Summary
Glycine receptors play a major role in mediating fast inhibitory neurotransmission in the spinal cord and brain stem, yet their high-resolution structures remain unsolved. We determined open-channel structures of the full-length transmembrane domain (TMD) of the human glycine receptor α1-subunit (hGlyR-α1) using NMR and electron micrographs. The hGlyR-α1 TMD spontaneously forms pentameric Cl−-conducting channels, with structures sharing overall topology observed in crystal structures of homologous bacterial and nematode pentameric ligand-gated ion channels (pLGICs). However, the mammalian hGlyR-α1 structures present several unique features, including a shorter helix of the pore-lining TM2 with helical unwinding near the C-terminal end, a TM3 helical kink at A288 that partially overlaps with the homologous ivermectin-binding site in GluCl, and a highly dynamic segment between S267(15′) of TM2 and A288 that likely affects allosteric modulations of channel function. The NMR structures provide new templates for identifying potential drug targets in GlyRs and other mammalian pLGICs.
Introduction
The glycine receptors are anion-selective channels and major inhibitory receptors in the human adult spinal cord and brain stem. They belong to the Cys-loop receptor superfamily which also includes nicotinic acetylcholine receptors (nAChRs), type A and type C γ-amino-butyric acid (GABAA and GABAC) receptors, and 5-hydroxy tryptamine type 3 (5-HT3) receptors. These membrane-embedded proteins mediate fast synaptic transmission in the peripheral and central nervous systems. They form pentameric ligand-gated ion channels (pLGICs) from five identical or homologous subunits. Each subunit contains an extracellular domain (ECD), a transmembrane domain (TMD), and an intracellular domain (ICD). ICD is involved in trafficking, localization, and modulation by secondary messengers, but is not essential for the ion-transport function of the channel (Jansen et al., 2008). Upon agonist binding to the ECD, the channels open transiently to allow selected ions to permeate through the membrane, causing a change in the cross-membrane potential (Betz and Laube, 2006; Pless and Lynch, 2008).
Currently available structures for the Cys-loop receptors include the 4-Å cryo-electron microscopy model of the muscle-type nAChR (Unwin, 2005), NMR structures of the TMD of the α4β2 nAChR (Bondarenko et al., 2012), and crystal structures of the α-bungarotoxin-bound α1 nAChR ECD (Dellisanti et al., 2007) and of a chimera from the acetylcholine binding protein (AChBP) and the α7 nAChR (Li et al., 2011). More recently, crystal structures have been solved for two prokaryotic homologues of Cys-loop receptors from the bacterium Erwinia chrysanthemi (ELIC) and cyanobacterium Gloebacter violaceus (GLIC) LGICs (Bocquet et al., 2009; Hilf and Dutzler, 2008, 2009) and for the ivermectin-bound nematode glutamate-gated chloride channel (GluCl) (Hibbs and Gouaux, 2011). Although these structures have greatly advanced the molecular understanding of Cys-loop receptors, no experimental structure is currently available for the TMD of any mammalian Cys-loop anion channels in an unliganded form.
The functional states of crystal structures are usually inferred from electrophysiology measurements under comparable conditions. The GluCl and GLIC structures are believed to represent either an open or desensitized conformation, whereas ELIC structures are thought to be in the closed conformation. Several hypothetical gating mechanisms have been proposed based on these structures (Corringer et al., 2010; Hibbs and Gouaux, 2011). However, it was recently discovered that crystallization conditions might bias the crystal structures into conformations that contradict electrophysiology results. A competitive antagonist-bound ELIC structure was found at the verge of channel opening (Pan et al., 2012b), yet the crystal structure of the mutation-stabilized open-channel ELIC is nearly identical to that of the closed ELIC (Gonzalez-Gutierrez et al., 2012). Anesthetic propofol inhibits GLIC current, but the crystal structure of the GLIC-propofol complex shows the same open channel conformation as that observed in GLIC (Nury et al., 2011). These complications highlight the limitation of crystal structures in revealing functional states of Cys-loop receptors and the need for complementary structural approaches.
Recent advances in high-field NMR spectroscopy have made it possible to determine polytopic helical TM structures for proteins ranging from an integral membrane enzyme, phototaxis receptor sensory rhodopsin, to various ion channels (Bondarenko et al., 2012; Gautier et al., 2010; Pielak et al., 2011; Van Horn et al., 2009; Verardi et al., 2011). Proteins examined in micelles and lipid bilayers often show similar structures, at least at the tertiary level (Gautier et al., 2010; Verardi et al., 2011). Lysophospholipids have been found particularly suitable for yielding good quality NMR spectra and retaining protein functionalities (Koehler et al., 2010; Krueger-Koplin et al., 2004). We report here the NMR structures of the full-length TMD of the human glycine receptor α1 subunit (hGlyR-α1), determined in the lysophospholipid lyso-1-palmitoylphosphotidylglycerol (LPPG). Electron microscopy (EM) and functional measurements show that the TMD forms pentameric and spontaneously Cl−-conducting channels. The NMR data revealed structural and dynamic features that are unique to the hGlyR-α1 TMD and may be shared by other anion-selective Cys-loop receptors. The functional relevance of the TMD structures is validated in a recent study (Duret et al., 2011) that the hGlyR-α1 TMD in a chimera with the GLIC ECD functions as an anion-selective channel and mirrors the pharmacological profile of the authentic hGlyR-α1.
Results
The hGlyR-α1 TMD forms spontaneously open Cl− channels
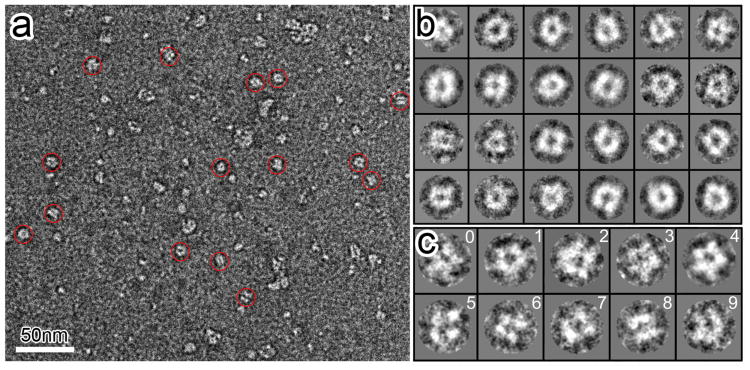
A protein encompassing the entire sequence (Figure S1) of the hGlyR-α1 TMD was expressed and reconstituted in LPPG lipid micelles for structure determination using EM and high-resolution NMR. Unlike the GlyR ECD that assembles randomly into dimers and higher-order oligomers (Haeger et al., 2010), the full-length hGlyR-α1 TMD spontaneously assembles into pentameric structures in LPPG lipid micelles. The negatively stained EM images (Figure 1) show face-on projections of pentamers. A small population of tetramers is also discernible, in accordance with the tetrameric sub-conductance state measureable in the authentic GlyR from mouse spinal cord neurons (Twyman and Macdonald, 1991). Circular averaging of all face-on pentamer images yielded a ring diameter of ∼45 Å for the peak intensities (Figure S2), corresponding to a distance of ∼26 Å between electron density centers of two adjacent subunits and ∼43 Å between two non-adjacent subunits.
Figure 1. Electron microscopy analyses of the hGlyR-α1 TMD oligomeric complexes.
(a) A raw electron micrograph of negatively stained hGlyR-α1 TMD oligomers in LPPG (scale bar, 50 nm). Representative particles are indicated with red circles. (b) Selected 2D class averages of hGlyR-α1 TMD oligomers from 210 particle images. Class averages show doughnut-shaped particles with a central channel and several oligomeric states, including pentamer and tetramer. (c) Representative raw particle images corresponding to the pentameric (0-3) and tetrameric (5-7) configurations. An average of the raw particles from panel 0-3 is shown in panel 4. Panel 8 and 9 show side views of the particles. See also Figure S2.
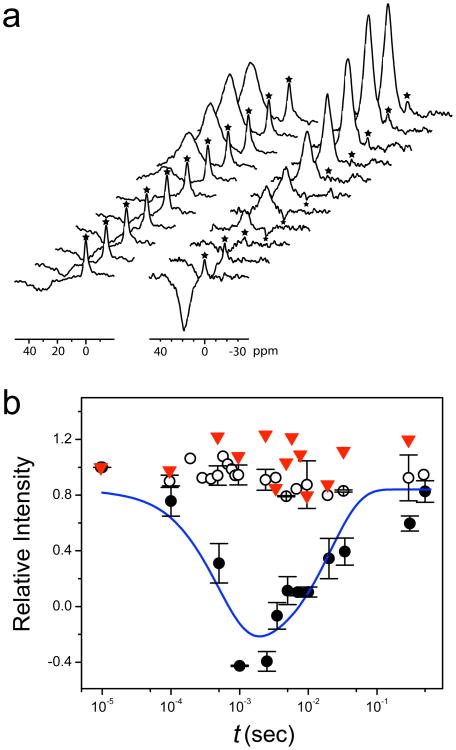
We also reconstituted the same expressed protein into large unilamellar vesicles (LUV) made of L-α phosphatidylcholine (PC) and phosphatidylglycerol (PG) lipids. Function of the hGlyR-α1 channel for Cl− transport was measured by NMR magnetization-inversion transfer experiments (MIT) (Hinton et al., 1994; Tang et al., 1999) using Co(Gly)3 − as a Cl− shift reagent (Diven et al., 2003) to separate intra- and extra-vesicle 35Cl resonances. We found that the channels are not only spontaneously open in the absence of the agonist-binding ECD but also Cl− permeable (Figure 2a). At a nominal channel density of ∼20-2000 per vesicle, the unidirectional Cl− efflux and influx rates (Tang et al., 1999) are 1350 ± 460 and 560 ± 290 s−1, respectively. Moreover, the Cl− transport across the TM channels can be completely blocked in the presence of 1 mM picrotoxin (Figure 2b), indicating that the quaternary association of the TMD is preserved to form a functional channel with a pore geometry resembling that of the authentic open channel.
Figure 2. Channel functional measurements.
(a) Stack plots of 35Cl NMR spectra in Cl− flux measurements across largeunilamellar vesicles (LUV) by NMR magnetization inversion transfer (MIT) experiments (see Supplemental Information for experimental details). Left: control vesicles without the protein; Right: vesicles having the hGlyR-α1 TMD channels. The Cl− shift reagent Co(Gly)3− separates extra-vesicle Cl− signal from the intra-vesicle signal (marked by the asterisk *). The intensity of the intra-vesicle signal (*) changes as a function of the inversion-recovery time (t) due the exchange of intra- and extra-vesicle Cl−. (b) The rates of Cl− influx (ki) and efflux (ke) are determined by fitting the intensity changes as a function of t with a two-site exchange model (solid line, see fitting details in Supplemental Information). ○, LUV without protein; ●, LUV with protein; ▼, LUV with the same amount of protein and with 1 mM picrotoxin. Error bars show the standard error of the mean.
NMR structure of the hGlyR-α1 TMD
A bundle of 15 monomer structures of the hGlyR-α1 TMD with the lowest target function from the CYANA calculation (Figure 3) exhibits a typical four-helix-bundle fold, which has been observed in the TMD structures of the α4β2 nAChR (Bondarenko et al., 2012) and other known structures of pLGICs (Bocquet et al., 2009; Hilf and Dutzler, 2008; Pan et al., 2012a; Pan et al., 2012b). The tertiary (Figure 3a) and quaternary (Figure 3b) packing of the TM helices were determined from the long-range intra-subunit and inter-helical nuclear Overhauser effect (NOE) connectivity (Figures S3, S4), paramagnetic relaxation enhancement (PRE) restraints (Battiste and Wagner, 2000) (Figure S5), and diameter restraints derived from the EM images. The orientation of the TM2 helix relative to the membrane normal was determined using the residual dipole coupling of the TM2-TM3 helical segments in low-q bicelles as reported previously (Canlas et al., 2008). See Supplemental Experimental Procedures. The NMR structure statistics for the bundles of 15 monomers (PDB code: 2M6B) and 15 pentamers (PDB code: 2M6I) are summarized in Table 1. The pair-wise root-mean-square deviations (r.m.s.d) among the 15 lowest target-function pentamer structures are 0.50 Å and 0.91 Å for the backbone and all heavy atoms, respectively, in the four membrane-spanning helices. The overall helical content calculated from the bundle of the NMR structures is 58-62%, in excellent agreement with the value (58%) determined by the circular dichroism (CD) of hGlyR-α1 in LPPG.
Figure 3. NMR structures of the hGlyR-α1 TMD.
(a) A bundle of 15 lowest target function monomer structures of the hGlyR-α1 TMD (PDB code: 2M6B). The TM1, TM2, TM3, and TM4 helices are indicated in red, light green, green, and blue, respectively. (b) Top view of a bundle of 15 lowest target function pentameric structures of the hGlyR-α1 TMD (PDB code: 2M6I), calculated using CYANA with restraints generated from NMR and EM data. Pore lining residues are highlighted based on residue type: green-polar, gray-nonpolar, and blue-basic. (c) Side view of the bundle of 15 lowest target function pentameric structures of the hGlyR-α1 TMD. For clarity, only two subunits are shown. Pore lining residues are labeled and colored based on their residue type. (d) The pore profile calculated using the HOLE program (Smart et al., 1996). Dashed lines represent plus or minus one standard deviation determined from the bundle of 15 lowest target function structures. See also Figures S3, S4, and S5.
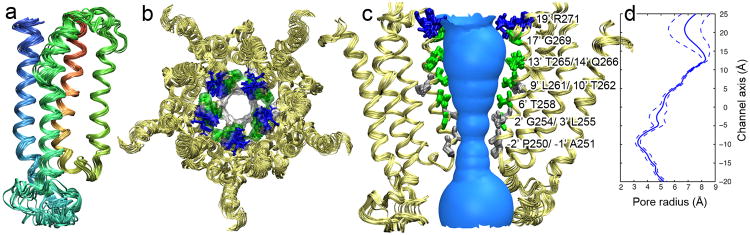
Table 1.
Summary of NMR structure statistics and restraints for the hGlyR-α1 TM domain in LPPG micelles. See also Figures S3, S4, and S5.
| NMR Distance & Dihedral Restraints | Monomer | Pentamer |
|---|---|---|
| Distance restraints | ||
| Total NOE | 1014 | 1014 × 5 |
| Intra-residue | 321 | 321 × 5 |
| Inter-residue | 693 | 693 × 5 |
| Sequential (|i-j| = 1) | 348 | 348 × 5 |
| Medium-range (|i-j| ≤ 4) | 324 | 324 × 5 |
| Long-range (|i-j| > 4) | 21 | 21 × 5 |
| Hydrogen bonds | 106 | 106 × 5 |
| Total dihedral angle restraints | 152 | 154 × 5 |
| Phi | 76 | 77 × 5 |
| Psi | 76 | 77 × 5 |
| PRE restraints | 219 | 226 × 5 |
| Upper | 107 | 114 × 5 |
| Lower | 112 | 112 × 5 |
| Inter-subunit distance restraints from EM and RDCa | ||
| Total constraints | 600 | |
| Upper | 300 | |
| Lower | 300 | |
|
| ||
| Structure Statistics | ||
|
| ||
| Violations (mean and s.d.) | ||
| Upper distance restraints (Å) | 0.0075 ± 0.0008 | 0.0157 ± 0.0010 |
| Lower distance restraints (Å) | 0.0016 ± 0.0010 | 0.0102 ± 0.0012 |
| Dihedral angle restraints (°) | 0.130 ± 0.010 | 0.338 ± 0.028 |
| Max. dihedral angle violation (°) | 1.29 | 3.32 |
| Max. distance restraint violation (Å) | 0.24 | 0.63 |
| Average pairwise r.m.s.d.b (Å) | ||
| Heavy | 1.04 ± 0.11c | 0.91 ± 0.14c |
| 2.27 ± 0.25d | 1.50 ± 0.36d | |
| Backbone | 0.67 ± 0.13c | 0.50 ± 0.17c |
| 1.66 ± 0.18d | 0.95 ± 0.30d | |
| Ramachandran Plot | ||
| Residues in most favored regions | 88.6% | 86.3% |
| Residues in allowed regions | 11.3% | 13.0% |
| Residues in disallowed regions | 0.1% | 0.7% |
|
| ||
| PDB code | 2M6B | 2M6I |
RDC values used to generate pentamer restraints were obtained from the previous study (Canlas et al., 2008).
The rmsd to the average coordinates was calculated from 15 structures.
Calculated over the helical TM regions (residues 220-241, 250-270, 289-305, 398-421)
Calculated over residues 215-425
Several structural features of the hGlyR-α1 TMD are worth noting. First, the pore-lining TM2 has a stable α-helix involving residues from P250 (−2′) to S267 (15′). Residues from 16′ to 18′ show an unwound helix exhibiting a slightly larger helical pitch (Figure 3a). The residues after 18′ in TM2 are nonhelical. In contrast, structures of several pLGICs, including the α4β2 nAChR determined by NMR (Bondarenko et al., 2012), show a longer TM2 α-helix that typically contains 23 residues (−2′ to 20′).
Second, unlike a straight helix observed in other pLGICs, the TM3 helix of hGlyR-α1 has a kink at A288 (Figure 3a). The kink changes the helix axis direction by ∼33°. It is also notable that fourth residue upstream from the kink is a conserved aspartate (D284). Aspartate is known to frequently locate at the i-4 position of TM helical bends (Langelaan et al., 2010).
Third, while most of the pore-lining residues in the hGlyR-α1 TMD structure (Figure 3c) agree well with homologous residues in the previously published pLGIC structures, one distinction is that the well-conserved L261 (9′) in our open-channel structures does not directly face the lumen of the pore, but T262 (10′) does.
Finally, the open channel pore of hGlyR-α1 has a cone shape with the smallest diameter of 6.2 Å defined by a hydrophobic girdle by P250 (−2′) and A251 (−1′) side chains situated at the cytoplasmic side of the membrane (Figure 3d). The positively charged R252(0′) side chains are tangential to the circumference of the pore. The constriction size of the hGlyR-α1 open pore is close to the estimated 6.2 Å for glycine and GABAA receptors based on the studies of ion permeability (Fatima-Shad and Barry, 1993).
Dynamics of the hGlyR-α1 TMD
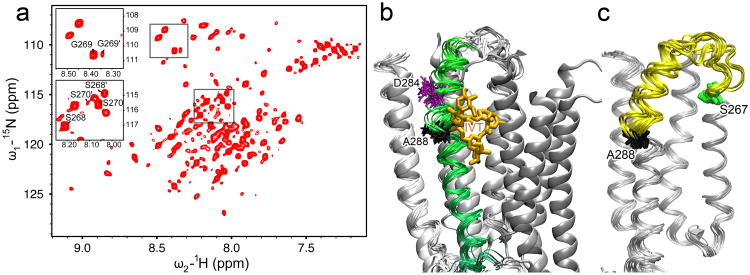
The dynamic characteristics of the TM2 and TM3 helices near the TM2-3 loop are observed not only in the bundle of structures (Figure 3a), but also directly in the high resolution NMR spectra, where two sets of NMR peaks are identifiable for several residues near the TM2 C-terminus, including S268(16′), G269(17′), and S270(18′) (Figure 4a). The data suggest that at least two conformations coexist in this region and they undergo slow exchange on the μs timescale used for NMR data acquisition. It should be noted that a similar minor conformation was also observed in an extended TM2 segment of GlyR (Yushmanov et al., 2003) and in the TM2-TM3 construct in lipid bicelles (Canlas et al., 2008). The NMR structures determined in the present study are associated with the major peaks. The structure in the minor conformation could not be determined because of insufficient NOESY connectivity. A considerable degree of conformational flexibility in the region of the TM2 and TM3 helices near the TM2-3 loop is also evident in the backbone dynamics (Figure S6), as measured by the 15N relaxation parameters (R1 and R2) and 15N-{1H} hetNOE of the major peaks. Nearly 60% of the hGlyR-α1 residues result in relatively high hetNOE values that are in agreement with the helical contents determined by CD, the chemical shift index, and the final structures. However, the segment from S267 (S15′) in TM2 to K276 (K24′) in the TM2-3 loop, and residues involved in the TM3 helical kink, such as W286, show smaller hetNOE and R2 values and relatively higher R1, suggesting high flexibility in the region.
Figure 4. Conformational dynamics around TM2-3 loop.
(a) A representative 1H-15N heteronuclear single quantum correlation spectrum of the hGlyR-α1 TMD in LPPG micelles at 40°C. Several residues at the C-terminus of TM2 show two sets of peaks, as exemplified in the insert for S268(16′), G269(17′), and S270(18′), indicating that two conformations coexist in this region and undergo slow conformational exchange. (b) The 15 lowest target function structures of the hGlyR-α1 TMD (ribbons) are aligned with the crystal structure of GluCl (cartoon, PDB code: 3rhw). TM3 of the hGlyR-α1 TMD is highlighted in green. Ivermectin (orange sticks) observed in GluCl partially overlaps with the kink at A288 (black sticks) in the NMR structures of the hGlyR-α1 TMD. The residue D284 (purple sticks), located 4 residues upstream of A288, may be responsible for the kink (Langelaan et al., 2010). (c) The segment showing high dynamics is highlighted in yellow between S267 (green) and A288 (black) in the bundle of 15 lowest target function NMR structures. See also Figure S6.
Discussion
It is remarkable that the hGlyR-α1 TMD alone, without the ICD and ECD, can self-assemble into a pentameric channel in the membrane-mimetic environment. It is perhaps even more remarkable that the assembled channels spontaneously conduct Cl−. These results, along with the previous finding on well-assembled and spontaneously open channels of the α4β2 nAChR TMD (Bondarenko et al., 2012), support a new hypothesis: the ECD serves as a regulator that creates an energy barrier between open and closed states and constrains the TM channel to the closed state. In the presence of the ECD but without agonist binding, the closed-channel (non-conducting) conformation is preferred and there is an energy barrier to the open state. In the absence of the ECD, however, either the open-channel (conducting) conformation has become preferred or the energy barrier between open- and closed-channel conformations has been relaxed to allow spontaneous channel opening. Our NMR spectra show that residues at the C-terminal end of TM2 exchange between two distinct conformations, possibly related to the open and closed states. The fact that NMR spectra can simultaneously capture two conformations in this region suggests that the free energy difference between the two conformations is not large.
The NMR-resolved structures of the hGlyR-α1 TMD show a general topological agreement with the pLGIC structures determined previously, but several notable differences may be functionally important. One distinct difference is the relatively short TM2 helix, which is about a half helical turn shorter than TM2 helices of ELIC (Hilf and Dutzler, 2008; Pan et al., 2012b), GLIC (Bocquet et al., 2009; Hilf and Dutzler, 2009; Pan et al., 2012a) and GluCl (Hibbs and Gouaux, 2011), but about two helical turns (7 residues) shorter than TM2 helices shown in the cryo-EM structure of nAChR (Unwin, 2005). One may wonder if a shorter TM2 helix is due to different methodologies, NMR vs. X-ray Our previously published NMR structures of the α4β2 nAChR TMD (Bondarenko et al., 2012) negate this possibility Both α4 and β2 show a TM2 helical length similar to that observed in ELIC, GLIC, and GluCl. The discrepancy in the TM2 helical length between hGlyR-α1 and other pLGICs occurs at the C-terminal end of TM2, where the helix ends at the 18′ residue (with slight unwinding after 15′) in hGlyR-α1, but at 20′ in other pLGICs. Furthermore, the TM2 residues S268(16′), G269(17′), and S270(18′) of hGlyR-α1 undergo slow exchange between two distinct conformations that have not been observed in the α4β2 nAChR using the same NMR method. These results demonstrate the unique structural flexibility at the EC end of the pore in hGlyR-α1. Compared to other pLGICs, glycine receptors are uniquely rich with serine residues (15′, 16′ 18′) near the TM2 C-terminus (Figure S1). It is known that the OH group of serine can weaken the helical backbone hydrogen bonds by constraining the carbonyl oxygen through the O· · ·H-O interaction (Ballesteros et al., 2000). The presence of a cluster of serines near the TM2 C-terminus may have contributed to the structural flexibility in the region.
It is also notable in the bundle of NMR structures (Figure 3) that R271(19′) shows conformational variation with a smaller population in a more extended helix and a larger population in an unwound conformation. Consequently, instead of facing the pore, R19′ in some structures is mostly tangent to the pore, where R271(19′) experiences a more hydrophobic environment. Indeed, such conformational flexibility was noted previously by tethering a rhodamine fluorophore to R271C (Pless et al., 2007), in which the experiment showed a population shift of the fluorescence probe at 19′ to a more hydrophobic environment upon channel opening, suggesting that conformational flexibility at the EC end of the pore is related to channel function. Ester substitution is expected to weaken the backbone hydrogen bonds and increase the flexibility of the pore-lining TM2 helix. Single-point amide-to-ester mutations at 13′, 16′, or 19′ of nAChR increased the receptor's sensitivity to agonist more than tenfold (England et al., 1999). A more recent study using electron paramagnetic resonance spectroscopy also observed greater conformational changes at the EC end of TM2 upon agonist binding (Velisetty et al., 2012).
The high flexibility of the TM2 C-terminus of hGlyR-α1 is likely coupled with the structural flexibility near the N-terminal helix of TM3. A helical kink (I285-A288) divides the TM3 domain into two α-helical segments: one from V277 to D284 and the other from V289 to V307 (Figure 3a and Figure S3). Three points about the kink are worth noting. First, statistically speaking, D284 is likely responsible for the kink formation. Analysis of nonredundant polypeptide chains revealed kinks in 64% of TM helices and aspartate showed notably high frequency at the i-4 position of the TM helical kink (Langelaan et al., 2010), though it remains unclear why aspartate promotes the helical disruption. Second, D284 is conserved in both glycine and GABAA receptors. It likely plays a similar structural and functional role in all anion-conducting Cys- loop receptors. Mutation of this conserved aspartate in the α1 GABAA receptor significantly reduced receptor activity (Bera et al., 2002). Third, while the TM3 helices in the crystal structure of the GluCl and ivermectin complex show no kink (Hibbs and Gouaux, 2011), the kink may exist in the absence of ivermectin. When we aligned the TM structures of GluCl and hGlyR-α1, it became clear that ivermectin partially overlapped with the kink observed in the NMR structure (Figure 4b), suggesting that ivermectin binding may have stabilized a straight helical conformation and that without ivermectin the flexibility would make it much more challenging to obtain high quality GluCl crystals for X-ray structure determination.
S267 and A288 mark the two ends of a dynamic region of the channel in our hGlyR-α1 TMD structures (Figure 4c). Intriguingly, mutations S267Y and A288W in the hGlyR-α1 TMD were found to substantially reduce general anesthetic and alcohol potentiation of GlyR responses (Mihic et al., 1997). Mutations at S267 showed that ethanol modulation was correlated with the volume but not the polarity or hydropathicity of the substituting side chains, suggesting that S267 itself is not directly involved in alcohol binding (Ye et al., 1998). These functional consequences may result from the reduced conformational flexibility in the region due to bulky substitution at the S267 position. In fact, our previous NMR study demonstrated that the S267Y mutation increased the α-helix length at the TM2 C-terminus (Tang et al., 2002). Mutation of A288 to an amino acid with a different size can also alter conformational flexibility in the region with functional consequences. Indeed, A288F and A288G have opposite functional impacts, with the former reducing and the latter increasing glycine-induced channel activation (Lynagh and Lynch, 2010). It is unlikely that glycine binding is affected by the mutations because the orthosteric agonist-binding site in the ECD is remote from A288. The changes in conformational flexibility due to mutations alter the channel's susceptible to allosteric activation.
Q266H is a TM2 mutation that causes hyperekplexia (Moorhouse et al., 1999), a human genetic disease characterized by a startle response to acoustic or tactile stimulations. The mutation reduces the ability of glycine and taurine to activate the channel and reduces the channel open times, implicating a change in gating kinetics. Moreover, the mutation does not affect ion accessibility to the channel lumen (Moorhouse et al., 1999), consistent with the Q266 side chain orientation in the NMR structure. Interestingly, Q266I also reduces glycine sensitivity and glycine-induced maximal currents (Borghese et al., 2012). Both H and I have a larger side chain than Q. Thus, Q266H and Q266I likely produce the same volume effects as in the S267 mutants, given the adjacency of Q266 and S267. Q has another unique property The amide of its side chain can contact its own backbone carbonyl oxygen (Figure S7). This contact competes with helical hydrogen bonding, weakening the helical propensity and adding structural flexibility to the region. Self-contacts within glutamine have been observed in other proteins (Pal and Sankararamakrishnan, 2008).
The NMR structures of the GlyR-α1 TMD provide valuable structural and dynamic templates for the design and discovery of modulators or therapeutics targeting glycine receptors. For example, our TMD structure revealed a specific binging site in the GlyR-α1 TM3 domain for Δ9-tetrahydrocannabinol (THC), the major ingredient of marijuana, and several other cannabinoids (Xiong et al., 2011; Xiong et al., 2012). NMR chemical shift titration further showed the direct involvement of residue S296 in THC binding. Hydrogen-bond interaction of S296 with the hydroxyl groups of THC is considered to be critical for the THC potentiation of α1- and α3-containing GlyR in mediating THC's analgesic effects. This important drug-binding site would not be discovered from any homology modeling using the crystal structures of lower species. It is foreseeable that the structures of the GlyR-α1 TMD reported here will offer useful insights for other potential drugs targeting mammalian Cys-loop anion channels.
Materials and Methods
Detailed experimental procedures can be found in the online supplemental information. Briefly full-length TMD of hGlyR-α1 was expressed and purified using the same methods as reported previously (Ma et al., 2005). In addition to uniform 15N-labeling and 15N ,13C-double labeling, specific 15N labeling of alanine, phenylalanine, leucine, isoleucine, and valine were performed to assist in chemical shift assignment. Samples for high-resolution NMR were prepared in LPPG micelles having a protein concentration of ∼500 μM with a protein-to-LPPG ratio of ∼1:200 (pH 5.8). The choice of LPPG was made based on protein sample stability, high NMR spectral quality, and pentameric channel formation as shown in EM. For EM measurements, serial dilutions were made from a stock solution of 54 μM protein and a protein-to-LPPG ratio of 1:50. To generate long-range distance restraints by PRE, the wild-type hGlyR-α1 TM (with one cysteine, C290) and two single-cysteine mutants (C290S/S296C and C290S/S308C) were prepared and labeled with [1-oxyl-2,2,5,5-tetramethylpyrroline-3-methyl]-methanethiosulfonate (MTSL). For functional Cl− flux measurements, hGlyR-α1 was reconstituted into 3:1 PC:PG LUV for MIT experiments.
NMR experiments were carried out at 40°C on Bruker Avance 600, 700, 800, or 900 MHz spectrometers equipped with triple-resonance inverse-detection cryoprobes (Bruker Instruments, Billerica, MA). A standard suite of 2D and 3D pulse sequences was used for resonance assignment, structure calculations, protein dynamics measurements, and Cl− flux measurements, as detailed in the online supplemental information. EM experiments were performed at 200 kV using a TF20 electron microscope (FEI, Hillsboro, OR) equipped with a 4K×4K Gatan CCD camera. CYANA 3.0 (Lopez-Mendez and Guntert, 2006) was used for structure calculations with a combination of angular and distance restraints from chemical shift, 3D NOESY, PRE, EM, and RDC measurements, as summarized in Table 1. The VMD program (Humphrey et al., 1996) was used for structure rendering, visualization, and analysis. The pore profiles were analyzed using the HOLE program (Smart et al., 1996).
Supplementary Material
Highlights.
The TM domain of hGlyR-α1 forms pentameric channels that spontaneously conduct Cl−
The hGlyR-α1 TM structures show a shorter TM2 helix and a kink at A288 of TM3
The segment between S267 of TM2 and A288 of TM3 is highly dynamic
The structures offer insights into genetic diseases and actions of drugs
Acknowledgments
The authors thank Ms. Ling Li for technical assistance in protein expression, Professor Peter Güntert for providing a pre-released version of CYANA 3.0 for pentamer structure calculation, and Ms. Sandra C. Hirsch for her editorial assistance. This work was supported by grants from the National Institutes of Health (R37GM049202, R01GM056257, R01GM069766, and R01 GM085043).
Footnotes
Supplemental Information: Supplemental information includes seven figures. Supplemental experimental procedures and supplemental references can also be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballesteros JA, Deupi X, Olivella M, Haaksma EEJ, Pardo L. Serine and threonine residues bend alpha-helices in the chi(1) = g(−) conformation. Biophysical Journal. 2000;79:2754–2760. doi: 10.1016/S0006-3495(00)76514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiste JL, Wagner G. Utilization of site-directed spin labeling and highresolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry. 2000;39:5355–5365. doi: 10.1021/bi000060h. [DOI] [PubMed] [Google Scholar]
- Bera AK, Chatav M, Akabas MH. GABA(A) receptor M2-M3 loop secondary structure and changes in accessibility during channel gating. J Biol Chem. 2002;277:43002–43010. doi: 10.1074/jbc.M206321200. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Bondarenko V, Mowrey D, Tillman T, Cui T, Liu LT, Xu Y, Tang P. NMR structures of the transmembrane domains of the alpha4beta2 nAChR. Biochim Biophys Acta. 2012;1818:1261–1268. doi: 10.1016/j.bbamem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Xiong W, Oh SI, Ho A, Mihic SJ, Zhang L, Lovinger DM, Homanics GE, Eger EI, 2nd, Harris RA. Mutations M287L and Q266I in the glycine receptor alpha1 subunit change sensitivity to volatile anesthetics in oocytes and neurons, but not the minimal alveolar concentration in knockin mice. Anesthesiology. 2012;117:765–771. doi: 10.1097/ALN.0b013e31826a0d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlas CG, Ma D, Tang P, Xu Y. Residual dipolar coupling measurements of transmembrane proteins using aligned low-q bicelles and high-resolution magic angle spinning NMR spectroscopy. J Am Chem Soc. 2008;130:13294–13300. doi: 10.1021/ja802578z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Baaden M, Bocquet N, Delarue M, Dufresne V, Nury H, Prevost M, Van Renterghem C. Atomic structure and dynamics of pentameric ligand-gated ion channels: new insight from bacterial homologues. J Physiol. 2010;588:565–572. doi: 10.1113/jphysiol.2009.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Diven CF, Wang F, Abukhdeir AM, Salah W, Layden BT, Geraldes CF, Mota de Freitas D. Evaluation of [Co(gly)3]- as a 35Cl- NMR shift reagent for cellular studies. Inorg Chem. 2003;42:2774–2782. doi: 10.1021/ic0258680. [DOI] [PubMed] [Google Scholar]
- Duret G, Van Renterghem C, Weng Y, Prevost M, Moraga-Cid G, Huon C, Sonner JM, Corringer PJ. Functional prokaryotic-eukaryotic chimera from the pentameric ligand-gated ion channel family. Proc Natl Acad Sci U S A. 2011;108:12143–12148. doi: 10.1073/pnas.1104494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England PM, Zhang Y, Dougherty DA, Lester HA. Backbone mutations in transmembrane domains of a ligand-gated ion channel: implications for the mechanism of gating. Cell. 1999;96:89–98. doi: 10.1016/s0092-8674(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Fatima-Shad K, Barry PH. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc Biol Sci. 1993;253:69–75. doi: 10.1098/rspb.1993.0083. [DOI] [PubMed] [Google Scholar]
- Gautier A, Mott HR, Bostock MJ, Kirkpatrick JP, Nietlispach D. Structure determination of the seven-helix transmembrane receptor sensory rhodopsin II by solution NMR spectroscopy. Nat Struct Mol Biol. 2010;17:768–774. doi: 10.1038/nsmb.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gutierrez G, Lukk T, Agarwal V, Papke D, Nair SK, Grosman C. Mutations that stabilize the open state of the Erwinia chrisanthemi ligand-gated ion channel fail to change the conformation of the pore domain in crystals. Proc Natl Acad Sci U S A. 2012;109:6331–6336. doi: 10.1073/pnas.1119268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger S, Kuzmin D, Detro-Dassen S, Lang N, Kilb M, Tsetlin V, Betz H, Laube B, Schmalzing G. An intramembrane aromatic network determines pentameric assembly of Cys-loop receptors. Nat Struct Mol Biol. 2010;17:90–98. doi: 10.1038/nsmb.1721. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- Hinton JF, Newkirk DK, Fletcher TG, Shungu DC. Application of the magnetization-inversion-transfer technique to the transport of 7Li+, 23Na+, and 39K+ ions through the gramicidin channel and the M2 delta transmembrane domain of the nicotinic acetylcholine receptor. (Series B).Journal of magnetic resonance. 1994;105:11–16. doi: 10.1006/jmrb.1994.1093. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jansen M, Bali M, Akabas MH. Modular design of Cys-loop ligand-gated ion channels: functional 5-HT3 and GABA rho1 receptors lacking the large cytoplasmic M3M4 loop. J Gen Physiol. 2008;131:137–146. doi: 10.1085/jgp.200709896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J, Sulistijo ES, Sakakura M, Kim HJ, Ellis CD, Sanders CR. Lysophospholipid micelles sustain the stability and catalytic activity of diacylglycerol kinase in the absence of lipids. Biochemistry. 2010;49:7089–7099. doi: 10.1021/bi100575s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger-Koplin RD, Sorgen PL, Krueger-Koplin ST, Rivera-Torres IO, Cahill SM, Hicks DB, Grinius L, Krulwich TA, Girvin ME. An evaluation of detergents for NMR structural studies of membrane proteins. J Biomol NMR. 2004;28:43–57. doi: 10.1023/B:JNMR.0000012875.80898.8f. [DOI] [PubMed] [Google Scholar]
- Langelaan DN, Wieczorek M, Blouin C, Rainey JK. Improved helix and kink characterization in membrane proteins allows evaluation of kink sequence predictors. J Chem Inf Model. 2010;50:2213–2220. doi: 10.1021/ci100324n. [DOI] [PubMed] [Google Scholar]
- Li SX, Huang S, Bren N, Noridomi K, Dellisanti CD, Sine SM, Chen L. Ligand-binding domain of an alpha(7)-nicotinic receptor chimera and its complex with agonist. Nat Neurosci. 2011;14:1253–1259. doi: 10.1038/nn.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Mendez B, Guntert P. Automated protein structure determination from NMR spectra. J Am Chem Soc. 2006;128:13112–13122. doi: 10.1021/ja061136l. [DOI] [PubMed] [Google Scholar]
- Lynagh T, Lynch JW. A glycine residue essential for high ivermectin sensitivity in Cys-loop ion channel receptors. Int J Parasitol. 2010;40:1477–1481. doi: 10.1016/j.ijpara.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Ma D, Liu Z, Li L, Tang P, Xu Y. Structure and dynamics of the second and third transmembrane domains of human glycine receptor. Biochemistry. 2005;44:8790–8800. doi: 10.1021/bi050256n. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Moorhouse AJ, Jacques P, Barry PH, Schofield PR. The startle disease mutation Q266H, in the second transmembrane domain of the human glycine receptor, impairs channel gating. Mol Pharmacol. 1999;55:386–395. doi: 10.1124/mol.55.2.386. [DOI] [PubMed] [Google Scholar]
- Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- Pal TK, Sankararamakrishnan R. Self-contacts in Asx and Glx residues of highresolution protein structures: role of local environment and tertiary interactions. J Mol Graph Model. 2008;27:20–33. doi: 10.1016/j.jmgm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Mowrey D, Kong XP, Cohen A, Divito CB, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel GLIC bound with anesthetic ketamine. Structure. 2012a;20:1463–1469. doi: 10.1016/j.str.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Chen Q, Willenbring D, Yoshida K, Tillman T, Kashlan OB, Cohen A, Kong XP, Xu Y, Tang P. Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nature communications. 2012b;3:714. doi: 10.1038/ncomms1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielak RM, Oxenoid K, Chou JJ. Structural investigation of rimantadine inhibition of the AM2-BM2 chimera channel of influenza viruses. Structure. 2011;19:1655–1663. doi: 10.1016/j.str.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless SA, Dibas MI, Lester HA, Lynch JW. Conformational variability of the glycine receptor M2 domain in response to activation by different agonists. J Biol Chem. 2007;282:36057–36067. doi: 10.1074/jbc.M706468200. [DOI] [PubMed] [Google Scholar]
- Pless SA, Lynch JW. Illuminating the structure and function of Cys-loop receptors. Clin Exp Pharmacol Physiol. 2008;35:1137–1142. doi: 10.1111/j.1440-1681.2008.04954.x. [DOI] [PubMed] [Google Scholar]
- Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph. 1996;14:354–360. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Tang P, Hu J, Liachenko S, Xu Y. Distinctly different interactions of anesthetic and nonimmobilizer with transmembrane channel peptides. Biophys J. 1999;77:739–746. doi: 10.1016/S0006-3495(99)76928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang P, Mandal PK, Xu Y. NMR structures of the second transmembrane domain of the human glycine receptor alpha(1) subunit: model of pore architecture and channel gating. Biophys J. 2002;83:252–262. doi: 10.1016/S0006-3495(02)75166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Van Horn WD, Kim HJ, Ellis CD, Hadziselimovic A, Sulistijo ES, Karra MD, Tian C, Sonnichsen FD, Sanders CR. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science. 2009;324:1726–1729. doi: 10.1126/science.1171716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisetty P, Chalamalasetti SV, Chakrapani S. Conformational transitions underlying pore opening and desensitization in membrane-embedded Gloeobacter violaceus ligand-gated ion channel (GLIC) J Biol Chem. 2012;287:36864–36872. doi: 10.1074/jbc.M112.401067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verardi R, Shi L, Traaseth NJ, Walsh N, Veglia G. Structural topology of phospholamban pentamer in lipid bilayers by a hybrid solution and solid-state NMR method. Proc Natl Acad Sci U S A. 2011;108:9101–9106. doi: 10.1073/pnas.1016535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cheng K, Cui T, Godlewski G, Rice KC, Xu Y, Zhang L. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat Chem Biol. 2011;7:296–303. doi: 10.1038/nchembio.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Cui T, Cheng K, Yang F, Chen SR, Willenbring D, Guan Y, Pan HL, Ren K, Xu Y, Zhang L. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J Exp Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Koltchine VV, Mihic SJ, Mascia MP, Wick MJ, Finn SE, Harrison NL, Harris RA. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position alpha267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.