Abstract
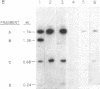
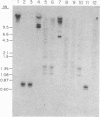
Crithidia fasciculata cells were treated with a plasmid (pDK96) containing pBR322 sequences, a Leishmania tarentolae maxicircle autonomously replicating sequence, and the bacterial gene for aminoglycoside 3' phosphotransferase I inserted between the yeast alcohol dehydrogenase 1 promotor and terminator sequences. Resistant colonies were selected on agar plates containing paromomycin and screened for vector DNA by hybridization. Approximately 1% of the resistant colonies contained detectable vector DNA, which was present as extrachromosomal closed circular molecules ranging in copy number from 1 to 160 per cell. The plasmids could be recovered from Escherichia coli transformed to ampicillin resistance with Crithidia total cell DNA. Most of the recovered plasmids were a deleted product of pDK96, which lacked the maxicircle autonomously replicating sequence and contained a unique fragment of Crithidia nuclear DNA present at a low copy number in the wild-type genome. The plasmid DNA in resistant Crithidia was unstable even under selective conditions and was lost within 30 cell divisions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G. Expression of genes in yeast using the ADCI promoter. Methods Enzymol. 1983;101:192–201. doi: 10.1016/0076-6879(83)01014-9. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Gibson W. C., Osinga K. A., Michels P. A., Borst P. Trypanosomes of subgenus Trypanozoon are diploid for housekeeping genes. Mol Biochem Parasitol. 1985 Sep;16(3):231–242. doi: 10.1016/0166-6851(85)90066-0. [DOI] [PubMed] [Google Scholar]
- Glassberg J., Miyazaki L., Rifkin M. R. Isolation and partial characterization of mutants of the trypanosomatid Crithidia fasciculata and their use in detecting genetic recombination. J Protozool. 1985 Feb;32(1):118–125. doi: 10.1111/j.1550-7408.1985.tb03025.x. [DOI] [PubMed] [Google Scholar]
- Hirth K. P., Edwards C. A., Firtel R. A. A DNA-mediated transformation system for Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7356–7360. doi: 10.1073/pnas.79.23.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Simpson L., Kayne P. S., Neckelmann N. Autonomous replication sequences in the maxicircle kinetoplast DNA of Leishmania tarentolae. Mol Biochem Parasitol. 1984 Nov;13(3):263–275. doi: 10.1016/0166-6851(84)90118-x. [DOI] [PubMed] [Google Scholar]
- KIDDER G. W., DUTTA B. N. The growth and nutrition of Crithidia fasciculata. J Gen Microbiol. 1958 Jun;18(3):621–638. doi: 10.1099/00221287-18-3-621. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Lanar D. E., Levy L. S., Manning J. E. Complexity and content of the DNA and RNA in Trypanosoma cruzi. Mol Biochem Parasitol. 1981 Sep;3(5):327–341. doi: 10.1016/0166-6851(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Leon W., Fouts D. L., Manning J. Sequence arrangement of the 16S and 26S rRNA genes in the pathogenic haemoflagellate Leishmania donovani. Nucleic Acids Res. 1978 Feb;5(2):491–504. doi: 10.1093/nar/5.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Simpson L., Rosenblatt H., Simpson A. M. Restriction map, partial cloning and localization of 9S and 12S kinetoplast RNA genes on the maxicircle component of the kinetoplast DNA of Leishmania tarentolae. Gene. 1979 May;6(1):51–73. doi: 10.1016/0378-1119(79)90085-4. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Riou G., Pautrizel R. Nuclear and kinetoplastic DNA from trypanosomes. J Protozool. 1969 Aug;16(3):509–513. doi: 10.1111/j.1550-7408.1969.tb02309.x. [DOI] [PubMed] [Google Scholar]
- Spithill T. W., Samaras N. The molecular karyotype of Leishmania major and mapping of alpha and beta tubulin gene families to multiple unlinked chromosomal loci. Nucleic Acids Res. 1985 Jun 11;13(11):4155–4169. doi: 10.1093/nar/13.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait A. Evidence for diploidy and mating in trypanosomes. Nature. 1980 Oct 9;287(5782):536–538. doi: 10.1038/287536a0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Simpson L. Studies on kinetoplast DNA. 3. Kinetic complexity of kinetoplast and nuclear DNA from Leishmania tarentolae. Biochim Biophys Acta. 1973 Sep 7;319(3):267–276. doi: 10.1016/0005-2787(73)90165-2. [DOI] [PubMed] [Google Scholar]
- Wünning I. U., Lipps H. J. A transformation system for the hypotrichous ciliate Stylonychia mytilus. EMBO J. 1983;2(10):1753–1757. doi: 10.1002/j.1460-2075.1983.tb01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]