Boer and Koolhaas 2005; Mendoza et al. 1999; Miczek et al. 1998b). Notably, in laboratory studies the anti-aggressive effects of most of 5-HT1A agonists are accompanied by nonspecific behavioral side effects including sedation, motor inactivity, stereotypic behavior or reduced social interest (de Boer and Koolhaas 2005; Miczek et al. 1998b; Olivier et al. 1995). However, some 5-HT1A agonists (i.e., alnespirone and S-15535) reduced aggressive behavior specifically without changing the other non-aggressive behaviors, at least in a wild-derived rat strain (de Boer et al. 1999; de Boer et al. 2000; de Boer and Koolhaas 2005). These compounds are suggested to have different effects on 5-HT receptors on presynaptic and postsynaptic terminals (de Boer and Koolhaas 2005), and thus it is possible that a subpopulation of 5-HT1A receptors on which those compounds acts is involved in anti-aggressive effect specifically.
The 5-HT1B receptor agonists seem more specific in their anti-aggressive effect than 5-HT1A agonists. In mice and rats, the systemic administration of 5-HT1B agonists (e.g. CP-94253, CP-93129, CGS-12066B) reduces aggressive behavior without sedation, or motor or sensory impairment (de Almeida et al. 2001a; de Almeida and Miczek 2002; de Boer and Koolhaas 2005; Fish et al. 1999; Miczek et al. 2002; Miczek et al. 2004; Mos et al. 1993; Olivier et al. 1990; Olivier 2004; Figure 3). These effects were antagonized by 5-HT1B/1D antagonist GR-127935, further confirming that the anti-aggressive effects of these compounds were mediated by 5-HT1B receptors (de Boer and Koolhaas 2005). However, there are no clinically approved drugs that target 5-HT1B receptors. There is an amino acid difference in the binding domain of 5-HT1B receptors of humans and rodents, and therefore the pharmacological sensitivity and specificity of 5-HT1B agonists in rodents may not compare with those in humans (Olivier 2004).
Figure 3.
A. Effects of social instigation on aggressive behavior by a resident mouse toward a male intruder. Bars represent the mean frequency ±SEM (vertical lines) of attack bites under control (light gray) and instigated (dark gray) conditions. Asterisks denote statistical significance from control (**P < 0.01). B. Preferential reduction of instigated aggressive behavior by the 5-HT1B agonist anpirtoline (left panel, filled circles) and CP-94,253 (right panel, filled squares). Symbols represent the mean frequency of attack bites, expressed as a percentage of vehicle (V) baseline, ±SEM. Light gray symbols represent non-instigated fighting and dark gray symbols represent instigated levels of fighting. Asterisks denote significance from vehicle baseline (P < 0.05). Adapted from Fish et al. (1999) and de Almeida and Miczek (2002).
The precise functions and sites of action for the anti-aggressive effects of 5-HT1A and 5-HT1B receptors still remain to be resolved. One site of inhibitory action for 5-HT is the 5-HT1 autoreceptors that are localized at either the somata or the synaptic terminal of serotonin neurons. Stimulation of 5-HT1 autoreceptors inhibits serotonergic neural activities and therefore reduces 5-HT impulse flow and release. On the other hand, 5-HT1 receptors are also localized at the postsynaptic terminal as heteroreceptors at the projection sites of serotonin neurons. By contrast, the effect of agonists that act on 5-HT1 heteroreceptors can be interpreted as increasing 5-HT neurotransmission.
Microdialysis studies have shown that both systemic and intra-raphé administration of 5-HT1A and 5-HT1B agonists decreased extracellular levels of 5-HT in the forebrain areas including striatum, hippocampus and frontal cortex (Adell et al. 2001; Bonvento et al. 1992; De Groote et al. 2003; Dekeyne et al. 2000; Gobert et al. 1998; Hjorth and Sharp 1991; Johnson et al. 2001; Knobelman et al. 2000; Kreiss and Lucki 1994; Sprouse and Aghajanian 1987). These data suggest that the inhibition of 5-HT release by 5-HT1A and 5-HT1B agonists is mediated by the 5-HT1 autoreceptors. Note that these findings suggest that compounds which decrease 5-HT neurotransmission concurrently reduce aggressive behavior, which challenges the serotonin deficiency hypothesis of aggression.
On the other hand, the importance of the 5-HT1A and 5-HT1B heretoreceptors on projection sites has been emphasized in studies using lesion or depletion of raphé 5-HT neurons either by systemic administration of the tryptophan hydroxylase inhibitor PCPA or by intracerebral injection of the 5-HT neurotoxin, 5,7-dihydroxytryptamine (5,7-DHT). Since these neurotoxic treatments destroy the function of serotonergic neurons, one can observe the effect of 5-HT1 agonists on postsynaptic receptors exclusively. If only somatodendritic and presynaptic autoreceptors are the site of action of 5-HT1A and 5-HT1B agonists, PCPA or 5,7-DHT treatment should eliminate the anti-aggressive effect of 5-HT1A and 5-HT1B agonists. However, lesions or depletion of 5-HT neurons did not affect the anti-aggressive effects of 5-HT1A and 5-HT1B agonists (de Almeida et al. 2001b; Miczek et al. 1998b) or even had pro-aggressive effects (Sanchez and Hyttel 1994; Sijbesma et al. 1991). While these data may suggest postsynaptic 5-HT1 receptors as critical sites of action, it is important to note that these pharmacological depletions spared a subpopulation of receptors.
The intracranial microinjection technique has been used to determine the critical brain regions underlying the anti-aggressive effects of 5-HT1A and 5-HT1B receptor agonists (Table 4). 5-HT in the mammalian central nervous system derives mainly from the dorsal and median raphé nuclei (DRN and MRN, respectively). Activation of 5-HT1A and 5-HT1B receptors in the DRN with microinfusion of selective receptor agonists consistently reduced aggressive behavior in rats and mice, but with concomitant reduction of motor activity and social interactions (Bannai et al. 2007; Faccidomo et al. 2008; Mos et al. 1993; Van Der Vegt et al. 2003). Infusion of a 5-HT1A agonist into the MRN also reduced aggressive behavior of lactating female rats (de Almeida and Lucion 1997). In projection sites of 5-HT neurons, 5-HT1B receptors likely modulate 5-HT release from synaptic terminals as autoreceptors, whereas both 5-HT1A and 5-HT1B receptors modulate postsynaptic neurons (Olivier et al. 1992). In most studies, local activation of 5-HT1A and 5-HT1B in projection regions (e.g., medial preoptic area, lateral septum, orbitofrontal cortex, anterior hypothalamus, medial hypothalamus, periacqueductal gray) reduced aggressive behavior in various procedures and species (see Table 4). Interestingly, under conditions that may escalate aggression, such as consumption of moderate doses of alcohol or maternal aggression, a 5-HT1A or 5-HT1B agonist further increased levels of aggressive behavior when infused into the medial prefrontal cortex (Faccidomo et al. 2008) or the medial septal area (de Almeida and Lucion 1997), respectively. Further studies are required to delineate the mechanisms for such pro-aggressive effects.
Table 4.
Modulation of aggressive behaviors after local infusion of drugs targeting 5-HT receptors in selected brain regions
| Brain Region | Type of Aggression Species |
Target; Drugs and Doses | Pharmacological Effects | References |
|---|---|---|---|---|
| DRN | Resident aggression, male rats | 5-HT1A: 8-OH-DPAT, 1–10 µg. 5-HT1A/5-HT1B: Eltopronazine, 1–30 µg (agonists) | ↓ aggressive behavior, with inactivity and decreased social interaction | Mos et al. 1993 |
| Resident aggression, male rats | 5-HT1A: Alnespirone, 25 µg (agonist) | ↓ aggression; no side effects | Van der Vegt et al.2003 | |
| Alcohol-escalated aggression, male mice | 5-HT1A: 8-OH-DPAT, 1.0 µg 5-HT1B: CP-94253, 1.0 µg (agonists) | 8-OH-DPAT and CP-94253: ↓ baseline aggression, with reduced motor activity; no effects on alcohol-related aggression | Faccidomo et al. 2008 | |
| Schedule-heightened aggression, male mice | 5-HT1B: CP-93129, 0.1–1.0 µg (agonist) | ↓ escalated aggression; reduced walking behavior | Bannai et al. 2007 | |
| Alcohol-escalated aggression, male mice | 5-HT1B: CP-93129, 0.1–1.0 µg (agonist) | ↓ baseline and alcohol-related aggression (0.5–1.0 µg); concomitant reduction in motor activity | Faccidomo et al. submitted | |
| Maternal aggression, rats | 5-HT1A: 8-OH-DPAT, 0.56 µg (agonist) | ↑ maternal aggression (0.56 µg); no motor effects DPAT-escalated aggression prevented by infusion of CP-93129 (1.0 µg) into the orbitofrontal cortex | Veiga et al. 2011 | |
| MRN | Maternal aggression, rats | 5-HT1A: 8-OH-DPAT, 0.2–2.0 µg (agonist) | ↓ maternal aggression; no side effects | De Almeida and Lucion, 1997 |
| PAG | Maternal aggression, rats | Dorsal PAG 5-HT1A: 8-OH-DPAT, 0.2–2.0 µg (agonist) | ↓ maternal aggression (0.2–2.0 µg); no side effects | de Almeida and Lucion, 1997 |
| Maternal aggression, rats | Dorsal PAG 5-HT2A/2C: α-methyl-5-HT maleate, 0.2–1.0 µg (agonist) 5-HT2A/2C: ketanserin, 1.0 µg (antagonist) | α-methyl-5-HT maleate: ↓ maternal aggression; no motor effects Ketanserin: no effects on aggression, decreased motor activity | de Almeida et al. 2005 | |
| Hypothalamic-stimulated defensive aggression, cats | PAG 5-HT1A: 8-OH-DPAT*, 0.016 ng - 1.0 µg 5-HT2C: DOI*, 3.57 ng-0.54 µg (agonists) | 8-OH-DPAT: ↓ defensive hissing (0.66 – 1.0 µg), effect prevented by antagonist p-MPPI. No motor effect DOI: facilitation of defensive hissing (0.54 µg) | Shaikh et al. 1997 | |
| Septal nuclei | Maternal aggression, rats | Medial septal nucleus 5-HT1A: 8-OH-DPAT, 0.2–2.0 µg (agonist) | ↑ maternal aggression (0.2–0.5 µg); reduced activity only with highest dose (2.0 µg) | de Almeida and Lucion, 1997 |
| Maternal aggression, rats | Medial septal nucleus 5-HT2a/2c: alpha-methyl-5-HT maleate, 0.2–1.0 µg (agonist) 5-HT2A/2C: ketanserin, 1.0 µg (antagonist) | No effects on aggressive or non-aggressive behaviors (agonist) Ketanserin: no effects on aggression, but decreased motor activity | de Almeida et al. 2005 | |
| Resident aggression, male mice (castrated males with hormonal replacement) | Lateral septal nucleus 5-HT1A: 8-OH-DPAT*, 0.33 ng 5-HT1B: CGS12066*,18.0 ng (agonists) | 8-OH-DPAT: no behavioral effects CGS: ↓ aggression with CGS, only in androgen-replacement condition; no side effects | Cologer-Clifford et al. 1997 | |
| Alcohol-escalated aggression, male mice | Lateral septal nucleus 5-HT1B: CP-94253, 1.0 µg (agonist) | No effects on alcohol-related or baseline aggression | Faccidomo et al. 2008 | |
| Hypothalamus | Resident aggression, male mice (castrated males with hormonal replacement) | Medial pre-optic area 5-HT1A: 8-OH-DPAT*, 0.16 ng 5-HT1B: CGS12066*,9.0 ng (agonists) | 8-OH-DPAT: ↓ aggression in androgen- and estrogen-replacement conditions; no motor effects □ CGS: ↓ aggression in androgen- and estrogen-replacement conditions ; no motor effects | Cologer-Clifford et al. 1997 |
| Vasopressin-escalated aggression, male hamsters | Anterior hypothalamus 5-HT1A: 8-OH-DPAT*, 0.03–3.28 ng 5-HT1B: CGS12066*, 4.5–45.0 ng (agonists) | 8-OH-DPAT: ↓ escalated aggression (0.33,3.28 ng); no apparent motor effects 5-HT1B: no effects on aggression (trends toward inhibition) | Ferris et al. 1999 | |
| PAG-stimulated defensive aggression, cats | Medial hypothalamus 5-HT1A: 8-OH-DPAT*, 0.03–1.0 µg 5-HT2C: DOI*,0.036–0.36 µg (agonists) | 8-OH-DPAT: ↓ defensive hissing (0.33–1.0 µg); effect prevented by antagonist p-MPPI DOI: facilitates defensive hissing (0.36 µg); effect prevented by antagonist LY-53,857 | Hassanain et al. 2003 | |
| Amygdala | Maternal aggression, rats | Corticomedial amygdaloid nucleus 5-HT1A: 8-OH-DPAT, 0.2–2.0 µg (agonist) | ↓ maternal aggression (0.5–2.0 µg); no side effects | De Almeida and Lucion, 1997 |
| Prefrontal cortex | Resident aggression, male mice | 5-HT1B: CP-94253, 1.0 µg (agonist) | No effects on aggressive or non-aggressive behaviors | de Almeida et al. 2006 |
| Alcohol-escalated aggression, male mice | 5-HT1B: CP-94253, 0.5–1.0 µg (agonist) | ↑ alcohol-related aggression (1.0 µg); mild increases in activity; no effects on baseline aggression | Faccidomo et al. 2008 | |
| Alcohol-escalated aggression, male mice | 5-HT1B: CP-93129, 0.1–1.0 µg (agonist) | ↓ baseline and alcohol-related aggression (0.1–1.0 µg); no motor effects | Faccidomo et al. submitted | |
| Orbitofrontal cortex | Resident aggression, male mice | 5-HT1B: CP-94253, 0.1–1.0 µg (agonist) | ↓ aggression (0.56–1.0 µg); no side effects. CP-94253 effects prevented by 1B antagonist, GR-127935. | De Almeida et al. 2006 |
| Maternal aggression, rats | 5-HT1B: CP-93129, 1.0 µg 5-HT1B: CP-94253, 0.56–1.0 µg (agonists) | CP-93129: ↓ maternal aggression (1.0 ug); no side effects CP-94253: no behavioral effect | Veiga et al. 2007 | |
| Alcohol-escalated aggression, male mice | 5-HT1B: CP-94253, 0.5–1.0 µg (agonist) | No effects on alcohol-related or baseline levels of aggression | Faccidomo et al. 2008 | |
| Social instigation-escalated aggression, male mice | 5-HT1A: 8-OH-DPAT, 0.1–1.0 µg 5-HT1B: CP-93129, 0.1–1.0 µg (agonists) | 8-OH-DPAT: ↓ instigated aggression (0.56–1.0 µg); no side effects CP-93129: ↓ instigated aggression (only 0.1 µg); no side effects | Centenaro et al. 2008 |
DRN = dorsal raphé nucleus; MRN = median raphé nucleus; PAG = periaqueductal gray area
doses calculated based on the following molecular weights (MW) of the compounds (as available at Sigma-Aldrich): 8-OH-DPAT hydrobromide, MW=328.29; CGS-12066 maleate salt, MW=450.41; DOI hydrochloride, MW=357.62.
Genetic approach
The 5-HT1B receptor was the first molecule to be linked to aggression by using the gene knockout technique. Male mice with disrupted 5-HT1B receptor expression (Htr1b−/−) increased isolation-induced aggressive behavior compared to the wild-type mice (Bouwknecht et al. 2001b; Saudou et al. 1994). However, aggressive behavior of the background strain of mice (129/Sv-ter) was very low, close to zero, and thus the frequency of attack bites in Htr1b−/− was low and the latency to initiate fighting was very long compared to other strains of mice. These mice displayed behavioral disinhibition in other behavioral tests including hyperlocomotor activity (Brunner et al. 1999; Ramboz et al. 1995), drug intake (Crabbe et al. 1996; Rocha et al. 1998), measures of anxiety-like behavior (Brunner et al. 1999; Malleret et al. 1999), and autonomic hyperreactivity to novelty (Bouwknecht et al. 2001a). Females of Htr1b−/− increased their aggressive behavior during the postpartum period (Brunner and Hen 1997). These results suggest a role for 5-HT1B receptors in the inhibition of some aggressive and impulsive behaviors. The level of tissue 5-HT concentration in Htr1b−/− mice was lower than wild-type in nucleus accumbens and spinal cord (Ase et al. 2000).
There are several polymorphisms in the human 5-HT1B receptor (HTR1B) gene. The most frequently studied HTR1B polymorphism in relation to aggressive phenotype is a single nucleotide polymorphism (SNP) G861C. This SNP had significant linkage with aggressive behavior in antisocial alcoholism (Lappalainen et al. 1998). Specifically, 861C, the SNP with lower 5-HT1B receptor expression (Huang et al. 1999), was related to antisocial behavior. This SNP is actually a synonymous polymorphism, in which no amino-acid change occurred, and thus this SNP seems not to have any function by itself. Thus, G861C polymorphism may have a linkage with other functional polymorphisms that causes the expression change of 5-HT1B receptors. Jensen et al. (2009) showed that a SNP (A1997G) in a non-coding regulatory region of the HTR1B gene is actually related to the 5-HT1B expression pattern. This SNP is in the binding site for the microRNA miR-96 that inhibits the translation or degrades the HTR1B mRNA. College students homozygous for the A-allele, who have reduced 5-HT1B receptor expression, reported a greater history of aggressive behaviors than G-allele individuals. Also, another SNP in the 5’UTR region of the HTR1B gene, A161T, was found to correlate significantly with a history of aggression in subjects who completed violent suicides (Zouk et al. 2007). Individuals with the T161 locus had more lifetime aggressive behaviors, and again this polymorphism was linked to reduced transcriptional activity of 5-HT1B receptors (Sun et al. 2002). However, these associations between the HTR1B polymorphisms (G861C, G261T, or C129T) and aggression and antisocial behavior are not seen in other studies (Huang et al. 1999; Kranzler et al. 2002; New et al. 2001; Sinha et al. 2003; Van den Berg et al. 2008). Based on the findings from pharmacological studies and gene knockout and polymorphism studies, it is likely that the 5-HT1B receptor has an important role in the inhibition of certain types of aggression.
In contrast to the strong pharmacological evidence implicating the 5-HT1A receptor in aggression, no linkage has yet been reported between the 5-HT1A gene polymorphism and aggression. Also, deletion of 5-HT1A gene (Htr1a−/−) reduced aggressive behavior in mice (Zhuang et al. 1999), which is the complete opposite of the findings with 5-HT1A agonists. However, there is evidence for a correlation between 5-HT1A receptor expression and aggression. A human PET study found a higher 5-HT1A receptor distribution in prefrontal cortex of subjects with higher aggression scores based on a self-report questionnaire (Witte et al. 2009). Also, mice selected for short latency to attack showed higher 5-HT1A receptor expression and receptor sensitivity (Korte et al. 1996; Van Der Vegt et al. 2001; van Riel et al. 2002; Veenema et al. 2005), while rats selected for higher defensive reactions showed reduced 5-HT1A receptor expression in several brain areas (Popova et al. 1998). It is possible that the polymorphisms which directly or indirectly affect 5-HT1A receptor transcription may be associated with either aggressive or defensive responses.
4-2. 5-HT2 family
Pharmacological approach
Atypical antipsychotic agents (e.g., risperidone) with significant antagonist action at 5-HT2A receptors have been successfully used to reduce aggressive outbursts in patients diagnosed with various neuropsychiatric disorders (Buckley et al. 1997; Buitelaar et al. 2001; Czobor et al. 1995; De Deyn et al. 1999; Fava 1997; Keck, Jr. et al. 2000; Zarcone et al. 2001). However, some reports cast doubt on the routine use of antipychotics (Swanson et al. 2008; Tyrer et al. 2008). In one study, the placebo control group showed the greatest reduction in aggressively challenging behavior compared to antipsychotic drug treatments in people with intellectual disability (Tyrer et al. 2008). In animal models, selective 5-HT2A antagonists (e.g., ketanserin, ritanserin and MDL 100907) reduce aggressive behaviors in a behaviorally non-specific manner (Rodriguez-Arias et al. 1998; Sakaue et al. 2002; Shih et al. 1999; White et al. 1991).
Activation of 5-HT2A and 5-HT2C receptors by DOI and other substituted phenylisopropylamines also reduces aggressive behavior in several species including flies, amphibians, mice and rats (Bonson et al. 1994; de Almeida and Lucion 1994; Johnson et al. 2009; Muehlenkamp et al. 1995; Olivier et al. 1995; Sanchez et al. 1993; Ten Eyck 2008). However, the anti-aggressive effects of 5-HT2 ligands are accompanied by sedative effects in the same dose range. Local infusion of a 5-HT2A/2C agonist into the PAG reduces maternal aggression in rats (de Almeida et al. 2005), whereas microinjections into the medial hypothalamus and into the PAG increased defensive aggression in cats (Hassanain et al. 2003; Shaikh et al. 1997; see Table 4). This latter effect is likely linked to the role of 5-HT2A/2C receptors in anxiety-like behavior (Lucki and Wieland 1990; Nogueira and Graeff 1995). The development of more selectively acting pharmacological tools will allow a more adequate differentiation of 5-HT2 receptor subtypes, and promises to dissociate the anti-aggressive and sedative effects.
Genetic approach
Platelet 5-HT2A receptor binding is increased in patients with personality disorders and in a psychiatric population with greater lifetime aggression scores (Coccaro et al. 1997; McBride et al. 1994). Positive correlation between impulsive physical aggression and 5-HT2A receptor expressions in orbitofrontal cortex has been reported using PET (Rosell et al. 2010) and a similar finding was reported in a postmortem study of suicide victims (Mann et al. 1986; Oquendo et al. 2006). However, another PET study reported opposite changes in 5-HT2A receptor expression (Meyer et al. 2008). It is possible that polymorphisms that affect the level of expression of 5-HT2A receptors can be associated with self-directed aggression. In some samples, a significant linkage was found between polymorphisms in the 5-HT2A receptor (HTR2A) gene, T102C, A1438G and His452Tyr, and aggressive-impulsive trait or adolescent-onset antisocial behavior in humans (Assal et al. 2004; Bjork et al. 2002; Burt and Mikolaiewski 2008; Nomura et al. 2006), but others have reported no such link between aggression and HTR2A polymorphisms (Khait et al. 2005; Van den Berg et al. 2008). Again, the successful pharmacotherapeutic management of aggressive patients using compounds with affinity for 5-HT2A receptors would suggest that violence-prone individuals may be characterized by distinctive HTR2A polymorphisms.
Linkage of a minor polymorphism in the 5-HT2B receptor (HTR2B) gene with antisocial behavior was reported recently. The HTR2B Q20* allele, which is found exclusively in the Finnish population, contains a stop codon in HTR2B and blocked the expression of the 5-HT2B receptor. Males with the Q20* allele are prone to show more impulsive violence or impulsive suicidal behavior interacting strongly with alcohol than control (Bevilacqua et al. 2010). Male mice with disrupted 5-HT2B receptors (Ht2b−/−) also showed increased impulsive behavior. Interestingly, these mice had three times higher testosterone level in the cerebrospinal fluid, which is also consisted with the Q20* individuals among humans.
5. Serotonin transporter (5-HTT)
Pharmacological approach
Aggressive behavior in humans and animals can be reduced and prevented by blocking serotonin transporter molecules, which in turn presumably increases 5-HT levels in the brain. Clinically, chronic treatment (i.e. >3 weeks) with selective serotonin reuptake inhibitors (SSRIs) has been shown to reduce aggressive outbursts and violent behavior in psychiatric patients (Barkan et al. 2006; Blader 2006; Bond 2005; Coccaro and Kavoussi 1997; New et al. 2004; Reist et al. 2003; Walsh and Dinan 2001). However, SSRIs have occasionally been reported to increase the incidence of aggressive and suicidal behavior, and the causes of these paradoxical effects remain unknown (Spigset 1999; Troisi et al. 1995).
Both acute and chronic SSRI treatment can produce a dose-dependent reduction in aggressive behavior in animal models (Carrillo et al. 2009; Delville et al. 1996; Olivier et al. 1989; Pinna et al. 2003). When given acutely to rodents and non-human primates, several SSRIs including fluoxetine, fluvoxamine and sertraline reduced aggression in various contexts (Abbadie et al. 1994; Carrillo et al. 2009; Cutler et al. 1997; Delville et al. 1996; Fairbanks et al. 2001; Ferris et al. 1997; Fuller 1996; Ho et al. 2001; Sanchez and Meier 1997). Mice treated with the SSRI citalopram daily for three weeks no longer showed increased levels of aggression after consuming a moderate dose of alcohol, and baseline levels of aggression also showed modest reductions (Caldwell and Miczek 2008). On the other hand, the very low levels of agonistic behavior typically seen in laboratory rats may be restored to species-typical levels by chronic SSRI treatment (Mitchell et al. 1991; Mitchell 2005; Mitchell and Redfern 1992; Mitchell and Redfern 1997). Thus, SSRIs are more likely to exhibit anti-aggressive effects in conditions of escalated agonistic behavior, such as fighting enhanced by alcohol (Caldwell and Miczek 2008).
Mechanistically, extracellular levels of 5-HT in the prefrontal cortex of rats are elevated by both acute and chronic administration of citalopram or the more potent isomer escitalopram, implying that SSRIs’ effects on aggression and other mood disorders is a result of increased cortical 5-HT (Ceglia et al. 2004). However, the anti-aggressive effects of fluoxetine, another SSRI, may be mediated primarily by acting on neurosteroids and GABA transmission, and only secondarily by elevating 5-HT (Pinna et al. 2003; Pinna et al. 2006). In addition, long-term SSRI treatment probably recruits both pre- and post-synaptic mechanisms and neuroplastic events that may also have therapeutic actions (Benmansour et al. 1999; Blier and de Montigny 1998; Ceglia et al. 2004; Pineyro et al. 1994).
Genetic approach
The serotonin-transporter-gene-linked polymorphic region (5-HTTLPR) is a variation in the length of the 5’-flanking transcriptional control region (promoter) of the 5-HTT gene that affects the gene’s transcriptional activity. The variation has been found in humans (Heils et al. 1996) and also in great apes and rhesus monkeys (Lesch et al. 1997). The short-length (s) allele reduces 5-HTT expression in vitro compared to the long length homozygote (l/l) and lowers the prolactin response to clomipramine in humans, indicating reduced 5-HT function (Heils et al. 1995; Lesch et al. 1996; Whale et al. 2000). Studies in humans have found that males and females with one or two copies of the s allele (s/s, s/l) exhibit more hostility, aggression, anxiety and depression and lower agreeableness than l/l homozygotes (Lesch and Merschdorf 2000). “Type 2” alcoholics who displayed high impulsivity and antisocial behaviors showed a higher frequency of the s allele than either “Type 1” alcoholics without antisocial behavior or healthy controls (Hallikainen et al. 1999). As in humans, rhesus monkeys with the s allele were more aggressive than l/l individuals (Jarrell et al. 2008; Lesch and Merschdorf 2000).
5-HTT polymorphism also exhibits a genotype-environment interaction. Rhesus monkeys with the s allele that were peer-reared without their mothers had lower CSF levels of 5-hydroxyindoleacetic acid (5HIAA) than l/l individuals, but this difference was not present in maternally reared monkeys (Bennett et al. 2002). Peer-reared male monkeys also showed both altered CSF 5HIAA levels and an increase in aggression-related behavior (Higley et al. 1991; Kraemer et al. 1989). Humans carrying the s allele exhibited more suicidal ideations or attempts in response to stressful life events than l/l homozygotes, but did not differ in less stressful situations (Caspi et al. 2003). Therefore, it is possible that animals with the s allele are more vulnerable to stressful challenges, and subsequently escalate their aggressive behaviors towards others and themselves. However, the presence of the s allele did not affect human aggression consistently across sexes (Cadoret et al. 2003) or cultures (Baca-Garcia et al. 2004).
Deletion of the 5-HTT gene in mice (Slc6a4) produced seemingly contrary results. Wild-type C57BL/6J mice attacked more and showed shorter latencies to start fighting in the resident-intruder test than homozygote and heterozygote 5-HTT knockouts (Holmes et al. 2002; Table 3). 5-HTT knockout mice have higher extracellular 5-HT concentrations and lower 5-HT uptake in the forebrain compared to wild type (Mathews 2004). Knockout mice with reduced or absent 5-HTT function also exhibited more than 50 phenotypic changes, including alterations of behavioral, physiological, morphological, and sensory functions (Murphy and Lesch 2008), and those pleiotropic changes in various other phenotypes may contribute to the reduction of aggression in these mice. Comparable findings on 5-HTT and aggression have also been reported in rats; 5-HTT knockout rats on a Wistar/Crl background showed reduced offensive behaviors and longer attack latencies compared to wild types (Homberg 2007). Thus genetic ablation of 5-HTT has consistently been shown to reduce aggressive behaviors in rodents.
6. Monoamine oxidase A (MAOA)
Pharmacological approach
Inhibition of MAOA leads to a reduction in the oxidative metabolism of monoamines, presumably making 5-HT and other monoamines more available in the brain. Although the importance of MAO inhibitors as antidepressants was soon recognized, only a few studies have evaluated the effects of MAO inhibitors on aggression in preclinical models (Miczek 1987). Non-selective inhibitors of both MAOA and MAOB (e.g. phenelzine, isocarboxazid, tranylcypromine) produce acute anti-aggressive effects only at doses that also produce sedation and alter other non-aggressive behaviors (DaVanzo et al. 1966; Sofia 1969; Valzelli et al. 1967; Welch and Welch 1968). Non-selective MAO inhibitors or selective MAOB inhibitors can be clinically useful for treating patients with personality disorders who exhibit suicidal tendencies and impulsive aggression, but the drugs also have a profile of undesirable side effects (Hollander 1999; Raj 2004).
Genetic approach
The gene for MAOA was the first to be identified as a possible determinant for pathological aggression in humans, and it has remained the focus of most genetic and epigenetic studies. A Dutch family with a syndrome of borderline mental retardation and dysregulated impulsive aggression was identified by Brunner and colleagues (Brunner et al. 1993b). Aggressive outbursts were seen in all affected males in the kindred, and some exhibited aberrant sexual behavior, attempted murder and arson. Linkage and sequence analyses identified one missense mutation in the MAOA gene on the × chromosome, so that MAOA function was completely disturbed; the affected males had more serotonin and lower levels of norepinephrine, dopamine, and 5-HT metabolites in their urine (Brunner et al. 1993a). MAOA is also an important factor in animal aggression. Aggressive behaviors (skin wounds among cage mates and briefer attack latency in the resident-intruder test) escalated in male mice with a disrupted MAOA gene on either C3H/He or 129Sv background compared to wild type mice (Cases et al. 1995; Scott et al. 2008). Mice with an MAOA deficiency also showed a large increase in 5-HT and norepinephrine and a slight elevation in dopamine in the brain and liver (Cases et al. 1995; Kim et al. 1997; Table 3); the behavioral changes in the MAOA-deficient mice are probably caused by this change in 5-HT function. Escalated aggression in MAOA mutant mice was blocked by 5-HT2A receptor antagonists such as ketanserin and MDL100907 (Shih et al. 1999). Some of the behavioral and brain structural abnormalities in the MAOA-deficient mice were ameliorated when 5-HT was depleted by PCPA early in the developmental process (Cases et al. 1995; Cases et al. 1996).
MAOA expression can also be affected by variable-number tandem repeat (VNTR) polymorphism on the upstream region of the MAOA gene. The number of tandem repeats determines MAOA levels: alleles with 3.5 or 4 repeats have 2–10 times higher transcription than 3 or 5 repeat alleles in vitro (Denney et al. 1999; Sabol et al. 1998). A powerful interaction between MAOA genotype and environment on aggressive behavior has been reported (Caspi et al. 2002; Figure 4). Individuals with low MAOA expression (MAOA-L) polymorphisms who suffered from abuse, neglect, or traumatic life events in the first 15 years of their lives were more likely to have a history of adolescent conduct disorder, violence and criminal arrests, and also scored higher on aggressive disposition in a self-report questionnaire compared to MAOA-L individuals without abuse or trauma, or individuals with higher MAOA expression (MAOA-H) (Caspi et al. 2002; Foley et al. 2004; Frazzetto et al. 2007; Kim-Cohen et al. 2006; Weder et al. 2009; Widom and Brzustowicz 2006). The effect of MAOA genotype disappeared if all subjects were lumped together regardless of rearing environment (Fresan et al. 2007), and MAOA-H individuals sometimes reported higher aggression in interviews and on questionnaires (Manuck et al. 2000; Manuck et al. 2002). The finding that individuals with the MAOA-L allele are vulnerable to environmental factors, showing a high propensity to engage in aggressive behaviors when in a stressful environment, is consistent in males but not females (Sjoberg et al. 2007). Rhesus monkeys have a similar repeat length variation polymorphism in the MAOA gene (rhMAOA-LPR) which is also linked to aggression. Monkeys with a low-activity allele who were reared by their mother showed more aggressive behavior and attained higher dominance rank than monkeys with the low-activity allele who were peer-reared separately from their parents (Newman et al. 2005). Higley and Suomi (1986) attributed this inhibition of aggression to increased fear and anxiety in peer-reared monkeys.
Figure 4.
Means on the composite index of antisocial behavior as a function of MAOA activity and a childhood history of maltreatment. MAOA activity is the gene expression level associated with allelic variants of the functional promoter polymorphism, grouped into low and high activity; childhood maltreatment is grouped into 3 categories of increasing severity. The antisocial behavior composite is standardized (z score) to a M = 0 and SD = 1; group differences are interpretable in SD unit differences (d). Reprinted with permission from Caspi et al. (2002).
Substantial differences in both volume and activity of limbic system and neocortical areas between MAOA-L and MAOA-H individuals have been found in neuroimaging studies (see Buckholtz and Meyer-Lindenberg 2008 for a review). In healthy male human volunteers with the MAOA-L variant, fMRI showed smaller limbic and orbitofrontal volumes and higher activity in amygdala and hippocampus during aversive recall (Meyer-Lindenberg et al. 2006), which may be related to violent behavior. Lower MAOA activity in cortical and subcortical brain areas is associated with elevated aggression as measured by a self-report questionnaire, with no effect of MAOA polymorphism (Alia-Klein et al. 2008). These data show that the MAOA activity is one determinant of the propensity to aggression. The interaction between MAOA polymorphism and stressful social experiences is especially critical, since it can escalate aggression and can also change relevant brain structures.
7. Modulation of serotonergic activity by other systems
The 5-HT neurons in the raphé nuclei are modulated by other amines, acids, peptides and steroids (Adell et al. 2002). Several efforts have been undertaken to uncover the nature of the neural systems that modulate 5-HT neurons to promote escalated aggressive behaviors. Here we will focus briefly on inhibitory and excitatory neurotransmitters and some neuropeptides in terms of their interaction with 5-HT system. Especially, dorsal raphé nucleus (DRN), the largest 5-HT nucleus in the brain, will be highlighted because of its important role for aggression (Bannai et al. 2007; Faccidomo et al. 2008; Jacobs and Cohen 1976; Koprowska and Romaniuk 1997; Mos et al. 1993; Sijbesma et al. 1991; Van Der Vegt et al. 2003; Vergnes et al. 1986).
7-1 Excitatory and Inhibitory Amino acids
γ-Aminobutyric acid (GABA)
GABA is the major inhibitory neurotransmitter in the brain. Large numbers of GABA interneurons and distal GABAergic afferents are found in the DRN (Belin et al. 1983; Gervasoni et al. 2000; Nanopoulos et al. 1982; Wang et al. 1992), and both GABAA and GABAB receptors are expressed in the DRN (Bowery et al. 1987). In vivo electrophysiology studies have shown that the activation of either GABAA or GABAB receptors can inhibit the cell firing of serotonergic neurons (Colmers and Williams 1988; Gallager and Aghajanian 1976; Innis and Aghajanian 1987; Judge et al. 2004). On the other hand, in vivo microdialysis studies have shown that the 5-HT release may be differentially modulated by GABAA receptors and GABAB receptors depending on the projection sites (Tao et al. 1996). In addition, microinjection of a GABAB receptor agonist into the DRN can induce either increases or decreases of 5-HT neuronal activity (Abellan et al. 2000; Takahashi et al. 2010b; Tao et al. 1996).
Systemic administrations of positive modulators of GABAA receptors such as benzodiazepines, barbiturates, and neurosteroids exert dose-dependent biphasic effects on aggressive behaviors, from escalation to inhibition (Miczek et al. 2003). Low to moderate doses of GABAA positive modulators escalate aggression in mice, rats, pigs, and monkeys (Arnone and Dantzer 1980; Cole and Wolf 1970; Ferrari et al. 1997; Fish et al. 2001; Gourley et al. 2005; Miczek 1974; Miczek and O’Donnell 1980; Mos and Olivier 1989; Rodgers and Waters 1985; Weerts and Miczek 1996). On the other hand, pharmacological activation of GABAA receptors in the DRN inhibits aggressive behaviors in rats (Van Der Vegt et al. 2003) but has no effect in mice (Takahashi et al. 2010a). However, we found an interaction between alcohol and GABAA receptor in the DRN on aggressive behavior. Only under the influence of alcohol, local administration of GABAA receptor agonist muscimol in the DRN also heightened aggressive behaviors (Takahashi et al. 2010a). Therefore, GABAA modulation of serotonergic neurons may connect to drug-induced escalated aggression (e.g., alcohol, benzodiazepines) but not to species-typical aggression.
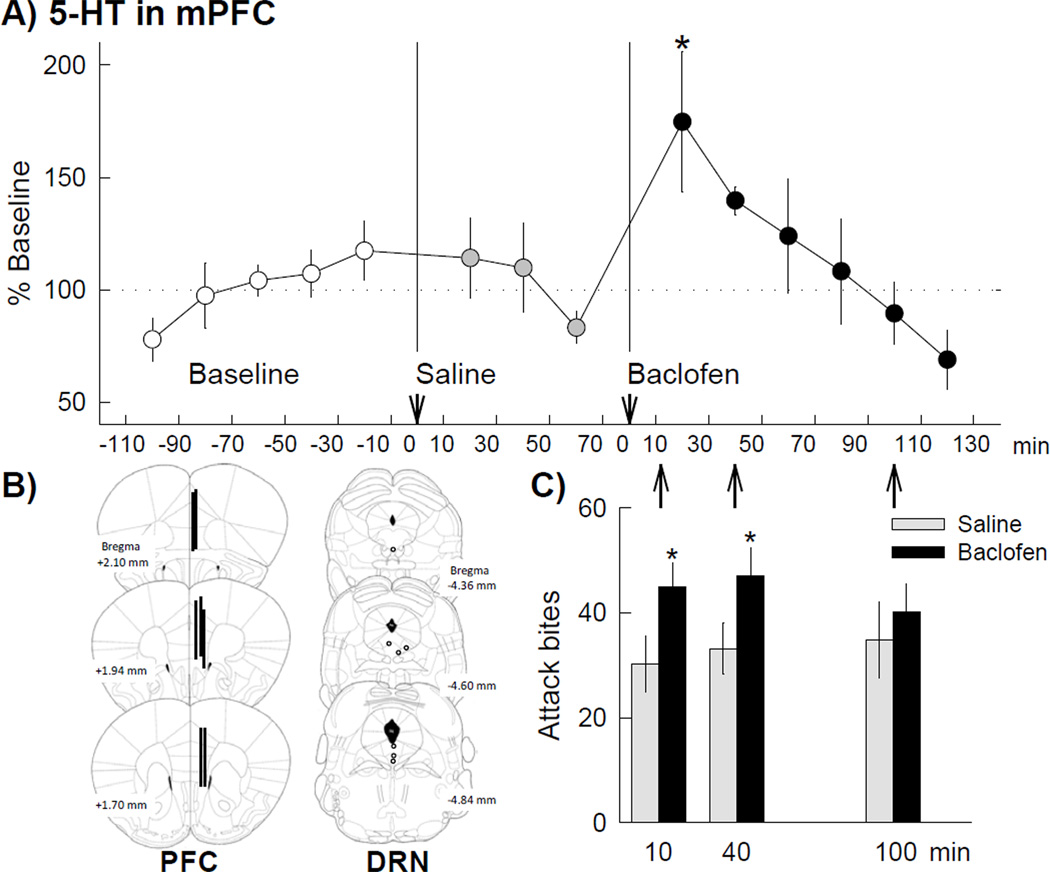
GABAB receptors in the DRN are also involved in the escalation of aggression in mice. Pharmacological activation of GABAB receptors in the DRN escalates inter-male aggression (Takahashi et al. 2010b). The pro-aggressive effect of GABAB agonist baclofen was blocked by selective agonists of GABAB receptors (phaclofen, CGP54626). Additionally, the systemic administration of baclofen also escalates aggression in male mice. Therefore, both GABAA and GABAB receptors are involved in escalated aggression via different mechanism to modulate different types of aggression. In vivo microdialysis showed that GABAB activation in the DRN increased extracellular 5-HT level in the medial prefrontal cortex (Takahashi et al. 2010b; Figure 5). This result suggests that the phasic activation of 5-HT system may be able to promote certain types of escalated aggressive behaviors in mice.
Figure 5. Extracellular 5-HT concentration in the medial prefrontal cortex (mPFC) of mice after GABAB receptor activation in the dorsal raphe nucleus (DRN).
(A) Baclofen microinjected into the DRN increased the 5-HT level in the mPFC whereas saline injection did not change the 5-HT level. Twenty-minute samples were collected: 5 samples for baseline, 3 samples after saline injection, and 6 samples after baclofen (0.06 nmol) injection. Data are means ± SEM expressed as percentage of baseline (n=7); * = p < 0.05 compared to baseline. (B) Histological representation of probe placement in the mPFC for the microdialysis (vertical bars: 2mm probe membrane) and drug injection site in the DRN (circles). (C) The effect of 0.06 nmol baclofen (black bars) or saline (gray bars) on attack bites at different post-injection intervals (10, 40 and 100 min, corresponding to fractions 9, 11, and 14 in the microdialysis, respectively). Escalated attack bites were observed both 10 and 40 minutes after the intra-DRN baclofen injection. Values are means ± SEM; * = p < 0.05compared to corresponding vehicle control. From Takahashi et al. 2010b
Glutamate
The DRN receives glutamate input by the descending projections from the lateral habenula, periaqueductal gray, lateral hypothalamus, interpeduncular nucleus and medial prefrontal cortex (Aghajanian and Wang 1977; Behzadi et al. 1990; Kalen et al. 1986; Maciewicz et al. 1981). Both the N-metyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolaproprionate/kainate (AMPA/kainate), ionotropic receptors for glutamate, are localized on serotonergic neurons and increase the 5-HT release in the DRN and its projection areas (Celada et al. 2001; Pallotta et al. 1998; Tao et al. 1996; Tao and Auerbach 2000; Vandermaelen et al. 1986). Metabotropic receptors of glutamate (mGluRs) are also found in the DRN, but their function on serotonergic neurotransmission and aggressive behavior needs to be characterized by further investigations. Systemic administrations of classic antagonists of NMDA receptors, including phencyclidine (PCP) and dizocilpine (MK-801), can increase aggressive behavior (Burkhalter and Balster 1979; Krsiak 1974; McAllister 1990; Musty and Consroe 1982; Rewerski et al. 1971; Wilmot et al. 1987), while other studies find that these compounds are suppressive and sedative due to their marked side-effects (Belozertseva and Bespalov 1999; Lang et al. 1995; Miczek and Haney 1994; Tyler and Miczek 1982). Memantine and neramaxane block the channel of NMDA receptors with different characteristics and promote alcohol-heightened aggression in resident mice confronting an intruder. Anatomically discrete analysis is required to identify the sites of action for NMDA receptors that produce escalated aggressive behavior. The 5-HT system is one of the candidates, especially the descending glutamatergic projection from the medial prefrontal cortex (mPFC) to the DRN will be interesting to investigate. The prefrontal cortex (PFC) is implicated in the emotion regulation including aggression (Davidson et al. 2000; Miczek et al. 2007). This mPFC-DRN glutamatergic projection is involved in the controllability or emotion regulation (Amat et al. 2005) and it is possible that this pathway is also involved in the regulation of aggression.
7-2 Neuropeptides
Corticotropin-releasing factor (CRF)
The DRN is innervated by CRF immunoreactive fibers, and is the site for both subtypes of CRF receptors, CRF1 and CRF2 (Chalmers et al. 1995; Potter et al. 1994; Swanson et al. 1983). CRF, CRF receptors and other peptides of the CRF family (i.e. urocortins), play key modulatory roles on DRN 5-HT neurons (Valentino and Commons 2005). Electrophysiological and microdialysis studies consistently report that i.c.v. or intra-DRN microinjections of CRF, or drugs targeting CRF receptors, exert potent modulatory control over 5-HT neural firing (Kirby et al. 2000; Lowry et al. 2000), and 5-HT output to limbic, striatal and prefrontal cortical regions (Amat et al. 2004; Amat et al. 2005; Forster et al. 2008; Lukkes et al. 2008; Meloni et al. 2008; Price et al. 1998; Price and Lucki 2001).
CRF, CRF1 and CRF2 receptors are implicated in maternal and inter-male aggression in mice and hamsters (D’Anna et al. 2005; Farrokhi et al. 2004; Gammie et al. 2004; Gammie et al. 2005; Gammie et al. 2007; Gammie and Stevenson 2006). In rats, i.c.v. or intra-amygdala infusions of low doses of CRF itself facilitated or induced pro-aggressive effects (Elkabir et al. 1990; Tazi et al. 1987). Under conditions of escalated aggression promoted by moderate doses of alcohol in male mice, CRF1 receptors are a promising target for pharmacological intervention. Systemic administration of the antagonists of CRF1 receptors reduce alcohol-heightened aggression, but also reduce baseline levels of aggressive behavior (Quadros et al. 2009b). When locally administered into the DRN, CRF1 antagonists (e.g., CP-154526 or MTIP) prevent the escalated levels of aggression observed after consumption of alcohol, with no detectable side effects on other behaviors. Remarkably, such anti-aggressive effects of CRF1 antagonists can be abolished with the infusion of 8-OH-DPAT into the DRN, which transiently slows 5-HT impulse flow. On the other hand, microinfusion of a CRF2 antagonist (e.g., Astressin-2B) into the DRN escalates aggressive behavior (Quadros et al. 2009a).
The modulation of aggressive behaviors by CRF systems depends on the species such as mice or rats, and type of aggression (species-typical, maternal or escalated aggression). Initial evidence suggests the 5-HT cells in the DRN as one of the critical sites for such modulation in escalated aggression that is promoted by alcohol, with presumably opposing roles for CRF1 and CRF2 receptors.
Neuropeptide Y (NPY)
NPY controls primarily food intake, energy balance, and metabolic regulation (Herzog 2003). In addition to the critical role for energy homeostasis, NPY is also implicated in aggressive behavior (Emeson and Morabito 2005). Male mice with deleted expression of Y1 receptor (Y1−/−) showed obesity and reduced energy homeostasis (Kushi et al. 1998). These animals also exhibited increased aggressive behaviors in the resident-intruder test (Karl et al. 2004). However, escalated aggression was observed only in the home cage but not in the novel environment in Y1−/− mice. Both NPY and its receptors can be found in the DRN (Martel et al. 1990; O’Donohue et al. 1985; Saria et al. 1984), and NPY inhibits both hyperpolarizing and depolarizing slow synaptic potentials in the DRN (Kombian and Colmers 1992). Y1−/− mice showed reduced expression of tryptophan hydroxylase mRNA in the raphé nuclei, and also 5-HT1A agonists could suppress the heightened aggression of Y1−/− mice (Karl et al. 2004). These results suggest that the disruption of Y1 receptor expression specifically increases territorial aggression by altering 5-HT function.
Arginine vasopressin (AVP)
AVP is a neuropeptide that modulates a variety of social behaviors including pair-bonding, social recognition, maternal behavior, and aggression (Albers and Bamshad 1998; Coccaro et al. 1998; Ferris et al. 1992; Goodson 2008; Koolhaas et al. 1990; Neumann et al. 2010; Winslow and Insel 1993). Selective antagonists of vasopressin V1a receptors (SRX251, [d(CH2)5Tyr(Me)AVP]) inhibited inter-male aggression (Ferris et al. 2006; Ferris and Potegal 1988), suggesting the involvement of V1a receptors in aggressive behaviors. Evidence points to a critical role of the interaction between AVP and 5-HT in certain types of aggressive behaviors. In humans, a positive correlation has been observed between AVP concentrations in the CSF and the life history of aggression, a composite measure of trait aggression. Also, there was a positive correlation between AVP concentrations and prolactin responses to a challenge with d-fenfluramine (Coccaro et al. 1998). This result indicates that individuals that have higher aggression ratings tend to have a high AVP concentration in the CSF and a hyporesponsive 5-HT system. Neuronal interactions between AVP containing neurons and 5-HT neurons are found in the anterior hypothalamus (Ferris et al. 1997; Ferris et al. 1999), and this AVP-5-HT link is implicated in aggression. Microinjection of AVP into the anterior or lateral hypothalamus increased aggressive behavior in hamsters, and systemic fluoxetine, a 5-HTT inhibitor, blocked the pro-aggressive effect of AVP (Delville et al. 1996; Ferris et al. 1997) Therefore, 5-HT may have an inhibitory effect on the AVP-heightened aggression. By contrast, mice with disrupted Ca2+ channel expression (Cav2−/−) showed escalated level of aggressive behavior and also higher AVP concentration in the CSF. However, these animals showed over-activation of the dorsal 5-HT neurons and increased 5-HT concentration in the hypothalamus (Kim et al. 2009a). Further investigation will uncover how AVP and 5-HT interact and whether there are specific types of aggression that require the activity of either 5-HT or AVP systems independently.
Oxytocin
Oxytocin, which has a structure similar to vasopressin, differing in only two amino acid positions, also plays an important role in the control of social behaviors like vasopressin. Administration of the agonists of oxytocin receptor increased aggressive behavior in lactating females (Bosch et al. 2005; Ferris et al. 1992) but reduced territorial aggression in males (Harmon et al. 2002). Mice in which the gene for oxytocin and its receptor were knocked out, showed enhanced aggressive behaviors in both males and lactating females (Ragnauth et al. 2005; Takayanagi et al. 2005; Winslow et al. 2000) but see (DeVries et al. 1997). In humans, the level of oxytocin in the CSF is inversely correlated with the life history of aggression (Lee et al. 2009). Oxytocin and its receptor are expressed in the DRN, and the effect of local infusion of oxytocin can facilitate passive avoidance in rats (Kovács et al. 1979). However, the exact interactions between the oxytocin and serotonergic systems in their modulatory effects on aggressive behavior remain to be discovered.
7-3 Other molecules that directly or indirectly affect 5-HT pathways and aggression
Tryptophan hydroxylase (TPH)
Variations in TPH activity can directly affect 5-HT neurotransmission. Polymorphisms in the TPH1 or TPH2 gene change the enzyme activity and 5-HT level (Jonsson et al. 1997) for TPH1 gene; (Cichon et al. 2008; Lin et al. 2007; Zhang et al. 2004; Zhang et al. 2005) for TPH2 gene). Two SNPs in tight linkage disequilibrium, A218C and A779C, located in the intron of TPH1 gene (which are often referred to as U (upper) and L (lower) allele), have been shown to be associated with state and trait anger as assessed by interview and self report questionnaires (Manuck et al. 1999; Rujescu et al. 2002). Lower CSF 5-HIAA levels have been observed in healthy men, but not women, with the U allele (Jonsson et al. 1997), whereas the lowest CSF 5-HIAA was observed in LL carriers diagnosed with antisocial alcoholism (Nielsen et al. 1994). Apparently, both U and L alleles may relate to different types of aggression. Hennig et al. (2005) performed a factor analysis on the Buss-Durkee Hostility Inventory (BDHI) and found two factors, named as “neurotic hostility” and “aggressive hostility”. Neurotic hostility is characterized by irritability, resentment, verbal hostility and guilt, whereas aggressive hostility represents a more “cold” aggression including assault and negativism but low guilt. Individuals with the UU allele showed higher aggressive hostility but slightly lower neurotic hostility (Hennig et al. 2005), whereas LL homozygote showed higher level of impulsivity in the measurements which focused more on neurotic hostility (New et al. 1998). This may explain controversial results about the association between the polymorphisms and aggression; depending on which dimensions of aggressive behavior are measured, the association pattern may change.
In mouse models, the focus has been on the Tph2 gene, because Tph2 is preferentially expressed in the brain, whereas Tph1 is more widely distributed throughout the body (Walther et al. 2003). Mice with an R439H point mutation in the Tph2 gene have strongly reduced 5-HTP, 5-HT and its acidic metabolite levels in (Beaulieu et al. 2008). This mutant mouse showed increased aggressive behaviors in the social interaction test in a neutral arena where typically very low levels of aggression are seen (Beaulieu et al. 2008). In contrast, another polymorphism in Tph2, C1473G, which inhibits Tph2 activity in the midbrain, reduced the intensity of aggression in mice (Osipova et al. 2009). The mouse data on the R439H mutation in the Tph2 gene may be relevant to a large association study in children with attention-deficit/hyperactivity disorder (ADHD), which identified a TPH2 gene polymorphism associated with impulsivity (Oades et al. 2008).
Pet-1 (also known as Fev)
Pet-1, one of the transcription factors, is specifically expressed in the serotonergic raphé neurons, and has a critical role in 5-HT neural development. Deletion of Pet-1 expression reduced 5-HT levels in the forebrain, and also depleted expression of TPH, 5-HTT, and the vesicular monoamine transporter 2 (Vmat2). In the resident-intruder test, Pet-1 knockout mice (Pet-1−/−) engaged in a higher frequency and intensity of attacks toward a conspecific male (Hendricks et al. 2003). These increases in aggressive behavior in Pet-1−/− mice are embedded in broad behavioral disruptions that also extended to maternal and anxiety-like behaviors (Hendricks et al. 2003; Lerch-Haner et al. 2008);), and it is possible that those other behavioral changes promote indirectly aggressive behaviors. Since Pet-1 expresses specifically in the 5-HT neurons, the promoter or enhancer regions of this gene are useful for gene manipulation of 5-HT neurons (Scott et al. 2005).
Brain-derived neurotrophic factor (BDNF)
BDNF has several important roles in neuronal survival, development, differentiation and plasticity. Mice with decreased BDNF expression including knockout (BDNF+/−) and conditional knockout (BDNF2L/1LNes-cre and BDNF2L/2LCk-Cre) all showed increased inter-male aggression (Chan et al. 2006; Lyons et al. 1999). Higher extracellular levels of 5-HT in the hippocampus was observed in BDNF+/− mice compared to wild-type (Deltheil et al. 2008). However, fluoxetine could reduce heightened aggressive behavior in BDNF+/− mice (Lyons et al. 1999). All mutants changed 5-HT2A receptor expression, however BDNF+/− showed increased 5-HT2A expression in the lateral frontal cortex and hypothalamus (Lyons et al. 1999) whereas BDNF2L/1LNes-Cre and BDNF2L/2LCk-Cre exhibit reduced 5-HT2A receptor expression in the prefrontal cortex (Chan et al. 2006; Rios et al. 2006). A SNP in the BDNF gene, Val66Met, has attracted considerable interest because of its association with mood disorders and hippocampal function in humans (Egan et al. 2003; Neves-Pereira et al. 2002). Knock-in mice with this human Met allele also showed increased aggressive behavior and changed the response to SSRI treatment (Chen et al. 2006). Conditional BDNF knockout mice using kainate receptor promoter lack BDNA expression especially in hippocampal CA3 area. These mice also showed heightened aggression compared to wild-type, suggesting an important role of hippocampal BDNF in aggression (Ito et al. 2011). In contrast to the consistent results on aggressive behavior among BDNF mutant mice, studies on polymorphisms of the BDNF gene in aggressive behavior in humans remain to be resolved. No association was observed between Val66Met polymorphism and proneness to violence in a Chinese male sample (Tsai et al. 2005). Other SNPs in the BDNF gene may be associated with high impulsivity in children with ADHD (Oades et al. 2008). A further role of BDNF is evident in neuroadaptions in the VTA-accumbens pathway of mice and rats that are repeatedly attacked and show evidence for defeat, submission and disrupted social interactions (Berton et al. 2006; Miczek et al. 2011).
Neuronal nitric oxide (nNOS)
Nitric oxide, a free radical gas which diffuses across membranes, is involved in several cellular functions (for review, see Calabrese et al. 2007). Mice lacking neuronal nitric oxide synthase (nNOS−/−) show various deficits in their physiological development and also behavior (Huang et al. 1993). nNOS−/− males, but not females, showed higher duration of aggressive behavior and also displayed much fewer submissive postures compared to wild-types (Nelson et al. 1995). Serotonergic dysfunctions were observed in the nNOS−/− mice, specifically reduced 5-HT turnover in the brain and deficient 5-HT1A and 5-HT1B receptor function (Chiavegatto et al. 2001). Escalated aggression in the nNOS−/− was rescued by 5-HTP treatment which increased 5-HT level and turnover. These findings point to an important role of nitric oxide for the normal 5-HT function, and thus increased aggression nNOS−/− may be induced by changing 5-HT activity.
α-Calcium-calmodulin kinase II (α-CaMKII)
α-CaMKII is a neural specific enzyme and has been shown to be involved in long-term potentiation (LTP) (Silva et al. 1992). Heterozygotes of α-CaMKII knockout mice showed escalated defensive aggression but not offensive aggression (Chen et al. 1994). In the resident-intruder test, resident α-CaMKII heterozygotes showed similar aggressive behavior as wild-type mice. In contrast, when the mutant was tested as an intruder, they exhibited highly defensive reactions toward the resident. Reduced 5-HT release was observed in the dorsal raphé of α-CaMKII mutant in vitro, and thus the changed 5-HT function may be associated with defensive aggression in this mouse.
8. Concluding remarks
Activation of 5-HT1A, 5-HT1B and 5-HT2a/2c receptors in mesocorticolimbic areas reduces species-typical and other aggressive behaviors. In contrast, agonists at 5-HT1A and 5-HT1B receptors in the medial prefrontal cortex or septal area can increase aggressive behavior under specific conditions. 5-HT exerts a complex role in aggression that depends on (1) the subtypes of receptors and the brain in which they are expressed, (2) the types of aggressive behaviors, and (3) the trait characteristics of the human subject or the mouse (i.e., Mus musculus is a pugnacious species and many inbred strains are quite placid).
Based on genetic studies, it is likely that the 5-HT1B receptor has an important role in the inhibition of aggression. For other receptor subtypes such as 5-HT1A or 5-HT2a receptors, the association between aggressive behavior and polymorphisms in receptor genes all failed to have a reliable relationship compared to relatively consistent pharmacological data. It may be important to determine where in the brain of aggressive individuals the gene expression is changed by the polymorphisms.
An important role of MAOA in aggressive behaviors is supported by genetic studies of deleterious mutation of MAOA gene in humans and knockout mice. In contrast to the serotonin deficiency hypothesis, these results suggest that chronically increased 5-HT levels due to reduced MAOA function - trait-like change - may promote or intensify escalated aggressive displays.
Consistent anti-aggressive effects of S SRI treatment in several species strongly point to the serotonin transporter as a promising therapeutic target for management of impulsive, escalated aggression. More studies are required to determine the precise neurobiological mechanisms recruited for the anti-aggressive effects of SSRIs to emerge, especially as a result of chronic treatment that induces pre- and post-synaptic neuroadaptive processes.
The genetic studies in human and nonhuman primates also suggest 5-HTT polymorphisms such as the short allele as a risk factor for violent traits, which seems to be particularly relevant in combination with environmental stress. Results from transgenic mice lacking 5-HTT are more difficult to interpret due to the wide variety of behavioral functions that are affected by the genetic manipulation.
Gene polymorphism studies of MAOA, 5-HTT and Tph2 revealed critical gene-environment interactions as a risk factor for violent traits. Individuals with a certain allele are particularly prone to engage in violent behavior when they have a history of early life maltreatment, but the effect disappeared when they are reared in an environment with low stress.
Modulatory mechanisms of serotonergic neurons that induce escalated aggression have gradually been identified. So far, evidence points to the modulations of activity of dorsal raphé nucleus by GABA, glutamate, CRF, and its auto-receptors as being particularly relevant to the display of escalated aggression. Genetic analyses of aggressive individuals have identified several molecules that affect the 5-HT system directly (e.g., Tph2, 5-HT1B, 5-HT transporter, Pet1, MAOA) or indirectly (e.g., Neuropeptide Y, αCaMKII, NOS, BDNF).
In the current phase of advanced molecular genetic studies, it is necessary to remind ourselves to match them with more sophisticated behavioral methodologies of aggression. It is evident that the neurogenetic mechanisms differentiate types and patterns of aggressive behavior requiring higher resolution. The current focus on brain serotonin takes advantage of the most intensively studied neural system that has been implicated in the neurogenetics of aggression. It remains astounding how such trace amounts of this indole amine can engender such profound changes in aggressive traits and states.
References
- Abbadie C, Honore P, Fournie-Zaluski MC, Roques BP, Besson JM. Effects of opioids and non-opioids on c-fos-like immunoreactivity induced in rat lumbar spinal cord neurons by noxious heat stimulation. Eur J Pharmacol. 1994;258:215–227. doi: 10.1016/0014-2999(94)90483-9. [DOI] [PubMed] [Google Scholar]
- Abellan MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Albers HE, Bamshad M. Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus) Prog Brain Res. 1998;119:395–408. doi: 10.1016/s0079-6123(08)61583-6. [DOI] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS. Brain monoamine oxidase a activity predicts trait aggression. J Neurosci. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, Panerai AE. Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci. 1998;10:3664–3672. doi: 10.1046/j.1460-9568.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Arnone M, Dantzer R. Effects of diazepam on extinction induced aggression in pigs. Pharmacol Biochem Behav. 1980;13:27–30. doi: 10.1016/0091-3057(80)90115-x. [DOI] [PubMed] [Google Scholar]
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT1A or 5-HT1B receptor knockout mice. J Neurochem. 2000;75:2415–2426. doi: 10.1046/j.1471-4159.2000.0752415.x. [DOI] [PubMed] [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Vaquero C, Diaz-Sastre C, García-Resa E, Saiz-Ruiz J, Fernández-Piqueras J, de Leon J. Lack of association between the serotonin transporter promoter gene polymorphism and impulsivity or aggressive behavior among suicide attempters and healthy volunteers. Psychiatry Res. 2004;126:99–106. doi: 10.1016/j.psychres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Barkan T, Peled A, Modai I, Weizman A, Rehavi M. Characterization of the serotonin transporter in lymphocytes and platelets of schizophrenia patients treated with atypical or typical antipsychotics compared to healthy individuals. Eur Neuropsychopharmacol. 2006;16:429–436. doi: 10.1016/j.euroneuro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3β in behavioral abnormalities induced by serotonin deficiency. Proc Nat Acad Sci U S A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Behzadi G, Kalen P, Parvopassu F, Wiklund L. Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective d-[3H] aspartate labelling of possible excitatory amino acid inputs. Neuroscience. 1990;37:77–100. doi: 10.1016/0306-4522(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Bell R, Hobson H. 5-HT1Areceptor influences on rodent social and agonistic behavior: A review and empirical study. Neurosci Biobehav Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Bespalov AY. Effects of NMDA receptor channel blockade on aggression in isolated male mice. Aggress Behav. 1999;25:381–396. [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Its causes, consequences and control. New York: Mc Graw Hill; 1993. Aggression. [Google Scholar]
- Bernstein IS, Rose RM, Gordon TP. Behavioral and environmental events influencing primate testosterone levels. J Hum Evol. 1974;3:517–525. [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, Belmer A, Hodgkinson CA, Dell’osso L, Suvisaari J, Coccaro E, Rose RJ, Peltonen L, Virkkunen M, Goldman D. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL. Serotonin 2a receptor T102C polymorphism and impaired impulse control. Am J Med Genet. 2002;114:336–339. doi: 10.1002/ajmg.10206. [DOI] [PubMed] [Google Scholar]
- Blader JC. Pharmacotherapy and postdischarge outcomes of child inpatients admitted for aggressive behavior. J Clin Psychopharmacol. 2006;26:419–425. doi: 10.1097/01.jcp.0000227356.31203.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Rodgers RJ, Hendrie CA, Hori K. ‘Taming’ of wild rats (Rattus rattus) by 5HT1A agonists buspirone and gepirone. Pharmacol Biochem Behav. 1988;31:269–278. doi: 10.1016/0091-3057(88)90345-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard CD. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Blanchard DC, Hall J. Ethanol effects on aggression of rats selected for different levels of aggressiveness. Pharmacology, Biochemistry and Behavior. 1987;27:641–644. doi: 10.1016/0091-3057(87)90187-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry. 1998;44:313–323. doi: 10.1016/s0006-3223(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Boles SM, Miotto K. Substance abuse and violence - A review of the literature. Aggress Violent Behav. 2003;8:155–174. [Google Scholar]
- Bond AJ. Antidepressant treatments and human aggression. Eur J Pharmacol. 2005;526:218–225. doi: 10.1016/j.ejphar.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Johnson RG, Fiorella D, Rabin RA, Winter JC. Serotonergic control of androgen-induced dominance. Pharmacol Biochem Behav. 1994;49:313–322. doi: 10.1016/0091-3057(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–104. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der GJ, Maes RA, Hen R, Olivier B. Absence of 5-HT1B receptors is associated with impaired impulse control in male 5-HT1B knockout mice. Biol Psychiatry. 2001a;49:557–568. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der GJ, Hijzen TH, Maes RA, Hen R, Olivier B. Corticosterone responses in 5-HT1B receptor knockout mice to stress or 5-HT1A receptor activation are normal. Psychopharmacology. 2001b;153:484–490. doi: 10.1007/s002130000598. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Brain P, Benton D. The interpretation of physiological correlates of differential housing in laboratory rats. Life Sci. 1979;24:99–115. doi: 10.1016/0024-3205(79)90119-x. [DOI] [PubMed] [Google Scholar]
- Brain PF. Hormones, drugs and aggression. Montreal, Canada: Eden Press; 1979. [Google Scholar]
- Brown GL, Goodwin FK. Cerebrospinal fluid correlates of suicide attempts and aggression. In: Mann JJ, editor. Psychobiology of Suicidal Behavior (Annals of the New York Academy of Sciences Vol. 487) New York: New York Academy of Sciences; 1986. pp. 175–220. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurology of impulsive behavior from serotonin receptor knockout mice. Ann N Y Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993a;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, Vanzandvoort P, Abeling NGGM, Vangennip AH, Wolters EC, Kuiper MA, Ropers HH, Vanoost BA. X-linked borderline mental-retardation with prominent behavioral disturbance -- phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet. 1993b;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Ibrahim ZY, Singer B, Orr B, Donenwirth K, Brar PS. Aggression and schizophrenia: Efficacy of risperidone. J Am Acad Psychiatry Law. 1997;25:173–181. [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman TM. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry. 2001;62:239–248. doi: 10.4088/jcp.v62n0405. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Balster RL. The effects of phencyclidine on isolation-induced aggression in mice. Psychol Rep. 1979;45:571–576. doi: 10.2466/pr0.1979.45.2.571. [DOI] [PubMed] [Google Scholar]
- Burt SA, Mikolaiewski AJ. Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggress Behav. 2008;34:437–445. doi: 10.1002/ab.20251. [DOI] [PubMed] [Google Scholar]
- Buss AH. The psychology of aggression. New York: Wiley and Sons; 1961. [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Langbehn D, Caspers K, Troughton EP, Yucuis R, Sandhu HK, Philibert R. Associations of the serotonin transporter promoter polymorphism with aggressivity, attention deficit, and conduct disorder in an adoptee population. Compr Psychiat. 2003;44:88–101. doi: 10.1053/comp.2003.50018. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Nakelski JS. On fighting in mice: Ontogenetic and experiential determinants. J Comp Physiol Psychol. 1971;74:354–364. doi: 10.1037/h0030584. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Miczek KA. Long-term citalopram maintenance in mice: selective reduction of alcohol-heightened aggression. Psychopharmacology. 2008;196:407–416. doi: 10.1007/s00213-007-0972-z. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology. 2009;205:349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, DeMaeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RM. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology. 2008;201:237–248. doi: 10.1007/s00213-008-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for α-calcium-calmodulin kinase II. Science. 1994;265:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Chen GL, Novak MA, Meyer JS, Kelly BJ, Vallender EJ, Miller GM. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in Rhesus monkeys: a retrospective analysis. Horm Behav. 2010;57:184–191. doi: 10.1016/j.yhbeh.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing DQ, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Heistad GT. Fixed-interval induced aggression. Psychon Sci. 1971;25:7–8. [Google Scholar]
- Cherek DR, Lane SD. Laboratory and psychometric measurements of impulsivity among violent and nonviolent female parolees. Biol Psychiatry. 1999;46:273–280. doi: 10.1016/s0006-3223(98)00309-6. [DOI] [PubMed] [Google Scholar]
- Chermack ST, Giancola PR. The relation between alcohol and aggression: An integrated biopsychosocial conceptualization. Clin Psychol Rev. 1997;17:621–649. doi: 10.1016/s0272-7358(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Winge I, Mattheisen M, Georgi A, Karpushova A, Freudenberg J, Freudenberg-Hua Y, Babadjanova G, Van Den Bogaert A, Abramova LI, Kapiletti S, Knappskog PM, McKinney J, Maier W, Abou Jamra R, Schulze TG, Schumacher J, Propping P, Rietschel M, Haavik J, Nothen MM. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5 ‘-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- Clotfelter ED, O’Hare EP, McNitt MM, Carpenter RE, Summers CH. Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens . Pharmacol Biochem Behav. 2007;87:222–231. doi: 10.1016/j.pbb.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Sheline YI, Berman ME, Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5- HT2A receptor binding. Neuropsychopharmacology. 1997;16:211–216. doi: 10.1016/S0893-133X(96)00194-7. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35:435–444. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole HF, Wolf HH. Laboratory evaluation of aggressive behavior of the grasshopper mouse (Onychomys) Journal of Pharmaceutical Sciences. 1970;59:969–971. doi: 10.1002/jps.2600590710. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Williams JT. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett. 1988;93:300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Cologer-Clifford A, Simon NG, Lu SF, Smoluk SA. Serotonin agonist-induced decreases in intermale aggression are dependent on brain region and receptor subtype. Pharmacol Biochem Behav. 1997;58:425–430. doi: 10.1016/s0091-3057(97)00295-5. [DOI] [PubMed] [Google Scholar]
- Connor DF, Steingard RJ. A clinical approach to the pharmacotherapy of aggression in children and adolescents. In: Understanding Aggressive Behavior in Children. Ann N Y Acad Sci. 1996;794:290–307. doi: 10.1111/j.1749-6632.1996.tb32529.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nature Genetics. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Schleidt WM, Contrera JF. Does social environment decrease propensity to fight in male mice? Behav Biol. 1975;15:73–83. doi: 10.1016/s0091-6773(75)92105-7. [DOI] [PubMed] [Google Scholar]
- Cutler MG, Rodgers RJ, Jackson JE. Behavioural effects in mice of subchronic buspirone, ondansetron, and tianeptine. I. Social interactions. Pharmacol Biochem Behav. 1997;56:287–293. doi: 10.1016/s0091-3057(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995;15:243–249. doi: 10.1097/00004714-199508000-00002. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, Welzl H, Papadimitriou S, Raffaele dB, Tiveron C, Tatangelo L, Pozzi L, Chapman PF, Knevett SG, Ramsay MF, Valtorta F, Leoni C, Menegon A, Wolfer DP, Lipp HP, Toniolo D. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11:2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:1061–1071. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- DaVanzo JP, Daugherty M, Ruckart R, Kang L. Pharmacological and biochemical studies in isolation-induced fighting mice. Psychopharmacologia. 1966;9:210–219. doi: 10.1007/BF02198481. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology. 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Faccidomo S, Fish E, Miczek KA. Inhibition of alcohol-heightened aggression by action at post-synaptic 5-HT1b receptors in male mice. Aggress Behav. 2001a;3:234–235. [Google Scholar]
- de Almeida RMM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res. 2005;38:597–602. doi: 10.1590/s0100-879x2005000400014. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Lucion A. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur J Neurosci. 1994;264:445–448. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline - a 5-HT1B receptor agonist. Neuropsychopharmacology. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA. Zolmitriptan--a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology. 2001b;157:131–141. doi: 10.1007/s002130100778. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM. Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: a comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J Pharmacol Exp Ther. 1999;288:1125–1133. [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM. Somatodendritic 5-HT1A autoreceptors mediate the anti-aggressive actions of 5-HT1A receptor agonists in rats: An ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology. 2000;23:20–33. doi: 10.1016/S0893-133X(00)00092-0. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, Lawlor BA. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJH, Laird JMA, Belmonte C, Cervero F, Hunt SP. Altered nocieption, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- De Groote L, Olivier B, Westenberg HG. Role of 5-HT1B receptors in the regulation of extracellular serotonin and dopamine in the dorsal striatum of mice. Eur J Pharmacol. 2003;476:71–77. doi: 10.1016/s0014-2999(03)02154-x. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA. Sexual dimorphism in the hormonal control of aggressive behavior of rats. Pharmacol Biochem Behav. 1981;14:89–93. doi: 10.1016/s0091-3057(81)80015-9. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Brocco M, Adhumeau A, Gobert A, Millan MJ. The selective serotonin 5-HT1A receptor ligand, S15535, displays anxiolytic-like effects in the social interaction and Vogel models and suppresses dialysate levels of 5-HT in the dorsal hippocampus of freely- moving rats. A comparison with other anxiolytic agents. Psychopharmacology. 2000;152:55–66. doi: 10.1007/s002130000449. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Guilloux JP, Nicolas L, Delomenie C, Reperant C, Le Maitre E, Leroux-Nicollet I, Benmansour S, Coudore F, David DJ, Gardier AM. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: studies in adult mice hippocampus. Pharmacol Biochem Behav. 2008;90:174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: Interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH. Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci. 1999;19:1–5. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male shin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Young WS, Nelson RJ. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Dompert WU, Glaser T, Traber J. 3H–TVX Q 7821: Identification of 5-HT1 binding sites as target for a novel putative anxiolytic. Naunyn Schmiedebergs Arch Pharmacol. 1985;328:467–470. doi: 10.1007/BF00692918. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Stribley JA, Fry DL, Hinrichs SH, Lockridge O. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Brain Res Dev Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eibl-Eibesfeldt I. Beiträge zur Biologie der Haus- und der Ährenmaus nebst einigen Beobachtungen an anderen Nagern. Z Tierpsychol. 1950;7:558–587. [Google Scholar]
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept. 1990;28:199–214. doi: 10.1016/0167-0115(90)90018-r. [DOI] [PubMed] [Google Scholar]
- Emeson RB, Morabito MV. Food fight: the NPY-serotonin link between aggression and feeding behavior. Sci STKE 2005. 2005 doi: 10.1126/stke.2772005pe12. pe12. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA. Escalated aggression after alcohol drinking in male mice: dorsal raphe and prefrontal cortex serotonin and 5-HT1B receptors. Neuropsychopharmacology. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplan JR, Mann JJ. CSF monoamines, age and impulsivity in wild grivet monkeys (Cercopithecus aethiops aethiops) Brain Behav Evol. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77:465–469. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20:427–451. doi: 10.1016/s0193-953x(05)70321-x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Parmigiani S, Rodgers RJ, Palanza P. Differential effects of chlordiazepoxide on aggressive behavior in male mice: the influence of social factors. Psychopharmacology. 1997;134:258–265. doi: 10.1007/s002130050448. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, Koppel G, Robert BF, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Pilapil CG, Hayden-Hixson D, Wiley RG, Koh ET. Functionally and anatomically distinct populations of vasopressinergic magnocellular neurons in the female golden hamster. J Neuroendocrinol. 1992;4:193–205. doi: 10.1111/j.1365-2826.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus) Behavioral Neuroscience. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Schuligoi R, Miczek KA, Hauser KF, Gerard C, Saria A. Alterations within the endogenous opioid system in mice with targeted deletion of the neutral endopeptidase (“enkephalinase”) gene. Regulatory Peptides. 2000;96:53–58. doi: 10.1016/s0167-0115(00)00200-7. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, DeBold JF, Miczek KA. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology. 2001;153:473–483. doi: 10.1007/s002130000587. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiat. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS ONE. 2007:2–e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresan A, Camarena B, Apiquian R, Aguilar A, Urraca N, Nicolini H. Association study of MAO-A and DRD4 genes in schizophrenic patients with aggressive behavior. Neuropsychobiology. 2007;55:171–175. doi: 10.1159/000106477. [DOI] [PubMed] [Google Scholar]
- Fuller RW. The influence of fluoxetine on aggressive behavior. Neuropsychopharmacology. 1996;14:77–81. doi: 10.1016/0893-133X(95)00110-Y. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur J Pharmacol. 1976;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Bethea ED, Stevenson SA. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007;8:17. doi: 10.1186/1471-2202-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KL. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav Brain Res. 2005;160:169–177. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Stevenson SA. Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav Brain Res. 2006;171:63–69. doi: 10.1016/j.bbr.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garattini S, Giacalone E, Valzelli L. Isolation, aggressiveness and brain 5-hydroxytryptamine turnover. J Pharm Pharmacol. 1967;19:338–339. doi: 10.1111/j.2042-7158.1967.tb08099.x. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Levinson CA, Corman MD, Godlaski AJ, Morris DH, Phillips JP, Holt JC. Men and women, alcohol and aggression. Exp Clin Psychopharmacol. 2009;17:154–164. doi: 10.1037/a0016385. [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L, Fernández-Teruel A, Escorihuela RM, Fredholm BB, Tobeña A, Pekny M, Johansson B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Audinot V, Newman-Tancredi A, Cistarelli L, Millan MJ. Simultaneous quantification of serotonin, dopamine and noradrenaline levels in single frontal cortex dialysates of freely-moving rats reveals a complex pattern of reciprocal auto- and heteroreceptor-mediated control of release. Neuroscience. 1998;84:413–429. doi: 10.1016/s0306-4522(97)00565-4. [DOI] [PubMed] [Google Scholar]
- Godlaski AJ, Giancola PR. Executive functioning, irritability, and alcohol-related aggression. Psychol Addict Behav. 2009;23:391–403. doi: 10.1037/a0016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, Linnoila M. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholics in Finland and the United States. Acta Psychiatrica Scandinavica. 1992;86:351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, DeBold JF, Yin W, Cook J, Miczek KA. Benzodiazepines and heightened aggressive behavior in rats: reduction by GABAA/α1 receptor antagonists. Psychopharmacology. 2005;178:232–240. doi: 10.1007/s00213-004-1987-3. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Swann AC, Moeller FG, Lane SD. Zolmitriptan and human aggression: interaction with alcohol. Psychopharmacology. 2010;210:521–531. doi: 10.1007/s00213-010-1851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. Behavioral responses to social stress in noradrenaline transporter knockout mice: effects on social behavior and depression. Brain Res Bull. 2002;58:279–284. doi: 10.1016/s0361-9230(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Haller J, Mikics E, Halasz J, Toth M. Mechanisms differentiating normal from abnormal aggression: glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for ‘pathological’ aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP, Kauhanen J, Syvalahti E, Hietala J, Tiihonen J. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4:385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Haney M, DeBold JF, Miczek KA. Maternal aggression in mice and rats towards male and female conspecifics. Aggress Behav. 1989;15:443–453. [Google Scholar]
- Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol. 2002;14:963–969. doi: 10.1046/j.1365-2826.2002.00863.x. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- Haug M, Wallian L, Brain PF. Effects of 8-OH-DPAT and fluoxetine on activity and attack by female mice towards lactating intruders. Gen Pharmacol. 1990;21:845–849. doi: 10.1016/0306-3623(90)90443-p. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W. Processes governing behavioral states of readiness. Adv Study Behav. 1974;5:173–200. [Google Scholar]
- Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, Riederer P, Lesch KP. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect. 1995;102:247–254. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hennig J, Reuter M, Netter P, Burk C, Landt O. Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behav Neurosci. 2005;119:16–25. doi: 10.1037/0735-7044.119.1.16. [DOI] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Hess WR. Das Zwischenhirn. Basel: Benno Schwabe & Co; 1954. [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ. Parental behavior in primates. In: Sluckin W, editor. Parental Behavior in Animals and Humans. Oxford,: Blackwell Press; 1986. pp. 152–207. [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- Ho HP, Olsson M, Westberg S, Hjorth L, Melke J, Eriksson E. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: an animal model of premenstrual irritability? Neuropsychopharmacology. 2001;24:502–510. doi: 10.1016/S0893-133X(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Hollander E. Managing aggressive behavior in patients with obsessive-compulsive disorder and borderline personality disorder. J Clin Psychiatry. 1999;60(Suppl 15):38–44. [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology. 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Homberg JR. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur J Neurosci. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang YY, Grailhe R, Arango V, Hen R, Mann JJ. Relationship of psychopathology to the human serotonin1B genotype and receptor binding kinetics in postmortem brain tissue. Neuropsychopharmacology. 1999;21:238–246. doi: 10.1016/S0893-133X(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL. Behavioral variation in wild house mice Mus domesticus Rutty: A quantitative assessment of female social organization. Anim Behav. 1987;35:1846–1857. [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. 2011;10:365–374. doi: 10.1111/j.1601-183X.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Cohen A. Differential behavioral effects of lesions of the median or dorsal raphe nuclei in rats: Open field and pain-elicited aggression. J Comp Physiol Psychol. 1976;90:102–108. doi: 10.1037/h0077262. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol Psychiatry. 2009;14:381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Rollema H, Schmidt AW, McHarg AD. Serotonergic effects and extracellular brain levels of eletriptan, zolmitriptan and sumatriptan in rat brain. Eur J Pharmacol. 2001;425:203–210. doi: 10.1016/s0014-2999(01)01151-7. [DOI] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD. Serotonin 5-HT2 and 5-HT1A-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009;158:1292–1300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, Goldman D, Spurlock G, Gustavsson JP, Nielsen DA, Linnoila M, Owen MJ, Sedvall GC. Tryptophan hydroxylase and catechol-O-methyltransferase gene polymorphisms: relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Eur Arch Psychiatry Clin Neurosci. 1997;247:297–302. doi: 10.1007/BF02922258. [DOI] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, Meisel RL. Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol Biochem Behav. 1997;58:349–353. doi: 10.1016/s0091-3057(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Ingram CD, Gartside SE. GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int. 2004;45:1057–1065. doi: 10.1016/j.neuint.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kalen P, Pritzel M, Nieoullon A, Wiklund L. Further evidence for excitatory amino acid transmission in the lateral habenular projection to the rostral raphe nuclei: lesion-induced decrease of high affinity glutamate uptake. Neurosci Lett. 1986;68:35–40. doi: 10.1016/0304-3940(86)90225-9. [DOI] [PubMed] [Google Scholar]
- Karl T, Lin S, Schwarzer C, Sainsbury A, Couzens M, Wittmann W, Boey D, von Horsten S, Herzog H. Y1 receptors regulate aggressive behavior by modulating serotonin pathways. Proc Natl Acad Sci U S A. 2004;101:12742–12747. doi: 10.1073/pnas.0404085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi R, Armstead P, Coccaro E. The neurobiology of impulsive aggression. Psychiatr Clin North Am. 1997;20:395–403. doi: 10.1016/s0193-953x(05)70319-1. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Strakowski SM, McElroy SL. The efficacy of atypical antipsychotics in the treatment of depressive symptoms, hostility, and suicidality in patients with schizophrenia. J Clin Psychiatry. 2000;61(Suppl 3):4–9. [PubMed] [Google Scholar]
- Khait VD, Huang YY, Zalsman G, Oquendo MA, Brent DA, Harkavy-Friedman JM, Mann JJ. Association of serotonin 5-HT2A receptor binding and the T102C polymorphism in depressed and healthy Caucasian subjects. Neuropsychopharmacology. 2005;30:166–172. doi: 10.1038/sj.npp.1300578. [DOI] [PubMed] [Google Scholar]
- Kim C, Jeon D, Kim YH, Lee CJ, Kim H, Shin HS. Deletion of N-type Ca2+ channel Cav2.2 results in hyperaggressive behaviors in mice. J Biol Chem. 2009a;284:2738–2745. doi: 10.1074/jbc.M807179200. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao SW, Maren S, Anagnostaras SG, Fanselow MS, DeMaeyer E, Seif I, Thompson RF. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Nat Acad Sci U S A. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Jahng JW, Min SK. Association between the serotonin transporter gene (5-HTTLPR) and anger-related traits in Korean schizophrenic patients. Neuropsychobiology. 2009b;59:165–171. doi: 10.1159/000218079. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Knobelman DA, Kung HF, Lucki I. Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT1A and 5-HT1B receptors. J Pharmacol Exp Ther. 2000;292:1111–1117. [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Colmers WF. Neuropeptide Y selectively inhibits slow synaptic potentials in rat dorsal raphe nucleus in vitro by a presynaptic action. J Neurosci. 1992;12:1086–1093. doi: 10.1523/JNEUROSCI.12-03-01086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM. Hypothalamically induced intraspecific aggressive behaviour in the rat. Exp Brain Res. 1978;32:365–375. doi: 10.1007/BF00238708. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Van der Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggress Behav. 1990;16:223–229. [Google Scholar]
- Koprowska M, Romaniuk A. Behavioral and biochemical alterations in median and dorsal raphe nuclei lesioned cats. Pharmacol Biochem Behav. 1997;56:529–540. doi: 10.1016/s0091-3057(96)00297-3. [DOI] [PubMed] [Google Scholar]
- Korte SM, Meijer OC, De Kloet ER, Buwalda B, Keijser J, Sluyter F, van Oortmerssen G, Bohus B. Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res. 1996;736:338–343. doi: 10.1016/0006-8993(96)00723-8. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Bohus B, Versteeg DH, De Kloet ER, de WD. Effect of oxytocin and vasopressin on memory consolidation: sites of action and catecholaminergic correlates after local microinjection into limbic-midbrain structures. Brain Res. 1979;175:303–314. doi: 10.1016/0006-8993(79)91009-6. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Hernandez-Avila CA, Gelernter J. Polymorphism of the 5-HT1B receptor gene (HTR1B): Strong within-locus linkage disequilibrium without association to antisocial substance dependence. Neuropsychopharmacology. 2002;26:115–122. doi: 10.1016/S0893-133X(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Differential regulation of serotonin (5-HT) release in the striatum and hippocampus by 5-HT1A autoreceptors of the dorsal and median raphe nuclei. J Pharmacol Exp Ther. 1994;269:1268–1279. [PubMed] [Google Scholar]
- Krsiak M. Isolation-induced timidity in mice as a measure of anxiolytic activity of drugs. Act Nerv Super (Praha) 1974;16:241–142. [PubMed] [Google Scholar]
- Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Cutter WJ, Rubia K, Brammer M, Daly EM, Craig MC, Cleare AJ, Murphy DG. 5-HT, prefrontal function and aging: fMRI of inhibition and acute tryptophan depletion. Neurobiol Aging. 2009;30:1135–1146. doi: 10.1016/j.neurobiolaging.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Lang A, Harro J, Soosaar A, Koks S, Volke V, Oreland L, Bourin M, Vasar E, Bradwejn J, Mannisto PT. Role of N-methyl-D-aspartic acid and cholecystokinin receptors in apomorphine-induced aggressive behaviour in rats. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:363–370. doi: 10.1007/BF00169076. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009;34:1567–1573. doi: 10.1016/j.psyneuen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Mueller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;264:1537–1551. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Leyhausen P. Cat behavior: predatory and social behavior of domestic and wild cats. New York: Garland STPM Press; 1979. [Google Scholar]
- Lin YMJ, Chao SC, Chen TM, Lai TJ, Chen JS, Sun HS. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in han chinese. Arch Gen Psychiatry. 2007;64:1015–1024. doi: 10.1001/archpsyc.64.9.1015. [DOI] [PubMed] [Google Scholar]
- Lindgren T, Kantak KM. Effects of serotonin receptor agonists and antagonists on offensive aggression in mice. Aggress Behav. 1987;13:87–96. [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Linnoila VMI, Virkkunen M. Aggression, suicidality, and serotonin. J Clin Psychiatry. 1992;53:46–51. [PubMed] [Google Scholar]
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer LK, Dulac C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucion AB, de Almeida RMM. On the dual nature of maternal aggression in rats. Aggress Behav. 1996;22:365–373. [Google Scholar]
- Lucki I, Wieland S. 5-Hydroxytryptamine-1A receptors and behavioral responses. Neuropsychopharmacology. 1990;3:481–493. [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R, Foote WE, Bry J. Excitatory projection from the interpeduncular nucleus to central superior raphe neurons. Brain Res. 1981;225:179–183. doi: 10.1016/0006-8993(81)90327-9. [DOI] [PubMed] [Google Scholar]
- Malick JB. The pharmacology of isolation-induced aggressive behavior in mice. In: Essman WB, editor. Current Developments in Psychopharmacology. New York: SP Medical and Scientific Books; 1979. pp. 1–27. [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and β-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45:603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Muldoon MF, Ferrell RE. Central nervous system serotonergic responsivity and aggressive disposition in men. Physiol Behav. 2002;77:705–709. doi: 10.1016/s0031-9384(02)00922-8. [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdelat-Parks BN, Liles LC, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Martel JC, Fournier A, St PS, Quirion R. Quantitative autoradiographic distribution of [125I]Bolton-Hunter neuropeptide Y receptor binding sites in rat brain. Comparison with [125I]peptide YY receptor sites. Neuroscience. 1990;36:255–283. doi: 10.1016/0306-4522(90)90367-d. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Mathews TA. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister KH. Ethological analysis of the effects of MK-801 upon aggressive male mice: similarity to chlordiazepoxide. Pharmacol Biochem Behav. 1990;37:101–106. doi: 10.1016/0091-3057(90)90048-m. [DOI] [PubMed] [Google Scholar]
- McBride PA, Brown RP, DeMeo M, Keilp J, Mieczkowski T, Mann JJ. The relationship of platelet 5-HT2 receptor indices to major depressive disorder, personality traits, and suicidal behavior. Biol Psychiatry. 1994;35:295–308. doi: 10.1016/0006-3223(94)90033-7. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Girasa KA, Allan AM, Miczek KA. 5-HT3 receptors, alcohol and aggressive behavior in mice. Behav Pharmacol. 2005;16:163–170. doi: 10.1097/00008877-200505000-00005. [DOI] [PubMed] [Google Scholar]
- McKinley JC, Hathaway SR, Meehl PE. The Minnesota Multiphasic Personality Inventory: the K-scale. J Consult Psychol. 1948;12:20–31. doi: 10.1037/h0061377. [DOI] [PubMed] [Google Scholar]
- McMillen BA, DaVanzo EA, Scott SM, Song AH. N-alkyl-substituted aryl-piperazine drugs: Relationship between affinity for serotonin receptors and inhibition of aggression. Drug Dev Res. 1988;12:53–62. [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Reedy CL, Cohen BM, Carlezon WA., Jr Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol Psychiatry. 2008;63:832–839. doi: 10.1016/j.biopsych.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza DL, Bravo HA, Swanson HH. Antiaggresive and anxiolytic effects of gepirone in mice, and their attenuation by WAY 100635. Pharmacol Biochem Behav. 1999;62:499–509. doi: 10.1016/s0091-3057(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Rusjan P, Clark M, Houle S, Woodside S, Arrowood J, Martin K, Colleton M. Serotonin2A receptor binding potential in people with aggressive and violent behaviour. J Psychiatry Neurosci. 2008;33:499–508. [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Nat Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Miczek KA. The psychopharmacology of aggression. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 19. Plenum, New York: New Directions in Behavioral Pharmacology; 1987. pp. 183–328. [Google Scholar]
- Miczek KA, Barros HM, Sakoda L, Weerts EM. Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998a;22:1698–1705. [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, DeBold JF, de Almeida RMM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Haney M. Psychomotor stimulant effects of d-amphetamine, MDMA and PCP: Aggressive and schedule-controlled behavior in mice. Psychopharmacology. 1994;115:358–365. doi: 10.1007/BF02245077. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology. 1998b;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Takahashi A, Covington HE, III, Yap JJ, Boyson CO, Shimamoto A, de Almeida RM. Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet Online First. 2011 doi: 10.1007/s10519-011-9462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Alcohol and chlordiazepoxide increase suppressed aggression in mice. Psychopharmacology. 1980;69:39–44. doi: 10.1007/BF00426519. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM. Alcohol and “bursts” of aggressive behavior: Ethological analysis of individual differences in rats. Psychopharmacology. 1992;107:551–563. doi: 10.1007/BF02245270. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ. Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol. 2005;526:147–162. doi: 10.1016/j.ejphar.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Fletcher A, Redfern PH. Is antidepressant efficacy revealed by drug-induced changes in rat behaviour exhibited during social interaction? Neurosci Biobehav Rev. 1991;15:539–544. doi: 10.1016/s0149-7634(05)80145-9. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Acute and Chronic Antidepressant drug treatments induce opposite effects in the social behavior of rats. J Psychopharmacology. 1992;6:241–257. doi: 10.1177/026988119200600218. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav Pharmacol. 1997;8:585–606. doi: 10.1097/00008877-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Takao K, Niki H. Differential effect of Fyn tyrosine kinase deletion on offensive and defensive aggression. Behav Brain Res. 2001;122:51–56. doi: 10.1016/s0166-4328(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Melanocortin-5 receptor deficiency reduces a pheromonal signal for aggression in male mice. Chem Senses. 2004;29:111–115. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B. Quantitative and comparative analyses of pro-aggressive actions of benzodiazepines in maternal aggression of rats. Psychopharmacology. 1989;97:152–153. doi: 10.1007/BF00442238. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. The effects of dorsal raphé administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol. 1993;238:411–415. doi: 10.1016/0014-2999(93)90877-k. [DOI] [PubMed] [Google Scholar]
- Muehlenkamp F, Lucion A, Vogel WH. Effects of selective serotenergic agonists on aggressive behavior in rats. Pharmacol Biochem Behav. 1995;50:671–674. doi: 10.1016/0091-3057(95)00351-7. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Musty RE, Consroe PF. Phencyclidine produces aggressive behavior in rapid eye movement sleep-deprived rats. Life Sci. 1982;30:1733–1738. doi: 10.1016/0024-3205(82)90307-1. [DOI] [PubMed] [Google Scholar]
- Nagtegaal MH, Rassin E. The usefulness of the thought suppression paradigm in explaining impulsivity and aggression. Pers Individ Dif. 2004;37:1233–1244. [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Caramaschi D. Animal violence demystified. Front Behav Neurosci. 2010;4:9. doi: 10.3389/fnbeh.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: Evidence from a family-based association study. Am J Hum Gene. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, O’Flynn K, Siever LJ. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- New AS, Gelernter J, Goodman M, Mitropoulou V, Koenigsberg H, Silverman J, Siever LJ. Suicide, impulsive aggression, and HTR1B genotype. Biol Psychiatry. 2001;50:62–65. doi: 10.1016/s0006-3223(01)01108-8. [DOI] [PubMed] [Google Scholar]
- New AS, Gelernter J, Yovell Y, Trestman RL, Nielsen DA, Silverman J, Mitropoulou V, Siever LJ. Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: A preliminary study. Am J Med Genet. 1998;81:13–17. doi: 10.1002/(sici)1096-8628(19980207)81:1<13::aid-ajmg3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan-hydroxylase polymorphism. Arch Gen Psychiatry. 1994;51:34–38. doi: 10.1001/archpsyc.1994.03950010034005. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Avgustinovich DF, Popova NK. Role of 5HT1A receptors in a variety of kinds of aggressive behavior in wild rats and counterparts selected for low defensiveness towards man. Aggress Behav. 1992;18:357–364. [Google Scholar]
- Nogueira RL, Graeff FG. Role of 5HT receptor subtypes in the modulation of dorsal periaqueductal gray generated aversion. Pharmacol Biochem Behav. 1995;52:1–6. doi: 10.1016/0091-3057(94)00402-5. [DOI] [PubMed] [Google Scholar]
- Noirot E, Goyens J, Buhot MC. Aggressive behavior of pregnant mice towards males. Horm Behav. 1975;6:9–17. doi: 10.1016/0018-506x(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kusumi I, Kaneko M, Masui T, Daiguji M, Ueno T, Koyama T, Nomura Y. Involvement of a polymorphism in the 5-HT2a receptor gene in impulsive behavior. Psychopharmacology. 2006;187:30–35. doi: 10.1007/s00213-006-0398-z. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Chronwall BM, Pruss RM, Mezey E, Kiss JZ, Eiden LE, Massari VJ, Tessel RE, Pickel VM, DiMaggio DA. Neuropeptide Y and peptide YY neuronal and endocrine systems. Peptides. 1985;6:755–768. doi: 10.1016/0196-9781(85)90180-9. [DOI] [PubMed] [Google Scholar]
- Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJS, Banaschewski T, Chen W, Anney RJL, Buitelaar JK, Ebstein RP, Franke B, Gill M, Miranda A, Roeyers H, Rothenberger A, Sergeant JA, Steinhausen HC, Taylor EA, Thompson M, Asherson P. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav Brain Funct. 2008;4:48. doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Nat Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-a gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998a;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-a gene disruption in male mice. Endocrinology. 1998b;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Oliveira-dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, Roder JC, Barnes CA, Siderovski DP, Penninger JM. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Nat Acad Sci U S A. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. Serotonin and aggression. Ann N Y Acad Sci. 2004;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Rasmussen D. Behavioural pharmacology of the serenic, eltoprazine. Rev Drug Metab Drug Interact. 1990;8:31–83. doi: 10.1515/dmdi.1990.8.1-2.31. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Tulp MTM, van der Poel AM. Animal models of anxiety and aggression in the study of serotonergic agents. In: Langer SZ, editor. Serotonin receptor subtypes: Pharmacological significance and clinical implications. Basel: Karger; 1992. pp. 67–79. [Google Scholar]
- Olivier B, Mos J, Van der Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Russo SA, Underwood MD, Kassir SA, Ellis SP, Mann JJ, Arango V. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biological Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Osipova DV, Kulikov AV, Popova NK. C1473G polymorphism in mouse tph2 gene is linked to tryptophan hydroxylase-2 activity in the brain, intermale aggression, and depressive-like behavior in the forced swim test. J Neurosci Res. 2009;87:1168–1174. doi: 10.1002/jnr.21928. [DOI] [PubMed] [Google Scholar]
- Pabis DJ, Stanislav SW. Pharmacotherapy of aggressive behavior. Ann Pharmacother. 1996;30:278–287. doi: 10.1177/106002809603000312. [DOI] [PubMed] [Google Scholar]
- Palanza P, Della Seta D, Ferrari PF, Parmigiani S. Female competition in wild house mice depends upon timing of female/male settlement and kinship between females. Anim Behav. 2005;69:1259–1271. [Google Scholar]
- Pallotta M, Segieth J, Whitton PS. N-methyl-d-aspartate receptors regulate 5-HT release in the raphe nuclei and frontal cortex of freely moving rats: differential role of 5-HT1A autoreceptors. Brain Res. 1998;783:173–178. doi: 10.1016/s0006-8993(97)01333-4. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Ferrari PF, Palanza P. An evolutionary approach to behavioral pharmacology: using drugs to understand proximate and ultimate mechanisms of different forms of aggression in mice. Neurosci Biobehav Rev. 1998;23:143–153. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40:1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Peeke HVS, Figler MH. Modulation of aggressive behavior in fish by alcohol and congeners. Pharmacol Biochem Behav. 1981;14(Sup 1):79–84. doi: 10.1016/s0091-3057(81)80013-5. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting in the Syrian golden hamster Mesocricetus auratus Waterhouse, and its relationship to serious fighting during postweaning development. Dev Psychobiol. 1988;21:323–337. doi: 10.1002/dev.420210404. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P, Dennis T, de Montigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Avgustinovich DF, Kolpakov VG, Plyusnina IZ. Specific [H-3]8-OH-DPAT binding in brain regions of rats genetically predisposed to various defense behavior strategies. Pharmacol Biochem Behav. 1998;59:793–797. doi: 10.1016/s0091-3057(97)00504-2. [DOI] [PubMed] [Google Scholar]
- Popova NK, Voitenko NN, Kulikov AV, Avgustinovich DF. Evidence for the involvement of central serotonin in mechanism of domestication of silver foxes. Pharmacol Biochem Behav. 1991;40:751–756. doi: 10.1016/0091-3057(91)90080-l. [DOI] [PubMed] [Google Scholar]
- Potegal M. Attack priming and satiation in female golden hamsters: tests of some alternatives to the aggression arousal interpretation. Aggress Behav. 1991;17:327–335. [Google Scholar]
- Potegal M, Tenbrink L. Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus) J Comp Psychol. 1984;98:66–75. [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LJ, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Miguel TT, DeBold JF, Miczek KA. 2009 Neuroscience Meeting Planner. Society for Neuroscience. Chicago, IL: 2009a. Opposing action of CRF1 vs. CRF2 receptors in the dorsal raphé: Modulation of alcohol-heightened aggression. Program No.445.5/T8. [Google Scholar]
- Quadros IM, Takahashi A, Miczek KA. Serotonin and aggression. In: Müller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; 2009b. pp. 687–714. [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. J Abnorm Child Psychol. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raj YP. Psychopharmacology of borderline personality disorder. Curr Psychiatry Rep. 2004;6:225–231. doi: 10.1007/s11920-004-0068-y. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Saudou F, Amara DA, Belzung C, Segu L, Misslin R, Buhot MC, Hen R. 5-HT1B receptor knock out: behavioral consequences. Behav Brain Res. 1995;73:305–312. doi: 10.1016/0166-4328(96)00119-2. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Giovenardi M, de Almeida RM. Drugs and aggression. Recent Pat CNS Drug Discov. 2008;3:40–49. doi: 10.2174/157488908783421456. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratey J, Sovner R, Parks A, Rogentine K. Buspirone treatment of aggression and anxiety in mentally retarded patients: a multiple-baseline, placebo lead-in study. J Clin Psychiatry. 1991;52:159–162. [PubMed] [Google Scholar]
- Reist C, Nakamura K, Sagart E, Sokolski KN, Fujimoto KA. Impulsive aggressive behavior: open-label treatment with citalopram. J Clin Psychiatry. 2003;64:81–85. [PubMed] [Google Scholar]
- Rewerski W, Kostowski W, Piechocki T, Rylski M. The effects of some hallucinogens on aggressiveness of mice and rats. I Pharmacology. 1971;5:314–320. doi: 10.1159/000136205. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr Serotonin type 3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Rios M, Lambe EK, Liu RJ, Teillon S, Liu JH, Akbarian S, Roffler-Tarlov S, Jaenisch R, Aghajanian GK. Severe deficits in 5-HT2A-mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Waters AJ. Benzodiazepines and their antagonists: A pharmacoethological analysis with particular reference to effects on “aggression”. Neurosci Biobehav Rev. 1985;9:21–35. doi: 10.1016/0149-7634(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Minarro J, Aguilar MA, Pinazo J, Simon VM. Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice. Eur Neuropsychopharmacol. 1998;8:95–103. doi: 10.1016/s0924-977x(97)00051-5. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Rosell DR, Thompson JL, Slifstein M, Xu X, Frankle WG, New AS, Goodman M, Weinstein SR, Laruelle M, Abi-Dargham A, Siever LJ. Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry. 2010;67:1154–1162. doi: 10.1016/j.biopsych.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudissaar R, Pruus K, Skrebuhhova T, Allikmets L, Matto V. Modulatory role of 5-HT3 receptors in mediation of apomorphine-induced aggressive behaviour in male rats. Behav Brain Res. 1999;106:91–96. doi: 10.1016/s0166-4328(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Bondy B, Gietl A, Zill P, Moller HJ. Association of anger-related traits with SNPs in the TPH gene. Mol Psychiatry. 2002;7:1023–1029. doi: 10.1038/sj.mp.4001128. [DOI] [PubMed] [Google Scholar]
- Rydén E, Thase ME, Straht D, Aberg-Wistedt A, Bejerot S, Landen M. A history of childhood attention-deficit hyperactivity disorder (ADHD) impacts clinical outcome in adult bipolar patients regardless of current ADHD. Acta Psychiatr Scand. 2009;120:239–246. doi: 10.1111/j.1600-0447.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Sowa C, Sakamoto Y, Nishihara B, Koyama Y, Baba A, Matsuda T. Modulation by 5-HT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol. 2002;89:89–92. doi: 10.1254/jjp.89.89. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. Adrenergic α2c receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci. 1998;18:3035–3042. doi: 10.1523/JNEUROSCI.18-08-03035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology. 1993;110:53–59. doi: 10.1007/BF02246950. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. Isolation-induced aggression in mice: Effects of 5-hydroxytryptamine uptake inhibitors and involvement of postsynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;264:241–247. doi: 10.1016/0014-2999(94)00470-6. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Psychopharmacology. 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Sano Y, Ornthanalai VG, Yamada K, Homma C, Suzuki H, Suzuki T, Murphy NP, Itohara S. X11-like protein deficiency is associated with impaired conflict resolution in mice. J Neurosci. 2009;29:5884–5896. doi: 10.1523/JNEUROSCI.5756-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A, Theodorsson-Norheim E, Lundberg JM. Evidence for specific neuropeptide Y-binding sites in rat brain synaptosomes. Eur J Pharmacol. 1984;107:105–107. doi: 10.1016/0014-2999(84)90098-0. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, Lemeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. NeuroReport. 2008;19:739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A, Stilli D, Musso E, Mainardi D, Parmigiani S. Offensive and defensive bite-target topographies in attacks by lactating rats. Aggress Behav. 1992;17:47–52. [Google Scholar]
- Shaikh MB, De Lanerolle NC, Siegel A. Serotonin 5-HT1A and 5-HT2/1C receptors in the midbrain periaqueductal gray differentially modulate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res. 1997;765:198–207. doi: 10.1016/s0006-8993(97)00433-2. [DOI] [PubMed] [Google Scholar]
- Shih JC, Ridd MJ, Chen K, Meehan WP, Kung MP, Seif I, De Maeyer E. Ketanserin and tetrabenazine aggression in mice lacking monoamine oxidase A. Brain Research. 1999;835:104–112. doi: 10.1016/s0006-8993(99)01478-x. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, De Kloet ER, Mos J, Van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase-II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Sinha R, Cloninger CR, Parsian A. Linkage disequilibrium and haplotype analysis between serotonin receptor 1B gene variations and subtypes of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:83–88. doi: 10.1002/ajmg.b.20064. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosicial factors. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Smuts BB. Sexual competition and mate choice. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1986. pp. 385–399. [Google Scholar]
- Sofia RD. Structural relationship and potency of agents which selectively block mouse killing (muricide) behavior in rats. Life Sci. 1969;8:1201–1210. doi: 10.1016/0024-3205(69)90049-6. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna) J Neuroendocrinol. 2003;15:150–160. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- Spielberg CD, Sharma S, Singh M. Development of Hindi edition of state-trait anxiety inventory. Indian J Psychol. 1973;48:11–20. [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors - reports from a spontaneous reporting system. Drug Saf. 1999;20:277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Steiniger F. Beiträg zur Soziologie und sonstigen Biologie der Wanderratte. Z Tierpsychol. 1950;7:356–379. [Google Scholar]
- Stoff DM, Vitiello B. Role of serotonin in aggression of children and adolesecents: biochemical and phamacological studies. In: Stoff DM, editor. Aggression and Violence: Genetic, Neurobiological and Biosocial Perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 101–123. [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Cremer H, Schachner M. Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule (NCAM) Eur J Neurosci. 1997;9:1117–1125. doi: 10.1111/j.1460-9568.1997.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, Yu WY, Cheng AT. Association study of novel human serotonin 5-HT1B polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51:896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Van Dorn RA, Volavka J, Monahan J, Stroup TS, Mcevoy JP, Wagner HR, Elbogen EB, Lieberman JA. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008;193:37–43. doi: 10.1192/bjp.bp.107.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, DeBold JF, Miczek KA. GABAA receptors in the dorsal raphé nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology. 2010a;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Quadros IM, de Almeida RM, Miczek KA. Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology. 2011;213:183–212. doi: 10.1007/s00213-010-2000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010b;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br J Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Personality. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Le Moal M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Regul Pep. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Ten Eyck GR. Serotonin modulates vocalizations and territorial behavior in an amphibian. Behav Brain Res. 2008;193:144–147. doi: 10.1016/j.bbr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Tompkins EC, Clemento AJ, Taylor DP, Perhach JL., Jr Inhibition of aggressive behavior in rhesus monkeys by buspirone. Res Commun Psychol Psychiatr Behav. 1980;5:337–352. [Google Scholar]
- Troisi A, Vicario E, Nuccetelli F, Ciani N, Pasini A. Effects of fluoxetine on aggressive behavior of adult inpatients with mental retardation and epilepsy. Pharmacopsychiatry. 1995;28:1–4. doi: 10.1055/s-2007-979593. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Liao DL, Yu YW, Chen TJ, Wu HC, Lin CH, Cheng CY, Hong CJ. A study of the association of (Val66Met) polymorphism in the brain-derived neurotrophic factor gene with alcohol dependence and extreme violence in Chinese males. Neurosci Lett. 2005;381:340–343. doi: 10.1016/j.neulet.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Tyler CB, Miczek KA. Effects of phencyclidine on aggressive behavior in mice. Pharmacol Biochem Behav. 1982;17:503–510. doi: 10.1016/0091-3057(82)90311-2. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Oliver-Africano PC, Ahmed Z, Bouras N, Cooray S, Deb S, Murphy D, Hare M, Meade M, Reece B, Kramo K, Bhaumik S, Harley D, Regan A, Thomas D, Rao B, North B, Eliahoo J, Karatela S, Soni A, Crawford M. Risperidone, haloperidol, and placebo in the treatment of aggressive challenging behaviour in patients with intellectual disability: a randomised controlled trial. Lancet. 2008;371:57–63. doi: 10.1016/S0140-6736(08)60072-0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valzelli L. About a “specific” neurochemistry of aggressive behavior. In: Delgado JMR, editor. Behavioral Neurochemistry. New York: Spectrum Publications, Inc.; 1977. pp. 113–132. [Google Scholar]
- Valzelli L, Bernasconi S. Aggressiveness by isolation and brain serotonin turnover changes in different strains of mice. Neuropsychobiology. 1979;5:129–135. doi: 10.1159/000117674. [DOI] [PubMed] [Google Scholar]
- Valzelli L, Giacalone E, Garattini S. Pharmacological control of aggressive behavior in mice. Eur J Pharmacol. 1967;2:144–146. doi: 10.1016/0014-2999(67)90040-4. [DOI] [PubMed] [Google Scholar]
- Van den Berg L, Vos-Loohuis M, Schilder MBH, van Oost BA, Hazewinkel HAW, Wade CM, Karlsson EK, Lindblad-Toh K, Liinamo AE, Leegwater PAJ. Evaluation of the serotonergic genes htr1A, htr1B, htr2A, and slc6A4 in aggressive behavior of Golden Retriever dogs. Behav Genet. 2008;38:55–66. doi: 10.1007/s10519-007-9179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vegt BJ, de Boer SF, Buwalda B, de Ruiter AJ, de Jong JG, Koolhaas JM. Enhanced sensitivity of postsynaptic serotonin-1A receptors in rats and mice with high trait aggression. Physiol Behav. 2001;74:205–211. doi: 10.1016/s0031-9384(01)00565-0. [DOI] [PubMed] [Google Scholar]
- Van Der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oortmerssen GA, Bakker TCM. Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behav Genet. 1981;11:115–126. doi: 10.1007/BF01065622. [DOI] [PubMed] [Google Scholar]
- van Riel E, Meijer OC, Veenema AH, Joels M. Hippocampal serotonin responses in short and long attack latency mice. J Neuroendocrinol. 2002;14:234–239. doi: 10.1046/j.0007-1331.2001.00770.x. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. The development of social structure in free-ranging rhesus monkeys. Behaviour. 1967;29:179–194. doi: 10.1163/156853967x00109. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Matheson GK, Wilderman RC, Patterson LA. Inhibition of serotonergic dorsal raphe neurons by systemic and iontophoretic administration of buspirone, a non-benzodiazepine anxiolytic drug. Eur J Pharmacol. 1986;129:123–130. doi: 10.1016/0014-2999(86)90343-2. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Sijtsma B, Koolhaas JM, De Kloet ER. The stress response to sensory contact in mice: genotype effect of the stimulus animal. Psychoneuroendocrinology. 2005;30:550–557. doi: 10.1016/j.psyneuen.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Social instigation and aggression in postpartum female rats: role of 5-HT1A and 5-HT1B receptors in the dorsal raphé nucleus and prefrontal cortex. Psychopharmacology. 2011;213:475–487. doi: 10.1007/s00213-010-2083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3:253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Depaulis A, Boehrer A. Parachlorophenylalanine-induced serotonin depletion increases offensive but not defensive aggression in male rats. Physiol Behav. 1986;36:653–658. doi: 10.1016/0031-9384(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36:307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Citrome L, McQuade RD, Carson WH, Kostic D, Hardy S, Marcus R. Efficacy of aripiprazole against hostility in schizophrenia and Schizoaffective disorder: Data from 5 double-blind studies. J Clin Psychiatry. 2005;66:1362–1366. doi: 10.4088/jcp.v66n1103. [DOI] [PubMed] [Google Scholar]
- Walsh MT, Dinan TG. Selective serotonin reuptake inhibitors and violence: a review of the available evidence. Acta Psychiatr Scand. 2001;104:84–91. doi: 10.1034/j.1600-0447.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscop y double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Miczek KA. Primate vocalizations during social separation and aggression: effects of alcohol and benzodiazepines. Psychopharmacology. 1996;127:255–264. [PubMed] [Google Scholar]
- Welch BL, Welch AS. Rapid modification of isolation-induced aggressive behavior and elevation of brain catecholamines and serotonin by the quick-acting monoamine-oxidase inhibitor pargyline. Commun Behav Biol. 1968;1:347–351. [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ. Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology. 2000;150:120–122. doi: 10.1007/s002130000432. [DOI] [PubMed] [Google Scholar]
- White SM, Kucharik RF, Moyer JA. Effects of serotonergic agents on isolation-induced aggression. Pharmacol Biochem Behav. 1991;39:729–736. doi: 10.1016/0091-3057(91)90155-u. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence”: Childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Wilmot CA, Vander Wende C, Spoerlein MT. The effects of phencyclidine on fighting in differentially housed mice. Pharmacol Biochem Behav. 1987;28:341–346. doi: 10.1016/0091-3057(87)90450-3. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233:101–107. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- Witte AV, Floel A, Stein P, Savli M, Mien LK, Wadsak W, Spindelegger C, Moser U, Fink M, Hahn A, Mitterhauser M, Kletter K, Kasper S, Lanzenberger R. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp. 2009;30:2558–2570. doi: 10.1002/hbm.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai K, Son LZ, Endou M, Sakurai E, Nakagawasai O, Tadano T, Kisara K, Inoue I, Watanabe T, Watanabe T. Behavioral characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience. 1998;87:479–487. doi: 10.1016/s0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- Yen CY, Stanger RL, Millman N. Ataractic suppression of isolation-induced aggressive behavior. Arch Int Pharmacodyn Ther. 1959;123:179–185. [PubMed] [Google Scholar]
- Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, Zheng QY, Smith RS, Bronson RT, Nelson RJ, Simpson EM. Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res. 2002;132:145–158. doi: 10.1016/s0166-4328(01)00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone JR, Hellings JA, Crandall K, Reese RM, Marquis J, Fleming K, Shores R, Williams D, Schroeder SR. Effects of risperidone on aberrant behavior of persons with developmental disabilities: I. A double-blind crossover study using multiple measures. Am J Ment Retard. 2001;106:525–538. doi: 10.1352/0895-8017(2001)106<0525:EOROAB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zeichner A, Pihl RO. Effects of alcohol and behavior contingencies on human-aggression. J Abnorm Psychol. 1979;88:153–160. doi: 10.1037//0021-843x.88.2.153. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KRR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:S52–S60. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Zitzman D, DeBold JF, Miczek KA. 2005 Neuroscience Meeting Planner, Program No. 76.15. Washington, DC: Society for Neuroscience; 2005. Positive modulation of the GABAA receptor heightens aggression in ovariectomized, nulliparous female mice. [Google Scholar]
- Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, Turecki G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive Behavior and suicide. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:996–1002. doi: 10.1002/ajmg.b.30521. [DOI] [PubMed] [Google Scholar]