Abstract
New methods are needed for the nondestructive measurement of tooth demineralization and remineralization and to monitor the progression of incipient caries lesions (tooth decay) for effective nonsurgical intervention and to evaluate the performance of anti-caries treatments such as chemical treatments or laser irradiation. Studies have shown that optical coherence tomography (OCT) has great potential to fulfill this role, since it can be used to measure the depth and severity of early lesions with an axial resolution exceeding 10-μm. It is easy to apply in vivo and it can be used to image the convoluted topography of tooth occlusal surfaces. In this paper we present early results from two clinical studies underway to measure the effect of fluoride intervention on early lesions. CP-OCT was used to monitor early lesions on enamel and root surfaces before and after intervention with fluoride varnish. The lesion depth and internal structure were resolved for all the lesions examined and some lesions had well defined surface zones of lower reflectivity that may be indicative of arrested lesions. Changes were also noted in the structure of some of the lesions after fluoride intervention.
Keywords: cross polarization optical coherence tomography, tooth demineralization, dental caries, root caries
1. INTRODUCTION
New tools are needed to non-destructively determine carious lesion depth and severity, assess lesion activity, differentiate between lesions caused by caries and those due to developmental defects and to measure the efficacy of chemical intervention. The National Institute of Dental and Craniofacial research has requested the validation of new technologies for the measurement of tooth surface demineralization or remineralization to serve as a likely surrogate end point in dental clinical trials 1. Several studies have demonstrated that polarization sensitive optical coherence tomography (PS-OCT) can be used to nondestructively measure the severity of subsurface demineralization in enamel and dentin and is therefore well suited for this role 2-13.
Since optical diagnostic tools exploit changes in the light scattering of the lesion they have great potential for the diagnosis of the current “state of the lesion”, i.e., whether or not the caries lesion is active and expanding or whether the lesion has been arrested and is undergoing remineralization. Therefore, new technologies must be able to determine whether caries lesions have been partially remineralized and have become arrested. The most important advantage of OCT over other competing technologies is that it has the ability to acquire depth resolved images of the lesion structure with high resolution in vivo. Ekstrand describes the patho-anatomical changes in enamel during caries initiation, progression and arrestment 14,15. Definitive features, such as a weakly scattering surface zone, are formed during the process of remineralization that are indicative of an arrested lesion. Kidd showed that there were structural differences in the histopathology of enamel lesions from young 16 and old permanent teeth. It is most likely that the lesions in older teeth better represent lower levels of lesion activity or arrested lesions. The lesions in the older teeth had larger and more definitive transparent surface zones than the lesions in younger teeth. Therefore the thickness of this transparent surface zone may serve as an indicator of lesion activity. Since PS-OCT can resolve changes in lesion structure, i.e., show a surface zone of reduced light scattering, we can postulate that it can be used as a quantitative diagnostic of the activity of the lesion or an indicator of an arrested lesion or a developmental defect that needs no further intervention. Developmental defects typical of mild fluorosis that are not undergoing active demineralization also show a very thick outer layer, that has a higher mineral content than the hypomineralized body of the defect17.
We developed an approach to quantifying the severity of demineralization by integrating the reflectivity of the orthogonal axis (⊥) or cross polarization image 6. This approach also has the added advantage of reducing the intensity of the strong reflection from the tooth surface that can prevent resolution of the lesion area near the tooth surface, which is important for measurement of the lesion surface zone that can potentially provide information about the lesion activity. A conventional OCT system cannot differentiate the strong reflectance from the tooth surface from increased reflectivity from the lesion itself 18, 19. Use of cross polarization images facilitates direct integration of the lesion reflectivity to quantify the lesion severity, regardless of the tooth topography and the difficult task of having to deconvolve the strong surface reflection from the lesion surface can be circumvented. Since the most important information about the lesion is near the surface, a cross polarization OCT system is invaluable for imaging the internal structure of early lesions. We have also demonstrated that automated algorithms can be applied successfully to calculate the depth of demineralization and the overall or integrated reflectivity from the zone of demineralization 20. This approach has significant advantages because OCT can be used to rapidly acquire 2D and 3D tomographic images of areas of early demineralization on tooth surfaces. In order to rapidly process the images and effectively quantify the lesion severity algorithms are needed to automatically extract lesion depth and severity information.
Both enamel lesions on tooth buccal and occlusal enamel surfaces and root caries lesion are being monitored in our study. We are imaging incipient enamel lesions on 20 test subjects at high risk for dental caries, i.e., each has multiple lesions on the facial and occlusal surfaces. It is anticipated that these test subjects will have a high percentage of active lesions that will respond to fluoride intervention. Test subjects are recruited from the UCSF predoctoral clinics that see ~33,000 patient visits per year. Up to four lesions on each test subject are being monitored for a period of 30 weeks. The OCT scans and the fluoride varnish will be repeated once every six weeks for thirty weeks, thus producing data at 0, 6, 12, 18, 24, 30 weeks. Our approach is modeled after the longitudinal remineralization study with QLF carried out by Tranaeus et al.21 in which they applied fluoride varnish at six-week intervals over a six month period in 13 test subjects (32 lesions) and observed a significant reduction in lesion area and concomitant increase in fluorescence radiance. We are also looking at another twenty test subjects with early root caries lesions.
The purpose of this paper is to present some of the early results from this study and discuss the challenges and anticipated outcome.
2. MATERIALS AND METHODS
2.1 Cross Polarization (CP) - OCT System
The cross-polarization system is Model IVS-3000-CP from Santec (Komaki,Aichi, Japan) and utilizes a swept laser source, Santec Model HSL-200-30 operating with a 30 kHz sweep rate. The Mac-Zehnder interferometer is integrated into the handpiece which also contains the microelectromechancial (MEMS) scanning mirror and the imaging optics. It is capable of acquiring complete tomographic images of a volume of 6 × 6 × 7 mm in less than a second. The imaging system along with close-up images of the scanning hand-piece are shown in Fig. 1. The handpiece is shown in Fig. 1, the body is 7 × 18 cm with an imaging tip that is 4 cm long and 1.5 cm across. This system operates at a wavelength of 1321-nm with a bandwidth of 111-nm with a measured resolution in air of 11.4 μm (3 dB). The lateral resolution is 80-μm (1/e2) with a transverse imaging window of 6 mm × 6 mm and a measured imaging depth of 7-mm in air. The extinction ratio was measured to be 32 dB.
Fig. 1.
Photographs of the 3D CP-OCT scanner showing the scanning handpiece along with the 6×6 mm scanning window at the end of the scanner.
2.1 Clinical Protocol
This is a two part study involving the use of CP-OCT to monitor existing early lesions over a period of 30 weeks during intervention with fluoride varnish. The first study involves 20 test subjects with early enamel lesions and the second study includes 20 test subjects with early root caries lesions. All the test subjects are solicited from UCSF clinics. The demographics of the patient population for the UCSF school of dentistry are listed below. By gender 50.7% are male and 49.3% female. The age distribution is 1.5% age 1-17, 9.1% age 18-24, 21.1% age 25-34, 24.3% age 35-44, 17.3% age 45-54, 10.5% 55-64, and 16.2% 65+. The Ethnic distribution is 49% White, 12% Black, 10% Asian and Pacific Islander, 11 % Hispanic (any race), and 19% other. Permission to recruit and carry out the testing has been obtained from the Committee on Human Research (CHR) at UCSF and subjects have been fully informed of the procedures. Test subjects that are identified as high caries risk with at least two new lesions will be invited to participate in the study. The test subjects will receive topical fluoride varnish treatments at six week intervals. Two scans of a selected 6 × 6 mm2 area encompassing the lesion are taken at each time point. Teeth are scanned just before fluoride varnish application and at 6-week intervals up to a period of 30-weeks. Digital photographs are taken at the beginning and end of the study. The inclusion and exclusion criteria are listed below.
(a) Inclusion criteria
These criteria will not be based on race or gender. Participants will be patients at the pre-doctoral dental clinic at UCSF and must be:
aged 12 to 60 years
able to give informed consent in English
willing to comply with all study procedures and protocols,
residing in San Francisco or nearby locales with community fluoridation (to eliminate water fluoridation as a potential confounding variable)
must have at least two suspected, non-cavitated enamel lesions or two non-cavitated root caries lesions
willing to waive a HIPAA acknowledgement that data will only be used for research
(b) Exclusion criteria
subjects suffering from systemic diseases
taking medications that may effect the oral flora or salivary flow (e.g., antibiotic use in the past three months, drugs associated with dry mouth/xerostomia)
other conditions that may decrease the likelihood of adherence to study protocol
in-office fluoride treatment within past 3 months
We developed a small sleeve made out of Delrin that fits over the window and prevents the brackets from coming in direct contact with the window of the scanner. Cellophane film is used as an infection control barrier on the handpiece which we found only minimally interferes with the images. The sleeve was placed in direct contact with the tooth surface and it served to aid the positioning process and prevent damage to the window covering the OCT scanner. It was sterilized by autoclave prior to each procedure.
3. RESULTS AND DISCUSSION
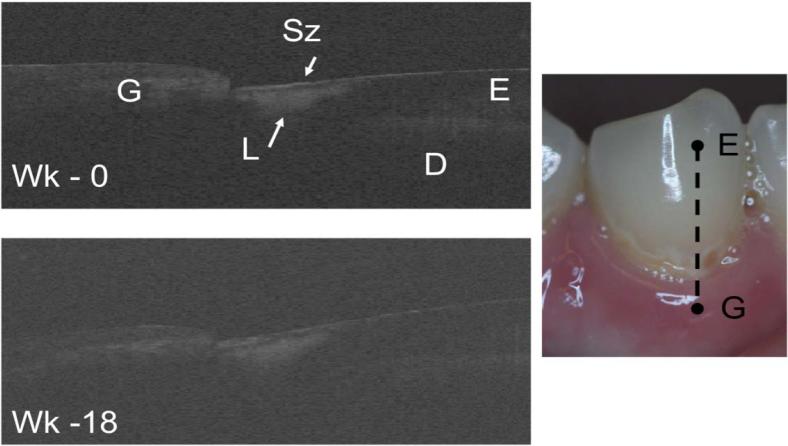
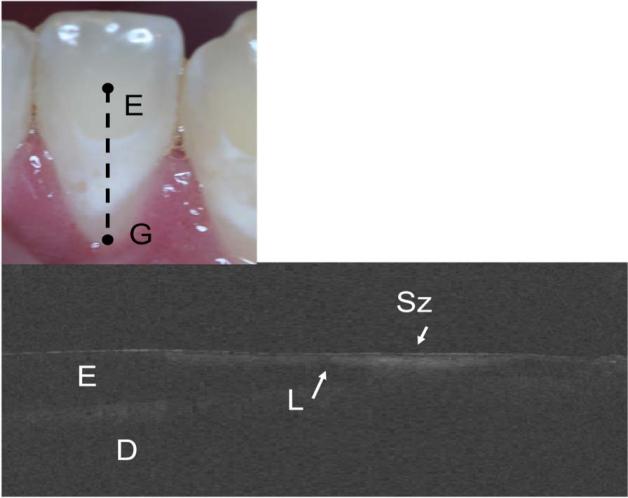
The study was initiated 8-months ago and we have recruited nearly one half of the required test subjects. Recruiting subjects with root caries lesions is more challenging than those with early enamel lesions. One of the first goals of this study is to demonstrate that OCT can provide useful images of the lesion internal structure. In vitro PS-OCT studies have demonstrated that for certain demineralization/remineralization models, the overall reflectivity of the lesion decreases after exposure and a distinct transparent surface zone is formed on the lesions surface after exposure to a remineralization regimen which can be measured with CP-OCT 8, 11. It was interesting to observe that a transparent surface zone was clearly observable on many of the lesions, both enamel and dentin (root) lesions, encountered in the study before intervention with fluoride varnish. Figure 2 shows an enamel lesion imaged at week 0 and 18 weeks with a well defined surface zone. Another cervical white spot lesion is shown in Fig. 3 that extends half the length of the facial surface. The severity of this shallow lesion looks fairly uniform in the photograph, however the CP-OCT image shows that the severity varies quite significantly over the length of the lesion and has a distinct surface zone over the whole length.
Fig. 2.
Two in vivo CP-OCT b-scans of a cervical enamel lesion taken at week 0 after 18 weeks. The Lesion (L) is clearly visible and it has a well defined surface zone (Sz) that is visible. The enamel (E), dentin (D) and the gingival (G) are demarcated in the image and the position of the scans are indicated on the photograph of the tooth.
Fig. 3.
In vivo CP-OCT b-scan of a cervical enamel lesion. The Lesion (L) is long and shallow and has a well defined surface zone (Sz) that is visible. The enamel (E), dentin (D) and the gingiva (G) are demarcated in the image and the position of the scans are indicated on the photograph of the tooth.
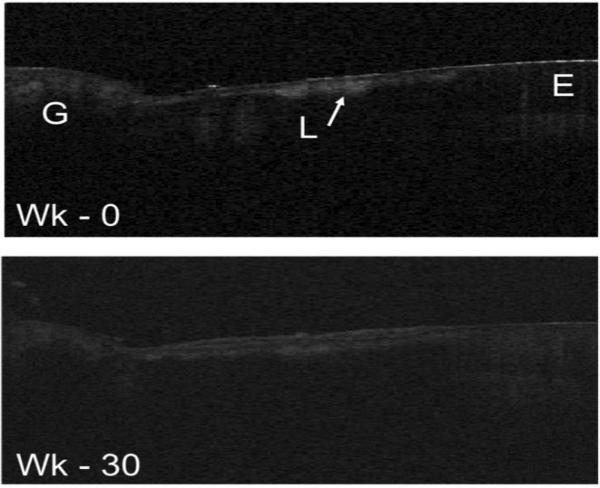
CP-OCT images from one test subject are shown in Fig. 4 before and after the completion of the study. In the initial scans (week 0) the lesions manifested similar structure to the lesions shown in Figs. 2 & 3 with a surface zone visible. After completion of the study all the lesions manifested an additional transparent layer over the existing lesion as shown in Fig. 4 (week 30). If the layer was plaque, calculus or precipitated mineral it would be highly scattering and there would not be a transparent layer. The transparent layer is most likely highly mineralized enamel caused by either the filling of existing pores by mineral or the deposition of an oriented (epitaxial) layer of mineral.
Fig. 4.
Two in vivo CP-OCT b-scans of a cervical enamel lesion taken at week 0 after 30 weeks. The Lesion (L) is clearly visible and it has a well defined surface zone that is visible. After fluoride therapy a double layer is visible in the lesion area.
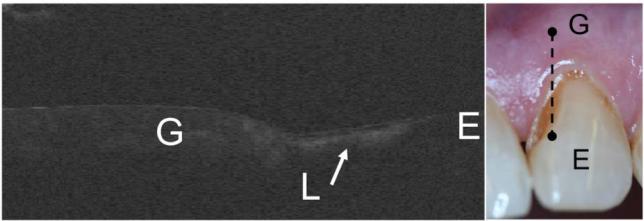
The structure of root caries lesions can also be clearly resolved in CP-OCT images. In addition, the remaining thickness of the cementum layer can be measured and the CP-OCT images show the surface topography of the root surface. Such images provide information about the exposed root surfaces and the degree of erosion that has occurred. Fig. 5 shows a CP-OCT image of an exposed root surface with a small lesion. A fairly large surface zone is present on the lesion. This scan was taken at week-0 and the thick surface zone may indicate the lesion is arrested.
Fig. 5.
A photograph and an in vivo CP-OCT b-scan of a root caries lesion. The root lesion (L) also has a well defined surface zone that is visible. The enamel (E), dentin (D) and the gingiva (G) are demarcated in the image and the position of the scan is indicated on the photograph of the tooth.
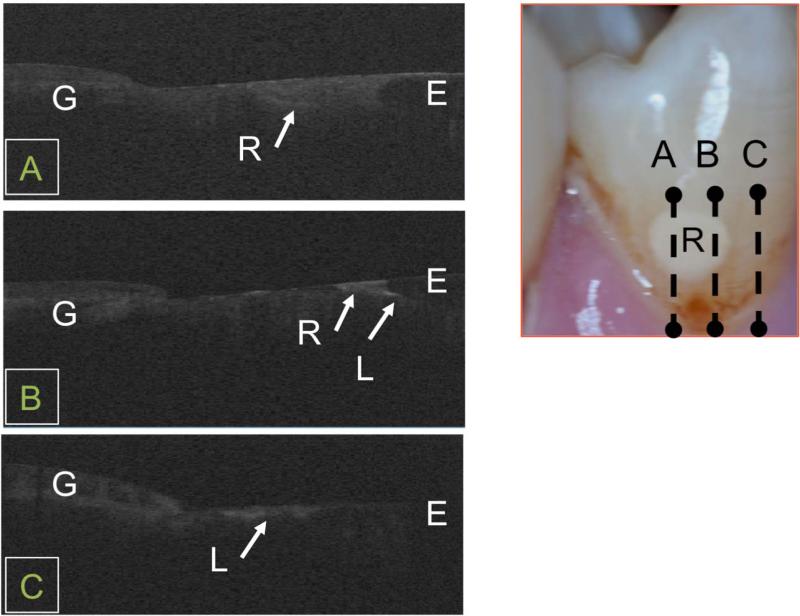
A few of the teeth examined had small restorations. The tooth shown in Fig. 6 had a small restoration on the facial surface along with a small lesion. The restoration is obvious in the photograph but the small enamel lesion is not. Three CP-OCT scans are shown one taken across the center of the restoration (A), one taken at the edge of the restoration (B) and the other scan (C) taken across the lesion. The most interesting image is the one taken near the edge of the restoration (B). This image shows a strong reflection along the DEJ next to the restoration at the position marked (L). Such a strong reflection at the DEJ suggests underlying decay/demineralization. This is most likely residual decay that was missed during the cavity preparation. It could also be secondary caries that developed after placement of the restoration.
Fig. 6.
In vivo CP-OCT b-scans taken at three positions on a tooth (A, B, C ) with a restoration and an enamel lesion. The full extent of the restoration (R) can be seen including decay peripheral to the restoration (L) along the DEJ. An enamel lesion (L) can also be seen on the same tooth at position C.
The in vivo CP-OCT images collected so far in this clinical study demonstrate the great potential of OCT for the clinical monitoring of caries lesions. The ability to monitor changes in lesion structure nondestructively in vivo offers many exciting possibilities. After collection of all the images the lesion depth and integrated reflectivity will be calculated at each time point. We postulate that the thickness of the transparent surface zone which was observed on many of the lesions imaged in this study will correlate with the degree of lesion activity. If this is the case, then such measurements are likely to provide a means of assessing lesion activity with a single measurement.
ACKNOWLEDGMENTS
This work was supported by NIH/NIDCR Grants R01-DE17869.
REFERENCES
- 1.NIH Diagnosis and Management of Dental Caries throughout Life. NIH Consensus Statement. 2001:1–24. [PubMed] [Google Scholar]
- 2.Baumgartner A, Dicht S, Hitzenberger CK, Sattmann H, Robi B, Moritz A, Sperr W, Fercher AF. Polarization-sensitive optical optical coherence tomography of dental structures. Caries Res. 2000;34:59–69. doi: 10.1159/000016571. [DOI] [PubMed] [Google Scholar]
- 3.Wang XJ, Milner TE, de Boer JF, Zhang Y, Pashley DH, Nelson JS. Characterization of dentin and enamel by use of optical coherence tomography. Applied Optics. 1999;38(10):2092–2096. doi: 10.1364/ao.38.002092. [DOI] [PubMed] [Google Scholar]
- 4.Everett MJ, Colston BW, Sathyam US, Silva LBD, Fried D, Featherstone JDB. Non-invasive diagnosis of early caries with polarization sensitive optical coherence tomography (PS-OCT) SPIE Proceeding. 1999;3593:177–183. [Google Scholar]
- 5.Feldchtein FI, Gelikonov GV, Gelikonov VM, Iksanov RR, Kuranov RV, Sergeev AM, Gladkova ND, Ourutina MN, Warren JA, Reitze DH. In vivo OCT imaging of hard and soft tissue of the oral cavity. Optics Express. 1998;3(3):239–251. doi: 10.1364/oe.3.000239. [DOI] [PubMed] [Google Scholar]
- 6.Fried D, Xie J, Shafi S, Featherstone JDB, Breunig T, Lee CQ. Early detection of dental caries and lesion progression with polarization sensitive optical coherence tomography. J. Biomed. Optics. 2002;7(4):618–627. doi: 10.1117/1.1509752. [DOI] [PubMed] [Google Scholar]
- 7.Jones RS, Fried D. Remineralization of enamel caries can decrease optical reflectivity. Journal of Dental Research. 2006;85(9):804–808. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RS, Darling CL, Featherstone JDB, Fried D. Remineralization of in vitro Dental Caries Assessed with Polarization Sensitive Optical Coherence Tomography. J Biomed Opt. 2006;11(1):014016. doi: 10.1117/1.2161192. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Darling C, Fried D. Polarization Sensitive Optical Coherence Tomographic Imaging of Artificial Demineralization on Exposed Surfaces of Tooth Roots. Dent. Mat. 2009;25(6):721–728. doi: 10.1016/j.dental.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manesh SK, Darling CL, Fried D. Nondestructive assessment of dentin demineralization using polarization-sensitive optical coherence tomography after exposure to fluoride and laser irradiation. J Biomed Mater Res B Appl Biomater. 2009;90(2):802–812. doi: 10.1002/jbm.b.31349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manesh SK, Darling CL, Fried D. Polarization-sensitive optical coherence tomography for the nondestructive assessment of the remineralization of dentin. Journal of Biomedical Optics. 2009;14(4):044002. doi: 10.1117/1.3158995. [DOI] [PubMed] [Google Scholar]
- 12.Louie T, Lee C, Hsu D, Hirasuna K, Manesh S, Staninec M, Darling CL, Fried D. Clinical assessment of early tooth demineralization using polarization sensitive optical coherence tomography. Lasers in Surgery and Medicine. 2010;42(10):738–745. doi: 10.1002/lsm.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staninec M, Douglas SM, Darling CL, et al. Nondestructive Clinical Assessment of Occlusal Caries Lesions using Near-IR Imaging Methods. Lasers in Surgery and Medicine. 2011;43(10):951–959. doi: 10.1002/lsm.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail AI. Visual and Visuo-tactile Detection of Dental Caries. J Dent Res. 2004;83(Spec Iss C):C56–C66. doi: 10.1177/154405910408301s12. [DOI] [PubMed] [Google Scholar]
- 15.ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11(5):277–286. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- 16.Kidd EA. The histopathology of enamel caries in young and old permanent teeth. British dental journal. 1983;155(6):196–198. doi: 10.1038/sj.bdj.4805177. [DOI] [PubMed] [Google Scholar]
- 17.Fejerskov O, Kidd E, editors. Dental Caries: The Disease and its Clinical Management. Blackwell; Oxford: 2003. [Google Scholar]
- 18.Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt. 2003;8(4):642–647. doi: 10.1117/1.1606685. [DOI] [PubMed] [Google Scholar]
- 19.Amaechi BT, Podoleanu AG, Komarov G, Higham SM, Jackson DA. Quantification of root caries using optical coherence tomography and microradiography: a correlational study. Oral Health Prev Dent. 2004;2(4):377–382. [PubMed] [Google Scholar]
- 20.Le MH, Darling CL, Fried D. Methods for calculating the severity of demineralization on tooth surfaces. SPIE Proceeding. 2009;7162:1–7. doi: 10.1117/12.816867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tranaeus S, Al-Khateeb S, Bjorkman S, Twetman S, Angmar-Mansson B. Application of quantitative light-induced fluorescence to monitor incipient lesions in caries-active children. A comparative study of remineralisation by fluoride varnish and professional cleaning. Eur J Oral Sci. 2001;109(2):71–75. doi: 10.1034/j.1600-0722.2001.00997.x. [DOI] [PubMed] [Google Scholar]