Abstract
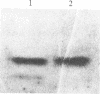
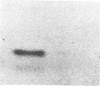
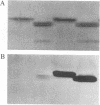
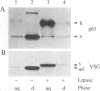
The variant surface glycoprotein (VSG) of the African trypanosomes is the major membrane protein of the plasma membrane of the bloodstream stage of the parasite. It is anchored in the plasma membrane by a glycolipid covalently bound to the C-terminal amino acid of the protein. The VSG is released through the action of a phosphatidylinositol-specific phospholipase C that removes dimyristoylglycerol and exposes the carbohydrate antigenic determinant common to all VSGs. Promastigotes of Leishmania have a predominant surface glycoprotein, termed p63, that is anchored in the plasma membrane in a similar way. A water-soluble form of p63 can be generated through the action of phosphatidylinositol-specific phospholipase C from trypanosomes or from Bacillus cereus. Either treatment exposes on the Leishmania p63 an antigenic determinant recognized by antibody prepared against the trypanosomal crossreacting determinant. These findings indicate that p63 and VSG have a common membrane anchor and are structurally related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Bülow R., Overath P. Synthesis of a hydrolase for the membrane-form variant surface glycoprotein is repressed during transformation of Trypanosoma brucei. FEBS Lett. 1985 Jul 22;187(1):105–110. doi: 10.1016/0014-5793(85)81223-0. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Allan L. M., Turner M. J. Purification and properties of the membrane form of variant surface glycoproteins (VSGs) from Trypanosoma brucei. J Protozool. 1984 Feb;31(1):53–60. doi: 10.1111/j.1550-7408.1984.tb04289.x. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Structure of the variant glycoproteins and surface coat of Trypanosoma brucei. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):3–12. doi: 10.1098/rstb.1984.0104. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Haldar K., Cross G. A. Trypanosoma brucei variant surface glycoprotein has a sn-1,2-dimyristyl glycerol membrane anchor at its COOH terminus. J Biol Chem. 1985 Apr 25;260(8):4963–4968. [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Ackermann K. E., Sherman W. R., Silman I. Identification of covalently bound inositol in the hydrophobic membrane-anchoring domain of Torpedo acetylcholinesterase. Biochem Biophys Res Commun. 1985 May 31;129(1):312–317. doi: 10.1016/0006-291x(85)91439-1. [DOI] [PubMed] [Google Scholar]
- Holder A. A. Carbohydrate is linked through ethanolamine to the C-terminal amino acid of Trypanosoma brucei variant surface glycoprotein. Biochem J. 1983 Jan 1;209(1):261–262. doi: 10.1042/bj2090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Cross G. A. Glycopeptides from variant surface glycoproteins of Trypanosoma Brucei. C-terminal location of antigenically cross-reacting carbohydrate moieties. Mol Biochem Parasitol. 1981 Feb;2(3-4):135–150. doi: 10.1016/0166-6851(81)90095-5. [DOI] [PubMed] [Google Scholar]
- Holder A. A. Glycosylation of the variant surface antigens of Trypanosoma brucei. Curr Top Microbiol Immunol. 1985;117:57–74. doi: 10.1007/978-3-642-70538-0_3. [DOI] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Miller E. N., Turner M. J. Analysis of antigenic types appearing in first relapse populations of clones of Trypanosoma brucei. Parasitology. 1981 Feb;82(1):63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. T-lymphocytes recognise Leishmania glycoconjugates. Parasitol Today. 1985 Aug;1(2):61–63. doi: 10.1016/0169-4758(85)90117-6. [DOI] [PubMed] [Google Scholar]
- Overath P., Czichos J., Stock U., Nonnengaesser C. Repression of glycoprotein synthesis and release of surface coat during transformation of Trypanosoma brucei. EMBO J. 1983;2(10):1721–1728. doi: 10.1002/j.1460-2075.1983.tb01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendashonga C. N., Black S. J. Humoral responses against Trypanosoma brucei variable surface antigen are induced by degenerating parasites. Parasite Immunol. 1982 Jul;4(4):245–257. doi: 10.1111/j.1365-3024.1982.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Turner M. J. Antigenic variation in its biological context. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):27–40. doi: 10.1098/rstb.1984.0106. [DOI] [PubMed] [Google Scholar]
- Turner M. J., Cardoso de Almeida M. L., Gurnett A. M., Raper J., Ward J. Biosynthesis, attachment and release of variant surface glycoproteins of the African trypanosome. Curr Top Microbiol Immunol. 1985;117:23–55. doi: 10.1007/978-3-642-70538-0_2. [DOI] [PubMed] [Google Scholar]
- Wightman P. D., Dahlgren M. E., Hall J. C., Davies P., Bonney R. J. Identification and characterization of a phospholipase C activity in resident mouse peritoneal macrophages. Inhibition of the enzyme by phenothiazines. Biochem J. 1981 Aug 1;197(2):523–526. doi: 10.1042/bj1970523. [DOI] [PMC free article] [PubMed] [Google Scholar]