Abstract
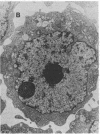
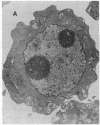
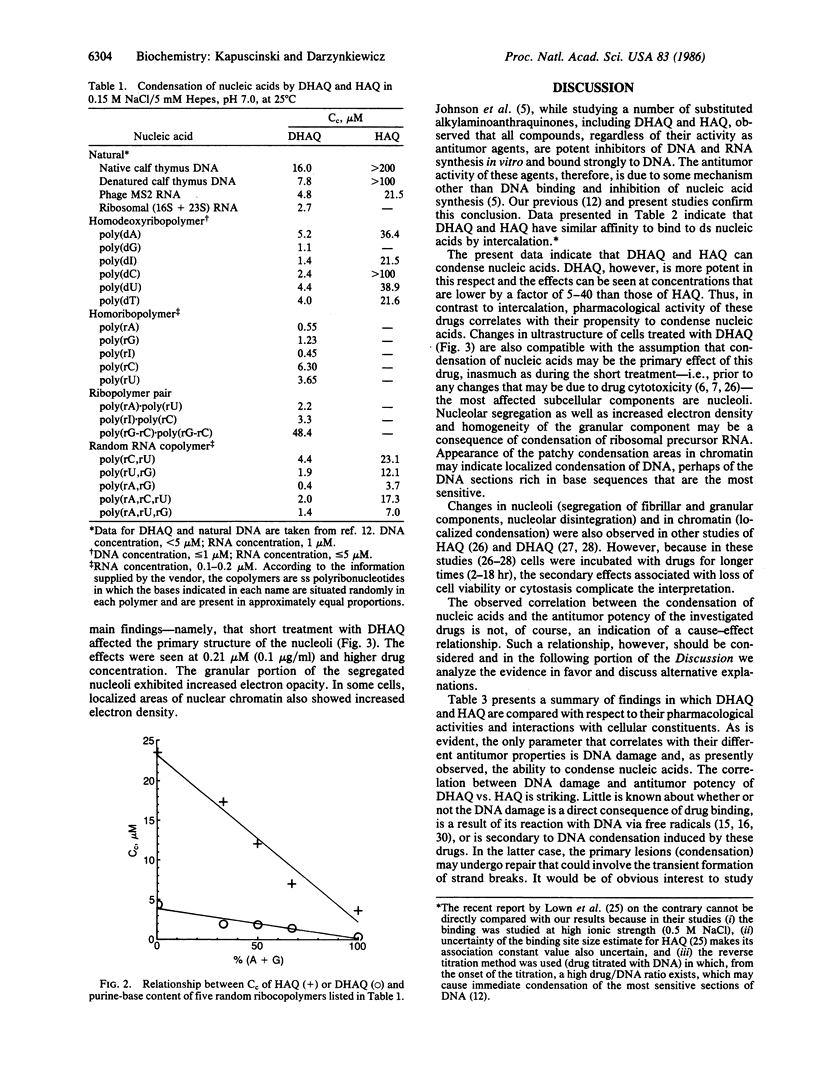
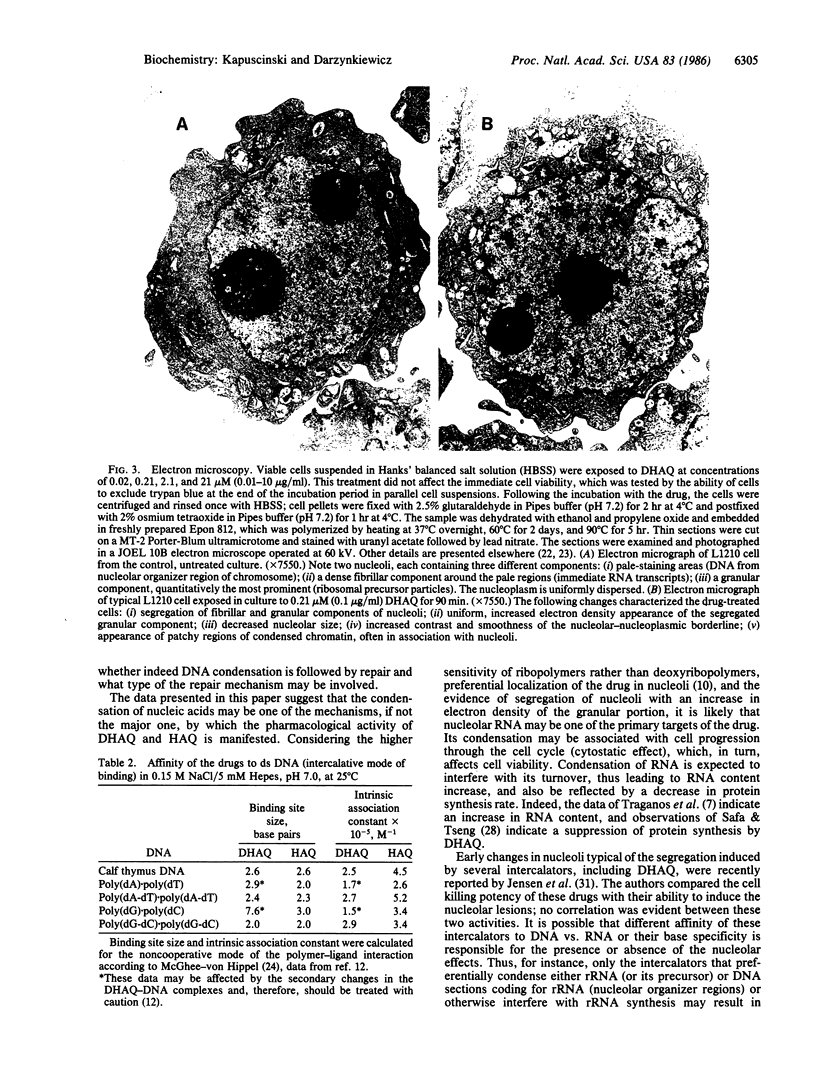
Ametantrone (HAQ) and mitoxantrone (DHAQ) are structurally similar antitumor drugs of the anthracenedione class. The cytostatic, cytotoxic, and antitumor activities of these drugs are different, with DHAQ being 10-100 times more potent, per molar basis. Both drugs are strong intercalators and intercalative modes of binding are suspected as relevant to their pharmacological activity. No significant differences, however, that could explain the differences in pharmacological activity are observed in their intercalative properties with respect to base specificity and binding affinity. A correlation, however, is evident between their potency and ability to condense nucleic acids inasmuch as DHAQ condenses nucleic acids at concentrations that are lower by a factor of 5-40 than those of HAQ and these effects can be observed at their pharmacological concentrations. The condensation is base- and sugar-specific and the long purine sequences of single-stranded RNA are the most sensitive. Electron microscopy of L1210 cells exposed a short time (90 min) to 0.21-21 microM DHAQ reveals segregation of nucleoli; the segregated granular portion shows increased electron opacity. In some preparations patchy areas of nuclear chromatin characterized by increased electron opacity can be seen. The results are compatible with the possibility that pharmacological effects of these antitumor drugs could involve condensation of nucleic acids, primarily of RNA in nucleoli.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au W. W., Butler M. A., Matney T. S., Loo T. L. Comparative structure-genotoxicity study of three aminoanthraquinone drugs and doxorubicin. Cancer Res. 1981 Feb;41(2):376–379. [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D., Kapuscinski J., Melamed M. R. Denaturation of RNA and DNA in situ induced by acridine orange. Exp Cell Res. 1983 Oct;148(1):31–46. doi: 10.1016/0014-4827(83)90185-4. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Kapuscinski J., Melamed M. R. Denaturation and condensation of DNA in situ induced by acridine orange in relation to chromatin changes during growth and differentiation of Friend erythroleukemia cells. Cytometry. 1985 May;6(3):195–207. doi: 10.1002/cyto.990060305. [DOI] [PubMed] [Google Scholar]
- Durr F. E., Wallace R. E., Citarella R. V. Molecular and biochemical pharmacology of mitoxantrone. Cancer Treat Rev. 1983 Dec;10 (Suppl B):3–11. doi: 10.1016/0305-7372(83)90016-6. [DOI] [PubMed] [Google Scholar]
- Evenson D. P., Darzynkiewicz Z., Staiano-Coico L., Traganos F., Melamed M. R. Effects of 9,10-anthracenedione, 1,4-bis[(2-[(2-hydroxyethyl)amino]-ethyl)amino]-diacetate on cell survival and cell cycle progression in cultured mammalian cells. Cancer Res. 1979 Jul;39(7 Pt 1):2574–2581. [PubMed] [Google Scholar]
- Evenson D. P., Traganos F., Darzynkiewicz Z., Staiano-Coico L., Melamed M. R. Effects of 9,10-anthracenedione, 1,4-bis[[2-[(2-hydroxyethyl)amino]-ethyl]amino]-, diacetate on cell morphology and nucleic acids of friend leukemia cells. J Natl Cancer Inst. 1980 Apr;64(4):857–866. [PubMed] [Google Scholar]
- Jensen C. G., Wilson W. R., Bleumink A. R. Effects of amsacrine and other DNA-intercalating drugs on nuclear and nucleolar structure in cultured V79 Chinese hamster cells and PtK2 rat kangaroo cells. Cancer Res. 1985 Feb;45(2):717–725. [PubMed] [Google Scholar]
- Johnson R. K., Zee-Cheng R. K., Lee W. W., Acton E. M., Henry D. W., Cheng C. C. Experimental antitumor activity of aminoanthraquinones. Cancer Treat Rep. 1979 Mar;63(3):425–439. [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Condensation of nucleic acids by intercalating aromatic cations. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7368–7372. doi: 10.1073/pnas.81.23.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Denaturation of nucleic acids induced by intercalating agents. Biochemical and biophysical properties of acridine orange-DNA complexes. J Biomol Struct Dyn. 1984 Jun;1(6):1485–1499. doi: 10.1080/07391102.1984.10507532. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Increased accessibility of bases in DNA upon binding of acridine orange. Nucleic Acids Res. 1983 Nov 11;11(21):7555–7568. doi: 10.1093/nar/11.21.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem Pharmacol. 1985 Dec 15;34(24):4203–4213. doi: 10.1016/0006-2952(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Melamed M. R. Interactions of acridine orange with nucleic acids. Properties of complexes of acridine orange with single stranded ribonucleic acid. Biochem Pharmacol. 1983 Dec 15;32(24):3679–3694. doi: 10.1016/0006-2952(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Melamed M. R. Luminescence of the solid complexes of acridine orange with RNA. Cytometry. 1982 Jan;2(4):201–211. doi: 10.1002/cyto.990020402. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z., Traganos F., Melamed M. R. Interactions of a new antitumor agent, 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]-ethyl]amino]-9,10-anthracenedione, with nucleic acids. Biochem Pharmacol. 1981 Feb 1;30(3):231–240. doi: 10.1016/0006-2952(81)90083-6. [DOI] [PubMed] [Google Scholar]
- Locher S. E., Meyn R. E. Relationship between cytotoxicity and DNA damage in mammalian cells treated with anthracenedione derivatives. Chem Biol Interact. 1983 Sep 15;46(3):369–379. doi: 10.1016/0009-2797(83)90020-0. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Hanstock C. C., Bradley R. D., Scraba D. G. Interactions of the antitumor agents mitoxantrone and bisantrene with deoxyribonucleic acids studied by electron microscopy. Mol Pharmacol. 1984 Jan;25(1):178–184. [PubMed] [Google Scholar]
- Lown J. W., Morgan A. R., Yen S. F., Wang Y. H., Wilson W. D. Characteristics of the binding of the anticancer agents mitoxantrone and ametantrone and related structures to deoxyribonucleic acids. Biochemistry. 1985 Jul 16;24(15):4028–4035. doi: 10.1021/bi00336a034. [DOI] [PubMed] [Google Scholar]
- Marx K. A., Ruben G. C. Studies of DNA organization in hydrated spermidine-condensed DNA toruses and spermidine-DNA fibres. J Biomol Struct Dyn. 1984 Mar;1(5):1109–1132. doi: 10.1080/07391102.1984.10507507. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Murdock K. C., Child R. G., Fabio P. F., Angier R. B., Wallace R. E., Durr F. E., Citarella R. V. Antitumor agents. 1. 1,4-Bis[(aminoalkyl)amino]-9,10-anthracenediones. J Med Chem. 1979 Sep;22(9):1024–1030. doi: 10.1021/jm00195a002. [DOI] [PubMed] [Google Scholar]
- Nishio A., Uyeki E. M. Cellular uptake and inhibition of DNA synthesis by dihydroxyanthraquinone and two analogues. Cancer Res. 1983 May;43(5):1951–1956. [PubMed] [Google Scholar]
- Novak R. F., Kharasch E. D. Mitoxantrone: propensity for free radical formation and lipid peroxidation--implications for cardiotoxicity. Invest New Drugs. 1985;3(2):95–99. doi: 10.1007/BF00174155. [DOI] [PubMed] [Google Scholar]
- Roboz J., Richardson C. L., Holland J. F. Comparison of the interaction of antineoplastic aminoanthraquinone analogs with DNA using competitive fluorescence polarization. Life Sci. 1982 Jul 5;31(1):25–30. doi: 10.1016/0024-3205(82)90396-4. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Chegini N., Tseng M. T. Influence of mitoxantrone on nucleic acid synthesis on the T-47D breast tumor cell line. J Cell Biochem. 1983;22(2):111–120. doi: 10.1002/jcb.240220205. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Tseng M. T. Inhibition of protein synthesis and cell proliferation in cultured human breast cancer cells treated with mitoxantrone. Cancer Lett. 1984 Oct;24(3):317–326. doi: 10.1016/0304-3835(84)90029-6. [DOI] [PubMed] [Google Scholar]
- Traganos F. Dihydroxyanthraquinone and related bis(substituted) aminoanthraquinones: a novel class of antitumor agents. Pharmacol Ther. 1983;22(2):199–214. doi: 10.1016/0163-7258(83)90004-9. [DOI] [PubMed] [Google Scholar]
- Traganos F., Evenson D. P., Staiano-Coico L., Darzynkiewicz Z., Melamed M. R. Action of dihydroxyanthraquinone on cell cycle progression and survival of a variety of cultured mammalian cells. Cancer Res. 1980 Mar;40(3):671–681. [PubMed] [Google Scholar]
- White R. J., Durr F. E. Development of mitoxantrone. Invest New Drugs. 1985;3(2):85–93. doi: 10.1007/BF00174154. [DOI] [PubMed] [Google Scholar]
- Zee-Cheng R. K., Cheng C. C. Antineoplastic agents. Structure-activity relationship study of bis(substituted aminoalkylamino)anthraquinones. J Med Chem. 1978 Mar;21(3):291–294. doi: 10.1021/jm00201a012. [DOI] [PubMed] [Google Scholar]