Abstract
Background
From birth, infants show a preference for the faces, gaze, and voices of others. In individuals with autism spectrum disorders (ASDs) these biases appear to be disturbed. The source of these disturbances is not well-understood, but recent efforts have shown that the spontaneous deployment of attention to social targets may be atypical as early as 6 months of age. The nature of this atypical behavior and the conditions under which it arises are currently unknown.
Methods
We used eye-tracking to examine the gaze patterns of 6-month-old infants (N=99) at high risk (HR; N=57) and low risk (LR; N=42) for developing ASD as they viewed faces that were (1) still, (2) moving and expressing positive affect, or (3) speaking. Clinical outcomes were determined through a comprehensive assessment at the age of 3 years. The scanning patterns of infants later diagnosed with ASD were compared to infants without an ASD outcome.
Results
Infants who later developed ASD spent less time looking at the presented scenes in general than other infants. When these infants looked at faces, their looking towards the inner features of faces decreased compared to the other groups only when the presented face was speaking.
Conclusions
Our study suggests that infants later diagnosed with ASD have difficulties regulating attention to complex social scenes. It also suggests that the presence of speech may uniquely disturb the attention of infants who later develop ASD at a critical developmental point when other infants are acquiring language and learning about their social world.
Keywords: Autism, eye-tracking, infants, speech, faces, social attention
Introduction
Hours after birth, typically developing (TD) newborns show preferences for face-like stimuli(1), eye-contact(2), and voices(3), suggesting that they come into the world already equipped with primitive biases that prepare them for integration into an exceedingly social world. By contrast, many children and even adults with autism spectrum disorders (ASDs) do not share these basic social predispositions(4–6). While many (not necessarily incompatible) hypotheses regarding the source of these disturbances in ASD have been proposed(6–8), recent views have highlighted the need to consider the disorder from a fundamental perspective of development(9), an approach that provides illumination not only of the pathogenesis of ASD but also of the essential genetic, neural, and behavioral underpinnings that comprise and contribute to the surprisingly robust social and communicative capabilities exhibited throughout early typical development.
Previous studies using eye-tracking have shown that adolescents and young adults with ASD look less at the faces of people engaged in complex social interactions(10) and take longer to locate faces in complex scenes(11). Similar studies in toddlers have shown that 2-year-olds with ASD preferentially attend to videos of geometric shapes over videos of children playing(12) and look less at the faces of actresses attempting to engage their attention using direct eye-contact and infant-directed speech(13; 14). These atypical patterns of attention towards social information have been linked with the communicative and social deficits that define ASDs.
A key question in the field of autism research is when and under what conditions do atypical attentional patterns towards social information begin to emerge. Interest in this stems from both theoretical and practical concerns. From a theoretical perspective, understanding the early ontogeny of the disorder provides a window into those critical processes that support the unique developmental progression that leads to communicative and social competence in typical development(15). From a practical perspective, increased understanding of the early prodromal symptoms of ASDs(16) may pave the way not only for earlier identification of ASD, but may also help to map out the relevant developmental targets for intervention, a goal which may help us to mitigate deleterious effects of the disorder on the quality of life of affected individuals (17–19).
However, identification of early prodromal symptoms of ASD has proven challenging(16; 20–22). The deficits that define the disorder are more easily identifiable in the 2nd year when the diminished social and communicative abilities and presence of stereotyped interests and repetitive behaviors(23) of many toddlers on the autism spectrum are more apparent(24–26). Before this age, the behavioral symptoms associated with ASD can be highly non-specific(16; 20; 21), hindering investigations of the early developmental manifestations of ASD because of the lack of a concurrent and definitive diagnosis.
Among methods used to examine the nature of autism before the first birthday (16), some of the most powerful involve prospective study designs that track infants from before the emergence of a full-blown behavioral syndrome (e.g. at 6-months of age) until their ASD status is relatively certain(16; 20; 21). Because large sample sizes would be required in the general population (at current prevalence estimates(27; 28), over 600 infants would need to be tracked to identify 10 who develop ASD), most prospective studies examine infants at high risk (HR) for developing ASD, such as infant siblings of children with a diagnosis of ASD (though see 29–31) as these infants, due to shared genetic liabilities, are at increased risk for ASD themselves. Recent studies of these HR infant siblings have found an over 10-fold increase in autism risk, with 18.7% of HR infant siblings developing ASD(32). Furthermore, over 30% of HR infants who do not develop ASD show some social, communicative, intellectual, or other delay.(32–36)
Prospective studies have shown that many HR siblings later diagnosed with ASD exhibit atypical patterns of attention at 12 months including decreased orienting towards their name(37; 38), reduced eye contact(37; 39–41), atypical exploration of objects(42), and decreased responsiveness to joint attention(40). These findings contrast with results at 6 months of age. Typically developing infants at 6 months display a wide array of socially-relevant skills including gaze following(43) and responsivity to direct gaze(44) and triadic interactions(45; 46), skills once considered to be promising potential markers for early emerging autism symptomology in HR infants(20; 21). Prospective studies to this point, however, have suggested few differences in social and communicative behaviors at 6 months between those who develop ASD and those who do not(35; 37; 39; 40; 47–52).
However, there is some evidence of more subtle behavioral manifestations of autism at 6 months of age in HR siblings. For example, Zwaigenbaum and colleagues noted decreased parent-reported activity levels in 6-month old HR infants who developed ASD (37) relative to other HR infants and low-risk (LR: no family history of ASD) controls. Flanagan and colleagues showed that 6-month old HR infants who developed ASD exhibited diminished postural control(53). Paul and colleagues showed relationships between atypical vocal production at 6 months and later autism symptoms(54). In addition, two recent eye-tracking studies have found attentional differences in infants who go on to develop ASD. In a study of an overlap task involving faces and objects, Elison and colleagues(55) found that 7-month-old infant siblings who developed ASD showed longer latencies to disengage and shift away from central stimuli. In our recent eye-tracking study, we showed that 6-month-old infants who later developed ASD demonstrated diminished spontaneous attention towards complex dynamic social scenes and to the face of an actress within those scenes(56).
Recent studies have also suggested that infants who develop ASD may exhibit neural level differences. A diffusion tensor imaging study by Wolff and colleagues found white-matter fiber tract irregularities in 6-month-old HR infants who developed ASD(57), a finding possibly related to morphological overgrowth in infants who develop ASD(58–62). A recent study by Elsabbagh and colleagues showed slower ERP P400 responses to faces with direct gaze as compared to averted gaze at 6 months in HR infants who developed ASD versus both other HR infants as well as LR controls(50).
Thus, emerging evidence suggests that even at 6 months behavioral and neural atypicalities may be present in infants who later are diagnosed with ASD. However, despite the prominence of studies of face perception in infancy research and the multitude of studies indicating atypical face scanning in older individuals with ASD (e.g. see 10; 13; 14; 63–65), there have been no studies that have linked specific patterns of looking towards faces in infancy to later-emerging ASD. One difficulty, as noted by Speer and colleagues, is that atypical patterns of attention towards facial features by individuals with ASD may arise only under specific, more socio-cognitively challenging contexts such as complex, dynamic social scenes(65). This effect is also observed in toddlers with ASD, who show a more atypical pattern of attention towards facial features in response to communicatively-informative actions by an actress but not in response to her solitary activities or to nonsocial motion(14).
In this study, we aimed to examine the effects of context on patterns of looking at faces in LR and HR 6-month-old infants. Unlike in our previous study(56), the faces in this study subtended wide visual angles affording a more fine-grained region-of-interest analysis. The faces were presented in three conditions: as a static image, as a dynamic video of an actress smiling, and as a dynamic video of a smiling actress speaking a nursery rhyme. These conditions reflected a sequence of increasingly complex and communicatively charged information, allowing us to examine whether, as in older toddlers(14) and children(65) with ASD, attentional profiles towards faces in infants who develop ASD become more atypical as socio-cognitive processing demands are increased. Through this process, we hope to obtain a better understanding of those attentional atypicalities towards social and communicative information which may constitute risk factors for the development of ASD, and, more broadly, to identify the links between the atypical social experiences implied by atypical attentional processes and the later emergence of autism symptoms.
Methods and Materials
Participants
Participants (N=122) were enrolled in a prospective study of face processing and were comprised of both younger siblings of children with ASD (high-risk, HR; N=68) and infants who had no family history of ASD in any 1st or 2nd degree relatives (low-risk, LR; N=54). In HR infants, the diagnosis of the older sibling was confirmed by clinical best estimate (CBE) together with the ADOS-G(66) and/or ADI-R(67). In LR infants, family history was ascertained through parent/caregiver interview via standardized questionnaires. The study protocol was approved by the University Institutional Review Board with parents signing informed consents.
At 6 months all infants were administered a developmental assessment (Mullen Scales of Early Learning(68)) and eye-tracking procedures. At a 24-month (28%) or 36-month assessment (72%), CBE diagnosis for HR infants were assigned by expert clinicians based on measures including developmental (Mullen(68)) and language assessments (CSBS(69) or PLS-4(70)), family and medical history, and the ADOS-G(66). The infants were classified into: (1) those presenting with frank symptoms of ASD (ASD, N=15: 14 HR, 1 LR); (2) HR infants with an atypical developmental presentation, transient or otherwise (HR-ATYP, N=35); (3) HR infants with no clinically significant symptoms (HR-TYP, N=19); and (4) LR infants with no clinical symptoms (LR-TYP, N=53). These criteria are identical to those used in our previous study(56) and reflect strict definitions of HR sibling “typical development”, including the lack of even transient developmental or broader autism phenotype concerns. Follow-up analyses with definitions of HR-ATYP that did not include transient concerns (resulting in HR-ATYP comprising 39.1% of the HR sample, comparable to proportions noted for elevated ASD symptoms or developmental concerns in(71)) did not change the results of our study.
Twenty-three of the 122 infants (18.9%) were excluded due to movement or inattention during calibration procedures. The remaining infants all contributed data to at least one condition and 82% contributed data to two or three conditions. The percentage of excluded participants did not differ by diagnosis (79.2% to 84.2%; χ2(3)=.32, p=.96). Similarly, there was no differential drop-out by condition (Static: χ2(3)=1.51, p=.68; Affective: χ2(3)=.2.17, p=.54; Speech: χ2(3)=3.12, p=.37). Excluded infants were younger than included infants (M=6.12 months, SD=.33 and M=6.32 months, SD=.49, respectively; t(46.8)=2.3, p=.02), but did not differ by gender (p=.59) or Mullen age equivalent (AE) scores (all p>.05).
The retained sample (N=99) included participants with ASD (N=12), HR-ATYP (N=29), HR-TYP (N=16), and LR-TYP (N=42). 93% of the sample was Caucasian and 88% non-Hispanic, with no between-group differences. At 6 months, diagnostic groups did not differ in chronological age or Mullen AE scores, with the exception of decreased fine motor performance in the HR-ATYP group relative to the LR-TYP group. At 24 months, toddlers with ASD showed the greatest level of social deficits compared to all other groups, and, together with the HR-ATYP group, showed lower verbal and nonverbal performance as compared to the HR-TYP and LR-TYP groups (Supplement: Table S1).
Stimuli
Stimuli were presented in three conditions. In the Static condition an image of a neutral female face(72) was shown. In the Affective condition a video of a smiling female face was shown. In the Speech condition a video of a female reciting a nursery rhyme was presented. Each stimulus was preceded by a 1.5s central target. To accentuate dynamic scene qualities, dynamic scenes (Affective and Speech conditions) included a single forward and backwards movement resembling a looming approach and retreat. The positions of the eyes and mouth were controlled between conditions. The display area (including background) extended 15.2 × 20.9 degrees of visual angle, with the face comprising 51–59% of the vertical and horizontal extent. All stimuli were presented together with an overlaid classical music soundtrack in order to enhance attention to the scene.
Considering that the study was focused on studying face recognition in young children, stimuli were shown until infants had looked at the stimulus for 20 seconds (see 63). Dynamic stimuli (approx. length 10s) were looped to meet the time criterion. In order to obtain comparability across presentations (which could include a partial second video iteration), and for the purposes of this study, only the first 10s of the Static condition and the first loop of dynamic stimuli were analyzed.
Procedure
Infants were seated in a car seat in front of a 24″ 16:9 widescreen LCD stimulus monitor in a dark and soundproof room. The center of their vision was aligned with the center of the monitor, with an eye-to-monitor distance of 75 cm. Once the child was settled, a 5-point calibration routine (colorful cartoons) was initiated. Following this, the stimuli described were presented. Gaze patterns were recorded on an iView X RED 60hz desktop mounted eye-tracking system and stimuli presented with Presentation (Neurobehavioral Systems, Inc.).
Analytic Strategy
Data Reduction
Gaze patterns were analyzed using standard area-of-interest (AOI) analysis techniques via custom software written in MATLAB (73) (see Figure 1). Regions were selected to capture areas known to be informative for facial recognition in young children with ASD(63; 74). For analyses on looking at the inner regions of the face we excluded 4 trials (2% of all trials) in which children looked at the scene less than 15% of the time. Periods in dynamic scenes where the face was in looming motion were excised (23.3% in the Affective condition and 16.7% in Speech).
Figure 1.

Areas-of-interest (AOIs) overlaid on frame from Speech condition.
The following dependent variables were examined: (1) %Scene - overall time spent looking at the scene, relative to the time the stimulus was shown. This variable represents overall attention to the stimuli, and has been shown to be diminished in infants who later develop ASD(56). (2) %InnerFace - time spent looking at inner areas of the face (eyes, nose, and mouth) relative to time spent looking at the scene, capturing attention towards face areas important for encoding facial identity in older, typically developing individuals(74). Attention to inner facial features is also diminished in young children with ASD(14; 56; 63). (3) %OuterPerson - time spent looking outer areas of the person (skin, hair, and body) relative to time looking at the scene. This variable captures attention towards areas on the person other than key areas of the face. (4) Eye-to-Mouth Ratio – eye-looking time / (eye-looking time + mouth-looking time)(47; 63; 75). This variable has been frequently studied in eye-tracking work on face scanning in ASD(47; 63; 75; 76) and captures discrepancies in attending towards the mouth over the eyes (see also 10; 13; 64; 77).
Together, this collection of variables captures differences in attention from the “top to bottom”, following general principles outlined in(78), with each successive layer refining our understanding of the nature of scanning patterns across infants with different outcomes.
Statistical Analyses
Dependent variables were analyzed in a 4 (diagnostic group) by 3 (experimental condition) linear mixed effects model with the experimental condition (Static, Affective, or Speech) as a within-group factor and diagnostic group (ASD, HR-ATYP, HR-TYP, LR-TYP) as a between-group factor. Planned contrasts between the ASD group and all other groups are reported using a Holm-Bonferroni correction. Preprocessing was done in Perl, Matlab, and R; final data analyses were implemented in SPSS.
Results
Looking at the scene
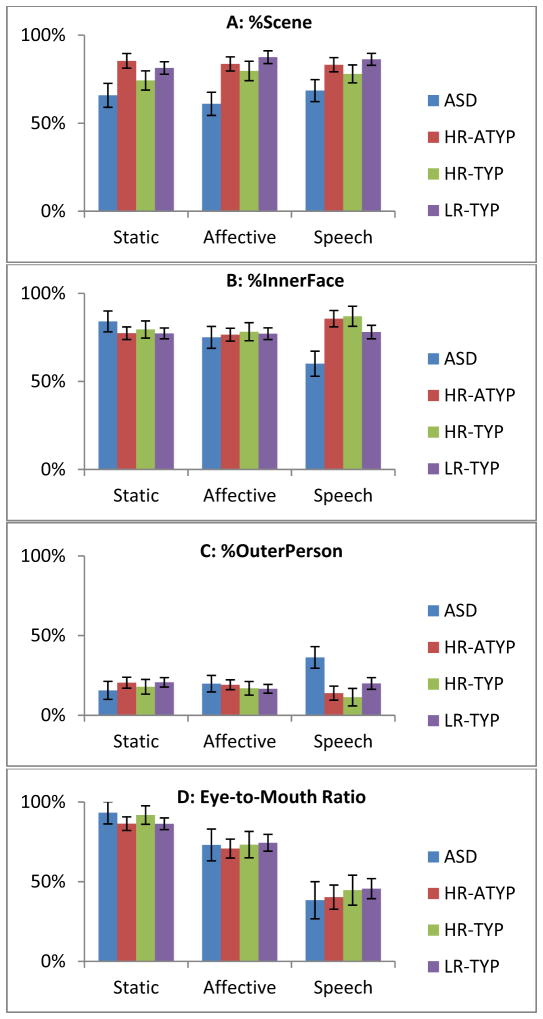
Analysis of %Scene indicated a group difference [F(3,92.0)=4.16, p=.008] but no condition (p=.656) or group x condition interaction (p=.620). Infants later diagnosed with ASD looked less at the scene as a whole as compared to HR-ATYP (p=.006, d=1.01) and LR-TYP (p=.004, d=1.19) infants and showed a trend to look less than HR-TYP infants (p=.083, d=.56) (Figure 2a; Supplement: Figure S1 Row 1)
Figure 2.
Face scanning variables by condition and group showing that infants who go on to develop ASD show: (A) general decreased attention to dynamic scenes with faces; (B) decreased attention to inner areas of the face (eyes, mouth, and nose) only in the Speech condition; (C) increased looking towards non-face areas of the person (skin, hair, and body) only in the Speech condition; but (D) condition-dependent increases in attention towards the mouth vs. the eye during Speech similar to that observed in other groups.
Looking at Inner Features of the Face
Analysis of %Inner showed no main effects of group (p=.533) or condition (p=.514), but indicated a group x condition interaction [F(6,68.7)=2.30, p=.044]. Post-hoc comparisons indicated that infants later diagnosed with ASD looked less at inner features of the face than other groups only in the Speech condition (HR-ATYP: p=.010, d=1.25; HR-TYP: p=.010, d=1.32; LR-TYP: p=.028, d=.60) and looked less at the inner features in the Speech condition than any other condition (vs. Affective: p=.048; vs. Static: p=.004) (Figure 2b; Supplement: Figure S1 Row 2). No other interactions were significant.
Looking at Outer features of the Person
Analysis of %Outer showed no effect of group (p=.521) or condition (p=.640), but indicated a group x condition interaction [F(6,61.7)=2.29, p=.046]. Infants later diagnosed with ASD looked more at outer features compared to controls only in the Speech condition (HR-ATYP: p=.018, d=1.12; HR-TYP: p=.018, d=1.22; LR-TYP: p=.038, d=.57) and looked more at outer features in the Speech condition than any other condition (vs. Affective: p=.011; vs. Static: p=.009) (Figure 2c).
Looking at the Eyes versus the Mouth
Analysis of Eye-to-Mouth Ratio showed no main effects of group (p=.954), a condition effect (F(2,72.6)=64.3, p<.001), and no group x condition interaction (p=.926). All infant groups looked at the eyes (relative to eyes and mouth) most in the Static condition (vs. Affective: p<.001, d=.62; vs. Speech: p<.001, d=1.52), followed by the Affective condition (vs. Speech: p<.001, d=.88), and least in the Speech condition (Figure 2d; Supplement: Figure S1 Row 3).
Discussion
The key findings of this study are that 6-month old infants who later develop ASD show marked reductions in attention towards both static and dynamic faces and, when they do attend to faces, they look less at the socially-informative inner features. However, while the former deficit does not appear to be condition-specific, diminished attention to the key facial features was found only in response to a face that was speaking. Despite these deficits, similarly to typically developing and developmentally delayed infants, infants later diagnosed with ASD show an elementary ability to modulate attention between the eyes and mouth that is contingent on the context in which faces are presented. These results have several implications.
First, observations of decreased overall levels of attention to complex social stimuli, consistent with our previous work in infants(56) and toddlers with ASD(14), suggest that already at 6 months, infants later diagnosed with ASD may have limited experience monitoring the faces of those around them. At present, it is not clear what the underlying neurophysiological and behavioral bases of these deficits are and whether they are restricted to social stimuli or extend to nonsocial stimuli as well. These questions will have to be answered though careful experimental decomposition of the complex stimuli used in this study and by extending the study to even younger infants at risk. However, the compounding influences of attention-driven experience and early brain plasticity are thought to lead to increasing specialization, proficiency, and preferences for social information throughout development(9; 79; 80). Our results suggest that at 6 months such processes have already been disrupted in infants who will develop ASD, potentially impairing further maturation of brain areas involved in processing of faces, affect, and speech.
Second, infants who later develop ASD attended less to inner areas of the face and focused more on areas of the speaker that provided less information regarding verbal cues. An eye-tracking study of 6-month old typically developing infants showed a speaking face (as compared to a still face) resulted in less looking at the eyes and more at the mouth but, no overall changes in combined looking at both features (81). Results of our study are consistent with this finding for infants who did not develop ASD, but not for those who did, suggesting that there may be a specific quality of speech which perturbs the scanning patterns of infants who later develop ASD.
Third, while allocation between eyes and mouth differed significantly by condition, this effect was similar in all groups, including infants later diagnosed with ASD. This finding is consistent with other studies(14; 52; 56; 81) and suggests that, like other infants, infants later diagnosed with ASD can adjust their scanning strategies based on the context in which the face is presented (e.g. static, smiling, or speaking). However, in infants who develop ASD, this preserved modulation is embedded within more general attentional deficits, especially in response to speaking faces.
In high-risk infant sibling research, most studies examining behavioral precursors to later socio-cognitive abilities in the context of live interactions in infant siblings around 6 months of age have not found differences between those who do and those who do not develop ASD(e.g. 35; 37; 39; 40; 49). It is possible that the increased saliency of live interactions provide additional attentional supports for infants who later develop ASD(82; 83), suggesting that, paradoxically, less salient experimental paradigms may help identify those features that constitute early markers of ASD. In addition, between-group differences in the allocation of attention towards socially-relevant events, which are often fleeting, may be particularly subtle. In this case, eye-tracking studies and studies of more fundamental attentional mechanisms may reveal behavioral differences where assessments of live interactions do not. Our current work supports these notions, as does our previous study(56), which identified generally reduced attention and diminished looking towards faces in the context of complex, dynamic social scenes involving a speaking actress, and the recent work by Elison and colleagues(55), which showed increased response latencies during an overlap task involving faces and objects(though see 84).
However, other eye-tracking studies have not found visual attention atypicalities in infants who develop ASD. Young and colleagues(47) used a live-video of mothers in a still-face procedure. It is possible that the added familiarity of the mother and/or tendency of mothers to adapt to their infants’ behaviors(82) may have contributed to lack of between-group differences. The work of Elsabbagh and colleagues(50–52) also showed no between-group differences through eye-tracking in infants between 6 and 10 months of age who developed ASD. In addition to focusing on a slightly different age range of participants, these studies used stimuli consisting of actresses repeatedly shifting gaze to the left and right(50), faces arranged in an array with objects(51), and dynamic videos of an actress who was not speaking(52). In terms of the distribution of attention between the eyes and mouth, our study is consistent with findings of no between-group effects and the preservation of context sensitivity in infants who develop ASD(52). However, our study deviates from the other work through our incorporation of a speech condition which separates infants who develop ASD from infants with other outcomes when considering attention towards key features of the face.
While it is possible that infants who develop ASD specifically “avoid” speaking faces, a lack of between-group effects in our previous study (where faces shifted between speaking and non-speaking) (56) may suggest a more nuanced phenomenon. It is important to note that our study also included a musical score as a backdrop to all presented face stimuli in order to increase infants’ attention, especially in the two conditions devoid of speech. In the speech condition, the presence of music may have increased the processing load associated with decoding audiovisual information, forcing the infants, as in real life, to select the most socially- or task-relevant stimuli for processing. Findings of speech-specific attentional difficulties in our study are consistent with reports of audio-visual integration difficulties in ASD(85–88) and HR infant siblings(89) as well as the intersensory processing impairment theory of autism proposed by Bahrick(8). Although the mechanisms of this phenomenon are not known at present, if expressed when typically developing infants are relying heavily on redundant audio-visual cues to facilitate development of speech perception(90; 91), difficulties in this domain may detrimentally impact social communication and language development.
Our study replicates and extends our earlier findings related to spontaneous monitoring of complex social scenes in 6-month-old later diagnosed with ASD(56) and toddlers with autism(14). The attention of infants later diagnosed with ASD appears generally depressed regardless of context and deviates from key facial features in the context of a speaking face. Based on extant evidence, it is not yet clear if differences in attention observed at 6 months are necessarily constrained to social stimuli or reflect more general information processing difficulties. However, our study suggests that already at 6 months infants who go on to develop ASD are experiencing a different social environment through their selective deployment of attention. Further work is necessary to clarify the nature of these difficulties as well as their impact on the development and outcomes of infants who will later develop ASD.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Child Health and Development, PO1 HD003008, Project 1 (PI: K.C.), National Institutes of Mental Health R01 MH087554 (PI:K.C.), NIMH grants #1R03MH086732 (PI: S.M.), R03 MH092618-01A1 (PI: F.S.), Expedition in Computing (award #1139078), and by the Associates of the Child Study Center. This publication was made also possible by CTSA Grant Number UL1 RR024139 from National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. We thank C. Saulnier, K. Tsatsanis, S. Kim, J. Koller, A. Steiner, T. Vernon, A. Snow, T. Goldsmith, K. Bearss, A. Carney, K. Bailey, E. Simmons, S. Austin, and R. Paul for their contribution to the sample characterization as well as J. Bradshaw, B. Butler, G. Chen, M. Coffman, J. Latz Davis, A. Dowd, R. Doggett, J. Garzarek, E. Gisin, M. Meltvedt, K. O’Loughlin, P. Ogston-Nobile, E. Prince, and J. Reed for assistance in data collection. We thank J. A. Kelley and A. P. Lin for their edits on this manuscript.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simion F, Leo I, Turati C, Valenza E, Dalla Barba B. Progress in Brain Research. Vol. 164. Elsevier; 2007. How face specialization emerges in the first months of life. In: C. von Hofsten and K. Rosander, editor; pp. 169–185. [DOI] [PubMed] [Google Scholar]
- 2.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. PNAS. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vouloumanos A, Hauser MD, Werker JF, Martin A. The tuning of human neonates’ preference for speech. Child Development. 2010;81:517–527. doi: 10.1111/j.1467-8624.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 4.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor K. Auditory processing in autism spectrum disorder: A review. Neuroscience & Biobehavioral Reviews. 2012;36:836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajendran G, Mitchell P. Cognitive theories of autism. Developmental Review. 2007;27:224–260. [Google Scholar]
- 8.Bahrick LE. Intermodal Perception and Selective Attention to Intersensory Redundancy: Implications for Typical Social Development and Autism. In: Bremner JG, Wachs TD, editors. The Wiley-Blackwell Handbook of Infant Development. Wiley-Blackwell; 2010. pp. 120–166. [Google Scholar]
- 9.Karmiloff-Smith A. Nativism versus neuroconstructivism: Rethinking the study of developmental disorders. Developmental Psychology. 2009;45:56–63. doi: 10.1037/a0014506. [DOI] [PubMed] [Google Scholar]
- 10.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual Fixation Patterns During Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals With Autism. Archives of General Psychiatry. 2002;59:809. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 11.Riby DM, Hancock PJ. Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. Journal of autism and developmental disorders. 2009;39:421–431. doi: 10.1007/s10803-008-0641-z. [DOI] [PubMed] [Google Scholar]
- 12.Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for Geometric Patterns Early in Life As a Risk Factor for Autism. Archives of general psychiatry. 2011;68:101. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones W, Carr K, Klin A. Absence of Preferential Looking to the Eyes of Approaching Adults Predicts Level of Social Disability in 2-Year-Old Toddlers With Autism Spectrum Disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 14.Chawarska K, Macari S, Shic F. Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry. 2012;53:903–913. doi: 10.1111/j.1469-7610.2012.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology. 2002;40:213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- 16.Yirmiya N, Charman T. The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics. Journal of Child Psychology and Psychiatry. 2010;51:432–458. doi: 10.1111/j.1469-7610.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- 17.Dawson G. Early Behavioral Intervention, Brain Plasticity, and the Prevention of Autism Spectrum Disorder. Development and Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 18.Boyd BA, Odom SL, Humphreys BP, Sam AM. Infants and Toddlers With Autism Spectrum Disorder: Early Identification and Early Intervention. Journal of Early Intervention. 2010;32:75–98. [Google Scholar]
- 19.Steiner AM, Gengoux GW, Klin A, Chawarska K. Pivotal Response Treatment for Infants At-Risk for Autism Spectrum Disorders: A Pilot Study. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: Studies of infants at risk. Neural Networks. 2010;23:1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behavioural Brain Research. 2013 doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 24.Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48:128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry. 2012:no–no. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50:1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baio J. Morbidity and Mortality Weekly Report. Surveillance Summaries. 3. Vol. 61. Centers for Disease Control and Prevention; 2012. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. [PubMed] [Google Scholar]
- 28.Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C, et al. Global Prevalence of Autism and Other Pervasive Developmental Disorders. Autism Research. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen IL, Gardner JM, Karmel BZ, Phan HTT, Kittler P, Gomez TR, et al. Neonatal Brainstem Function and 4-Month Arousal-Modulated Attention Are Jointly Associated With Autism. Autism Research. 2012:n/a–n/a. doi: 10.1002/aur.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmel BZ, Gardner JM, Meade LS, Cohen IL, London E, Flory MJ, et al. Early Medical and Behavioral Characteristics of NICU Infants Later Classified With ASD. Pediatrics. 2010;126:457–467. doi: 10.1542/peds.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, Gallagher N. Detecting, Studying, and Treating Autism Early: The One-Year Well-Baby Check-Up Approach. The Journal of Pediatrics. 2011;159:458–465. e6. doi: 10.1016/j.jpeds.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macari SL, Campbell D, Gengoux GW, Saulnier CA, Klin AJ, Chawarska K. Predicting Developmental Status from 12 to 24 Months in Infants at Risk for Autism Spectrum Disorder: A Preliminary Report. J Autism Dev Disord. 2012;42:2636–2647. doi: 10.1007/s10803-012-1521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landa RJ, Holman KC, Garrett-Mayer E. Social and Communication Development in Toddlers With Early and Later Diagnosis of Autism Spectrum Disorders. Arch Gen Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 35.Bedford R, Elsabbagh M, Gliga T, Pickles A, Senju A, Charman T, Johnson MH. Precursors to Social and Communication Difficulties in Infants At-Risk for Autism: Gaze Following and Attentional Engagement. J Autism Dev Disord. 2012;42:2208–2218. doi: 10.1007/s10803-012-1450-y. [DOI] [PubMed] [Google Scholar]
- 36.Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early Social, Imitation, Play, and Language Abilities of Young Non-Autistic Siblings of Children with Autism. J Autism Dev Disord. 2007;37:145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Nadig ASOS. A prospective study of response to name in infants at risk for autism. Arch Pediatr Adolesc Med. 2007;161:378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- 39.Ozonoff S, Iosif A-M, Baguio F, Cook IC, Hill MM, Hutman T, et al. A Prospective Study of the Emergence of Early Behavioral Signs of Autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:256–266. e2. [PMC free article] [PubMed] [Google Scholar]
- 40.Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, Sigman M. Behavioral Profiles of Affected and Unaffected Siblings of Children with Autism: Contribution of Measures of Mother–Infant Interaction and Nonverbal Communication. J Autism Dev Disord. 2011;41:287–301. doi: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbaro J, Dissanayake C. Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the Social Attention and Communication Study. Autism. 2012 doi: 10.1177/1362361312442597. [DOI] [PubMed] [Google Scholar]
- 42.Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12:457–471. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gredebäck G, Fikke L, Melinder A. The development of joint visual attention: a longitudinal study of gaze following during interactions with mothers and strangers. Developmental Science. 2010;13:839–848. doi: 10.1111/j.1467-7687.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- 44.Symons LA, Hains SMJ, Muir DW. Look at me: five-month-old infants’ sensitivity to very small deviations in eye-gaze during social interactions. Infant Behavior and Development. 1998;21:531–536. [Google Scholar]
- 45.Tremblay H, Rovira K. Joint visual attention and social triangular engagement at 3 and 6 months. Infant Behavior and Development. 2007;30:366–379. doi: 10.1016/j.infbeh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Striano T, Stahl D. Sensitivity to triadic attention in early infancy. Developmental Science. 2005;8:333–343. doi: 10.1111/j.1467-7687.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- 47.Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryson SE, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A Prospective Case Series of High-risk Infants who Developed Autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 49.Landa RJ, Gross AL, Stuart EA, Faherty A. Developmental Trajectories in Children With and Without Autism Spectrum Disorders: The First 3 Years. Child Development. 2012:n/a–n/a. doi: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, et al. Infant Neural Sensitivity to Dynamic Eye Gaze Is Associated with Later Emerging Autism. Current Biology. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elsabbagh M, Gliga T, Pickles A, Hudry K, Charman T, Johnson MH. The development of face orienting mechanisms in infants at-risk for autism. Behavioural Brain Research. 2012 doi: 10.1016/j.bbr.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH. What you see is what you get: contextual modulation of face scanning in typical and atypical development. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flanagan JE, Landa R, Bhat A, Bauman M. Head Lag in Infants at Risk for Autism: A Preliminary Study. Am J Occup Ther. 2012;66:577–585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- 54.Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: vocal production in infant siblings of children with ASD. J Child Psychol Psychiatry. 2011;52:588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, et al. White Matter Microstructure and Atypical Visual Orienting in 7-Month-Olds at Risk for Autism. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chawarska K, Macari S, Shic F. Decreased Spontaneous Attention to Social Scenes in 6-Month-Old Infants Later Diagnosed with Autism Spectrum Disorders. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chawarska K, Campbell D, Chen L, Shic F, Klin A, Chang JT. Early Generalized Overgrowth in Boys with Autism. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Jama. 2003;290:337. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 60.Elder L, Dawson G, Toth K, Fein D, Munson J. Head Circumference as an Early Predictor of Autism Symptoms in Younger Siblings of Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2008;38:1104–1111. doi: 10.1007/s10803-007-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic Resonance Imaging and Head Circumference Study of Brain Size in Autism: Birth Through Age 2 Years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 62.Mraz KD, Green J, Dumont-Mathieu T, Makin S, Fein D. Correlates of Head Circumference Growth in Infants Later Diagnosed With Autism Spectrum Disorders. J Child Neurol. 2007;22:700–713. doi: 10.1177/0883073807304005. [DOI] [PubMed] [Google Scholar]
- 63.Chawarska K, Shic F. Looking But Not Seeing: Atypical Visual Scanning and Recognition of Faces in 2 and 4-Year-Old Children with Autism Spectrum Disorder. JADD. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual Scanning of Faces in Autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 65.Speer LL, Cook AE, McMahon WM, Clark E. Face processing in children with autism: Effects of stimulus contents and type. Autism. 2007;11:265–277. doi: 10.1177/1362361307076925. [DOI] [PubMed] [Google Scholar]
- 66.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule: ADOS: Manual. Western Psychological Services; 2002. [Google Scholar]
- 67.Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 68.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 69.Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales: Developmental Profile. Paul H Brookes Publishing; 2002. [Google Scholar]
- 70.Zimmerman IL, Steiner VG, Pond RE. (PLS-4) San Antonio, TX: Harcourt Assessment, Inc; 2002. [Google Scholar]
- 71.Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, et al. Beyond Autism: A Baby Siblings Research Consortium Study of High-Risk Children at Three Years of Age. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:300–308. e1. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces. Pictoral face set available from the Department of Neurosciences, Karolinska Hospital; Stockholm, Sweden: 1998. [Google Scholar]
- 73.Shic F. PhD thesis. Yale University; 2008. Computational Methods for Eye-Tracking Analysis: Applications to Autism. [Google Scholar]
- 74.Hsiao JH, Cottrell G. Two Fixations Suffice in Face Recognition. Psychological Science. 2008;19:998–1006. doi: 10.1111/j.1467-9280.2008.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merin N, Young G, Ozonoff S, Rogers SJ. Visual Fixation Patterns during Reciprocal Social Interaction Distinguish a Subgroup of 6-Month-Old Infants At-Risk for Autism from Comparison Infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- 76.Bird G, Press C, Richardson DC. The Role of Alexithymia in Reduced Eye-Fixation in Autism Spectrum Conditions. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1183-3. [DOI] [PubMed] [Google Scholar]
- 77.Rice K, Moriuchi JM, Jones W, Klin A. Parsing Heterogeneity in Autism Spectrum Disorders: Visual Scanning of Dynamic Social Scenes in School-Aged Children. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51:238–248. doi: 10.1016/j.jaac.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shic F, Chawarska K, Bradshaw J, Scassellati B. Autism, Eye-Tracking, Entropy. 2008 7th IEEE International Conference on Development and Learning; Presented at the 2008 7th IEEE International Conference on Development and Learning (ICDL 2008); Monterey, CA. 2008. pp. 73–78. [Google Scholar]
- 79.Klin A, Jones W, Schultz R, Volkmar F. The Enactive Mind, or from Actions to Cognition: Lessons from Autism. Philosophical Transactions: Biological Sciences. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson MH, Grossman T, Cohen Kadosh K. Mapping functional brain development: Building a social brain through interactive specialization. Developmental psychology. 2009;45:151–159. doi: 10.1037/a0014548. [DOI] [PubMed] [Google Scholar]
- 81.Tenenbaum EJ, Shah RJ, Sobel DM, Malle BF, Morgan JL. Increased Focus on the Mouth Among Infants in the First Year of Life: A Longitudinal Eye-Tracking Study. Infancy. 2012:n/a–n/a. doi: 10.1111/j.1532-7078.2012.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Parent–infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities. 2012;33:924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Roseberry S, Hirsh-Pasek K, Parish-Morris J, Golinkoff RM. Live Action: Can Young Children Learn Verbs From Video? Child Development. 2009;80:1360–1375. doi: 10.1111/j.1467-8624.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elsabbagh M, Fernandes J, Jane Webb S, Dawson G, Charman T, Johnson MH. Disengagement of Visual Attention in Infancy Is Associated with Emerging Autism in Toddlerhood. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith EG, Bennetto L. Audiovisual speech integration and lipreading in autism. Journal of Child Psychology and Psychiatry. 2007;48:813–821. doi: 10.1111/j.1469-7610.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 86.Gelder B, de Vroomen J, van der Heide L. Face recognition and lip-reading in autism. European Journal of Cognitive Psychology. 1991;3:69–86. [Google Scholar]
- 87.Mongillo EA, Irwin JR, Whalen DH, Klaiman C, Carter AS, Schultz RT. Audiovisual Processing in Children with and without Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2008;38:1349–1358. doi: 10.1007/s10803-007-0521-y. [DOI] [PubMed] [Google Scholar]
- 88.Irwin JR, Tornatore LA, Brancazio L, Whalen DH. Can Children With Autism Spectrum Disorders “Hear” a Speaking Face? Child Development. 2011;82:1397–1403. doi: 10.1111/j.1467-8624.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guiraud JA, Tomalski P, Kushnerenko E, Ribeiro H, Davies K, Charman T, et al. Atypical Audiovisual Speech Integration in Infants at Risk for Autism. PLoS ONE. 2012;7:e36428. doi: 10.1371/journal.pone.0036428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhl PK, Meltzoff AN. The Intermodal Representation of Speech in Infants. Infant Behavior and Development. 1984;7:361–381. [Google Scholar]
- 91.Lewkowicz DJ, Hansen-Tift AM. Infants deploy selective attention to the mouth of a talking face when learning speech. PNAS. 2012;109:1431–1436. doi: 10.1073/pnas.1114783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.