Abstract
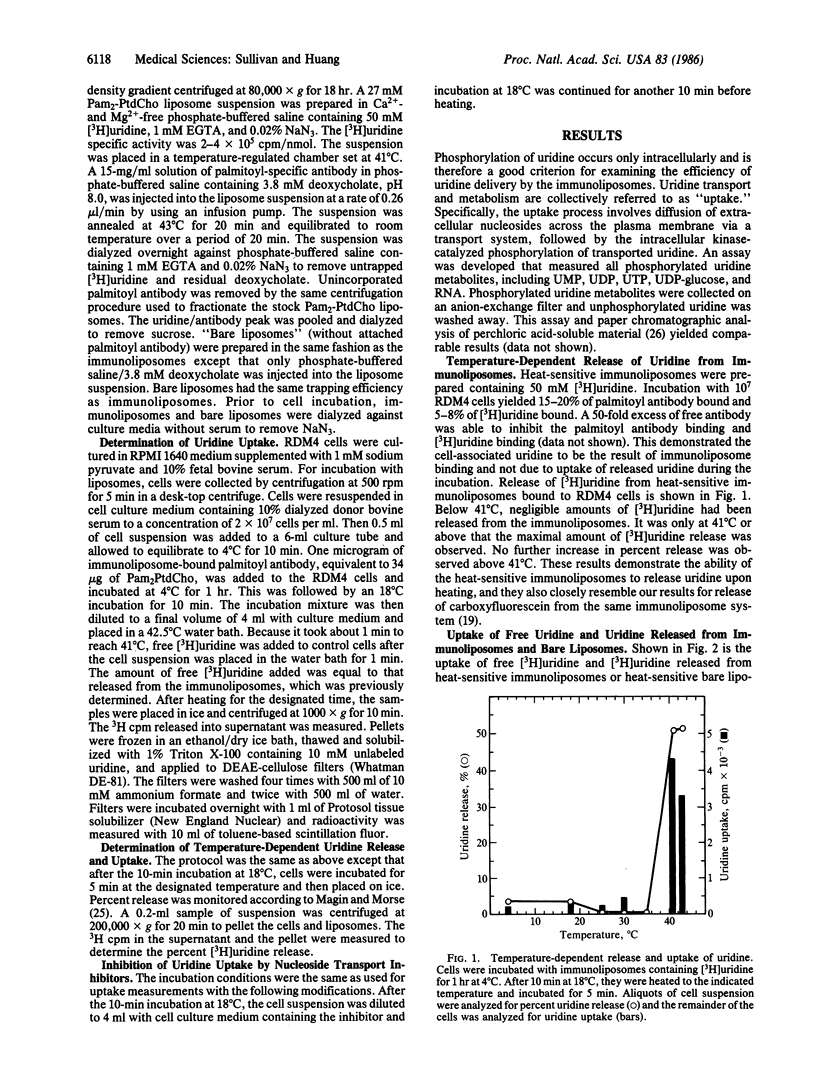
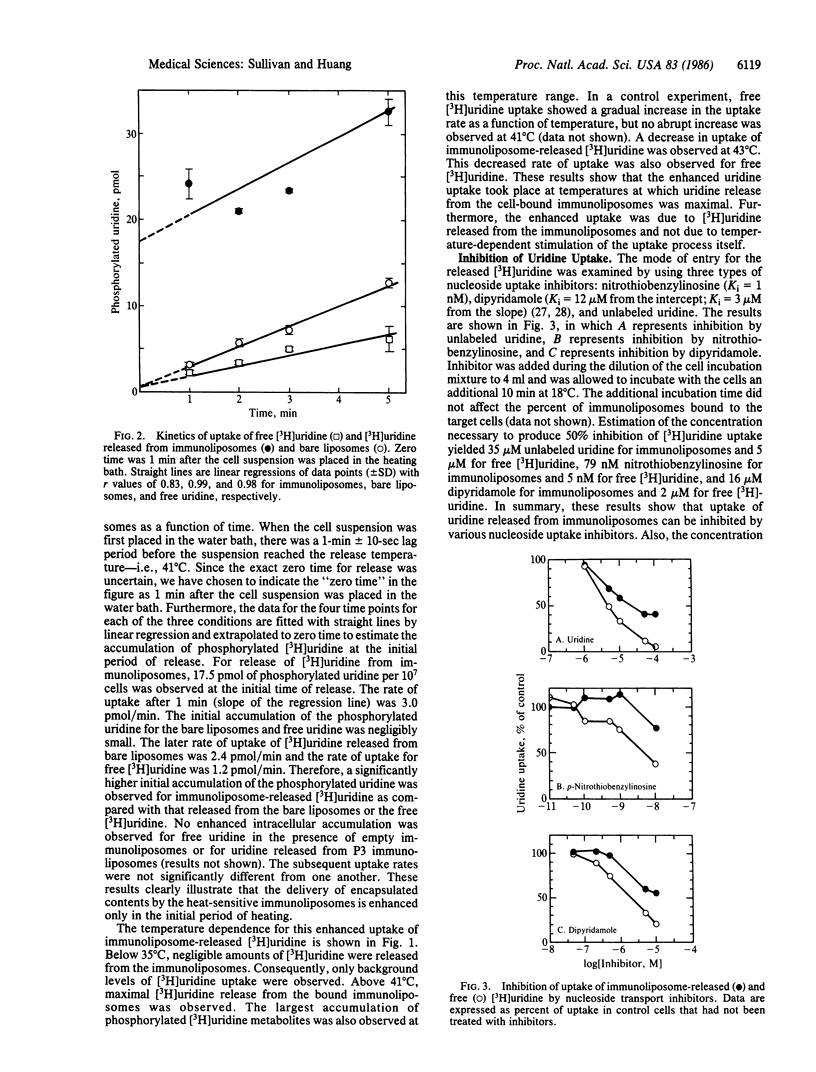
Heat-sensitive immunoliposomes are capable of releasing the entrapped content at the target cell surface upon a brief heating to the phase transition temperature of the liposome membrane. In this study we have examined the delivery efficiency of drugs entrapped in heat-sensitive immunoliposomes. Immunoliposomes composed of dipalmitoyl phosphatidylcholine with entrapped [3H]uridine were incubated with target cells at 4 degrees C. The cell-liposome mixture was then heated to 41 degrees C and the uptake of [3H]uridine into the intracellular pool of phosphorylated uridine-containing molecules was measured. The immunoliposomes showed maximal release of the uridine at 41 degrees C, the phase transition temperature of dipalmitoyl phosphatidylcholine liposomes. The largest accumulation of [3H]uridine in the target cells also took place at 41 degrees C. The initial level of uptake of [3H]uridine released from immunoliposomes by heating was greatly enhanced over that observed for free [3H]uridine and [3H]uridine released from liposomes without attached antibody. The nucleoside uptake inhibitors nitrothiobenzylinosine, dipyridamole, and unlabeled uridine were able to inhibit uptake of [3H]uridine released from immunoliposomes. This supports the hypothesis that the enhanced uptake is due to a heat-induced release of [3H]uridine at the cell surface followed by transport and phosphorylation of [3H]uridine by the target cells. These results indicate the feasibility of using the heat-sensitive immunoliposomes as a target-specific drug delivery system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blumenthal R., Ralston E., Dragsten P., Leserman L. D., Weinstein J. N. Lipid vesicle-cell interactions: analysis of a model for transfer of contents from adsorbed vesicles to cells. Membr Biochem. 1982;4(4):283–303. doi: 10.3109/09687688209065437. [DOI] [PubMed] [Google Scholar]
- Connor J., Huang L. Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J Cell Biol. 1985 Aug;101(2):582–589. doi: 10.1083/jcb.101.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Sullivan S., Huang L. Monoclonal antibody and liposomes. Pharmacol Ther. 1985;28(3):341–365. doi: 10.1016/0163-7258(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., van Galen M., Scherphof G. Effects of (dihydro)cytochalasin B, colchicine, monensin and trifluoperazine on uptake and processing of liposomes by Kupffer cells in culture. Biochim Biophys Acta. 1985 Apr 22;845(1):34–42. doi: 10.1016/0167-4889(85)90051-5. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Straubinger R. M., Baldwin P. A., Friend D. S., Papahadjopoulos D. Proton-induced fusion of oleic acid-phosphatidylethanolamine liposomes. Biochemistry. 1985 Jun 18;24(13):3091–3098. doi: 10.1021/bi00334a004. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Heath T. D., Montgomery J. A., Piper J. R., Papahadjopoulos D. Antibody-targeted liposomes: increase in specific toxicity of methotrexate-gamma-aspartate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1377–1381. doi: 10.1073/pnas.80.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heichal O., Ish-Shalom D., Koren R., Stein W. D. The kinetic dissection of transport from metabolic trapping during substrate uptake by intact cells. Uridine uptake by quiescent and serum-activated Nil 8 hamster cells and their murine sarcoma virus-transformed counterparts. Biochim Biophys Acta. 1979 Feb 20;551(1):169–186. doi: 10.1016/0005-2736(79)90363-8. [DOI] [PubMed] [Google Scholar]
- Huang A., Kennel S. J., Huang L. Interactions of immunoliposomes with target cells. J Biol Chem. 1983 Nov 25;258(22):14034–14040. [PubMed] [Google Scholar]
- Huang A., Tsao Y. S., Kennel S. J., Huang L. Characterization of antibody covalently coupled to liposomes. Biochim Biophys Acta. 1982 May 27;716(2):140–150. doi: 10.1016/0304-4165(82)90262-8. [DOI] [PubMed] [Google Scholar]
- Machy P., Pierres M., Barbet J., Leserman L. D. Drug transfer into lymphoblasts mediated by liposomes bound to distinct sites on H-2 encoded I-A, I-E, and K molecules. J Immunol. 1982 Nov;129(5):2098–2102. [PubMed] [Google Scholar]
- Magin R. L., Morse P. D., 2nd Rapid measurement of drug release from temperature-sensitive liposomes by electron paramagnetic resonance and radioisotope techniques. Biochim Biophys Acta. 1983 Nov 8;760(3):357–362. doi: 10.1016/0304-4165(83)90373-2. [DOI] [PubMed] [Google Scholar]
- Mezei M., Gulasekharam V. Liposomes--a selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980 May 5;26(18):1473–1477. doi: 10.1016/0024-3205(80)90268-4. [DOI] [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Transport and metabolism of deoxycytidine and 1-beta-D-arabinofuranosylcytosine into cultured Novikoff rat hepatoma cells, relationship to phosphorylation, and regulation of triphosphate synthesis. Cancer Res. 1978 Apr;38(4):978–989. [PubMed] [Google Scholar]
- Pool G. L., French M. E., Edwards R. A., Huang L., Lumb R. H. Use of radiolabeled hexadecyl cholesteryl ether as a liposome marker. Lipids. 1982 Jun;17(6):448–452. doi: 10.1007/BF02535225. [DOI] [PubMed] [Google Scholar]
- Schwenk M., Hegazy E., Lopez del Pino V. Uridine uptake by isolated intestinal epithelial cells of guinea pig. Biochim Biophys Acta. 1984 Dec 11;805(4):370–374. doi: 10.1016/0167-4889(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Sullivan S. M., Huang L. Preparation and characterization of heat-sensitive immunoliposomes. Biochim Biophys Acta. 1985 Jan 10;812(1):116–126. doi: 10.1016/0005-2736(85)90528-0. [DOI] [PubMed] [Google Scholar]
- Tümer A., Kirby C., Senior J., Gregoriadis G. Fate of cholesterol-rich liposomes after subcutaneous injection into rats. Biochim Biophys Acta. 1983 Oct 4;760(1):119–125. doi: 10.1016/0304-4165(83)90132-0. [DOI] [PubMed] [Google Scholar]
- Weinstein J. N., Magin R. L., Cysyk R. L., Zaharko D. S. Treatment of solid L1210 murine tumors with local hyperthermia and temperature-sensitive liposomes containing methotrexate. Cancer Res. 1980 May;40(5):1388–1395. [PubMed] [Google Scholar]
- Weinstein J. N., Magin R. L., Yatvin M. B., Zaharko D. S. Liposomes and local hyperthermia: selective delivery of methotrexate to heated tumors. Science. 1979 Apr 13;204(4389):188–191. doi: 10.1126/science.432641. [DOI] [PubMed] [Google Scholar]
- Yatvin M. B., Mühlensiepen H., Porschen W., Weinstein J. N., Feinendegen L. E. Selective delivery of liposome-associated cis-dichlorodiammineplatinum(II) by heat and its influence on tumor drug uptake and growth. Cancer Res. 1981 May;41(5):1602–1607. [PubMed] [Google Scholar]
- Yatvin M. B., Weinstein J. N., Dennis W. H., Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978 Dec 22;202(4374):1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]


