Abstract
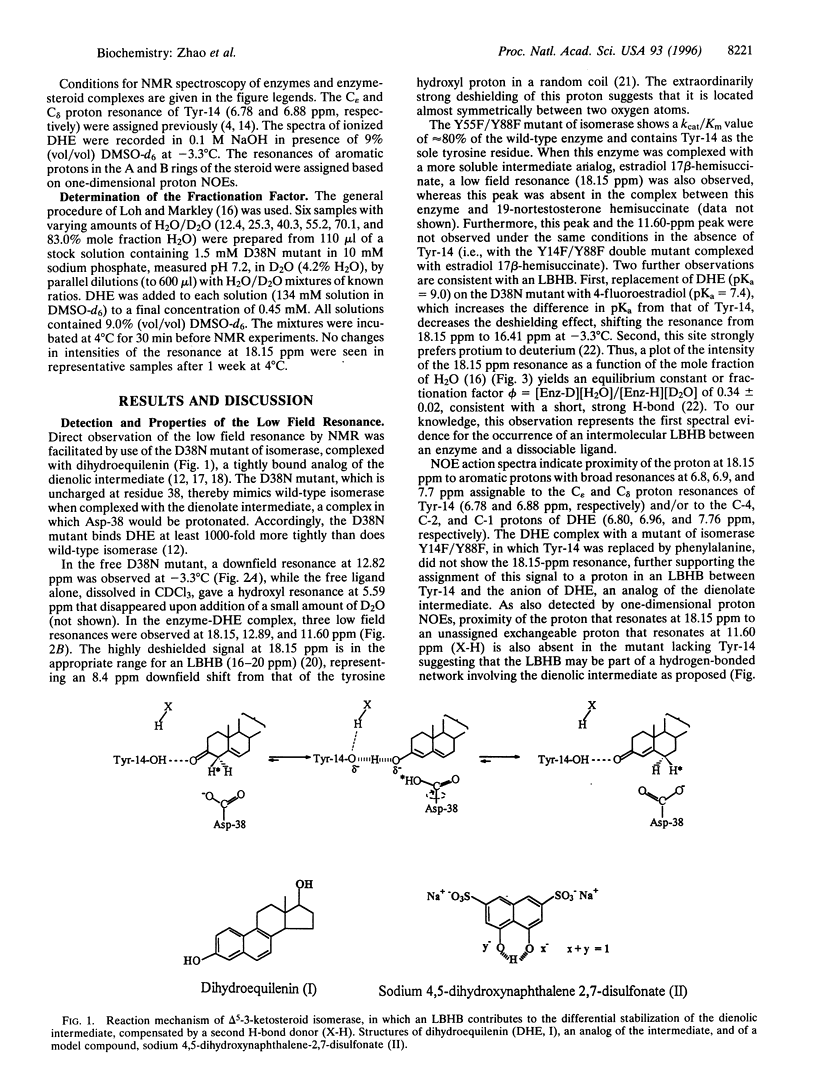
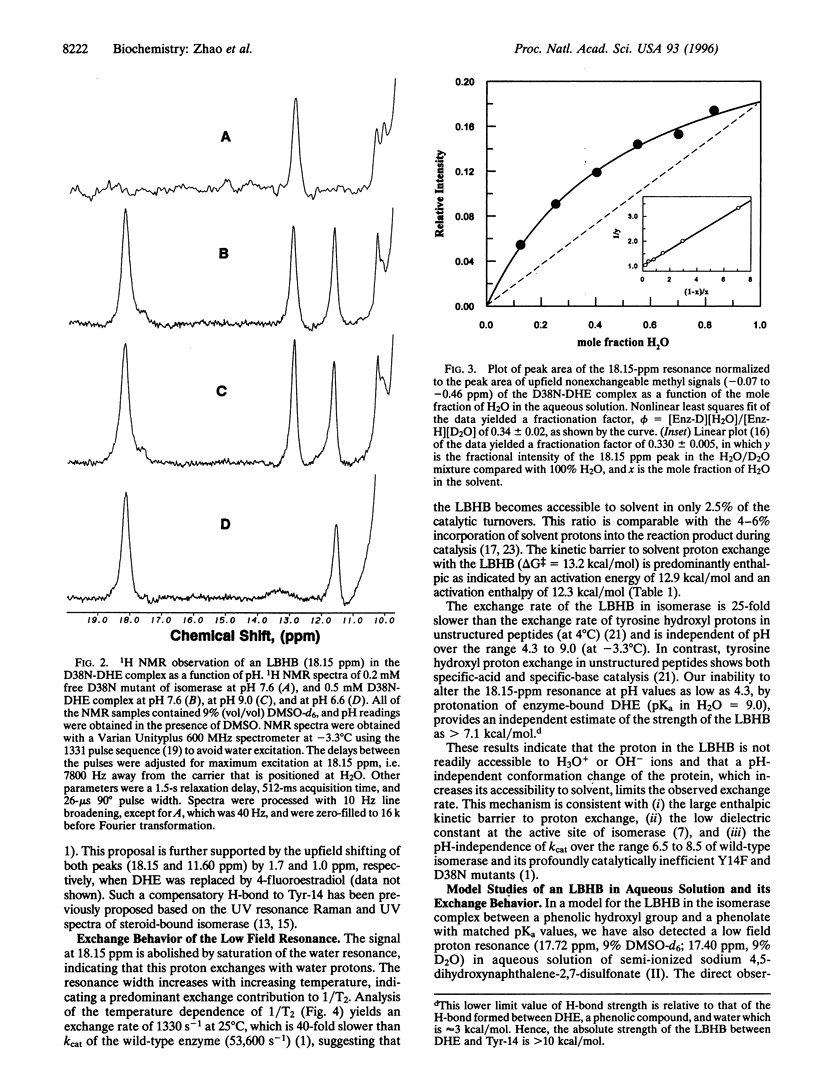
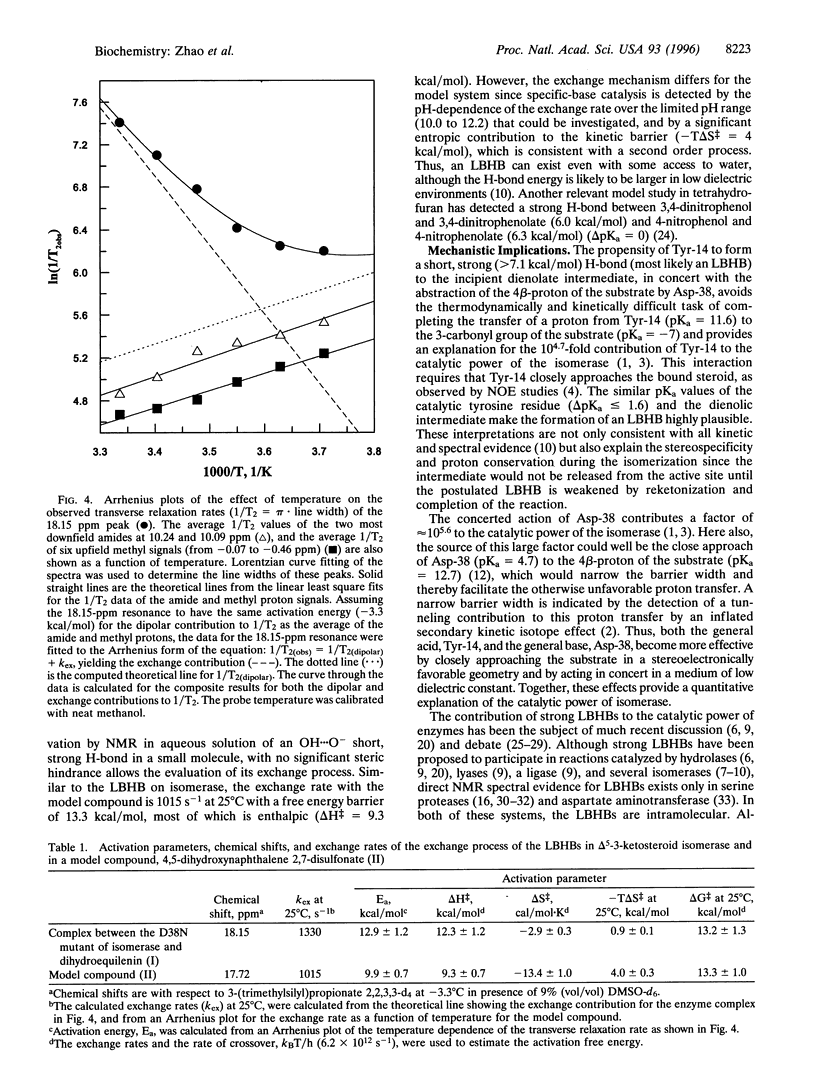
Delta 5-3-Ketosteroid isomerase (EC 5.3.3.1) promotes an allylic rearrangement involving intramolecular proton transfer via a dienolic intermediate. This enzyme enhances the catalytic rate by a factor of 10(10). Two residues, Tyr-14, the general acid that polarizes the steroid 3-carbonyl group and facilitates enolization, and Asp-38 the general base that abstracts and transfers the 4 beta-proton to the 6 beta-position, contribute 10(4.7) and 10(5.6) to the rate increase, respectively. A major mechanistic enigma is the huge disparity between the pKa values of the catalytic groups and their targets. Upon binding of an analog of the dienolate intermediate to isomerase, proton NMR detects a highly deshielded resonance at 18.15 ppm in proximity to aromatic protons, and with a 3-fold preference for protium over deuterium (fractionation factor, phi = 0.34), consistent with formation of a short, strong (low-barrier) hydrogen bond to Tyr-14. The strength of this hydrogen bond is estimated to be at least 7.1 kcal/mol. This bond is relatively inaccessible to bulk solvent and is pH insensitive. Low-barrier hydrogen bonding of Tyr-14 to the intermediate, in conjunction with the previously demonstrated tunneling contribution to the proton transfer by Asp-38, provide a plausible and quantitative explanation for the high catalytic power of this isomerase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin J. C., Zhao Q., Jordan T., Talalay P., Mildvan A. S., Spiro T. G. Ultraviolet resonance Raman spectroscopy of delta 5-3-ketosteroid isomerase revisited: substrate polarization by active-site residues. Biochemistry. 1995 Apr 4;34(13):4441–4447. doi: 10.1021/bi00013a037. [DOI] [PubMed] [Google Scholar]
- Brooks B., Benisek W. F. Mechanism of the reaction catalyzed by delta 5-3-ketosteroid isomerase of Comamonas (Pseudomonas) testosteroni: kinetic properties of a modified enzyme in which tyrosine 14 is replaced by 3-fluorotyrosine. Biochemistry. 1994 Mar 8;33(9):2682–2687. doi: 10.1021/bi00175a042. [DOI] [PubMed] [Google Scholar]
- Cleland W. W., Kreevoy M. M. Low-barrier hydrogen bonds and enzymic catalysis. Science. 1994 Jun 24;264(5167):1887–1890. doi: 10.1126/science.8009219. [DOI] [PubMed] [Google Scholar]
- Cleland W. W., Kreevoy M. M. Response. Science. 1995 Jul 7;269(5220):104–104. doi: 10.1126/science.269.5220.104. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Low-barrier hydrogen bonds and low fractionation factor bases in enzymatic reactions. Biochemistry. 1992 Jan 21;31(2):317–319. doi: 10.1021/bi00117a001. [DOI] [PubMed] [Google Scholar]
- Frey P. A. Response. Science. 1995 Jul 7;269(5220):104–106. doi: 10.1126/science.269.5220.104-a. [DOI] [PubMed] [Google Scholar]
- Frey P. A., Whitt S. A., Tobin J. B. A low-barrier hydrogen bond in the catalytic triad of serine proteases. Science. 1994 Jun 24;264(5167):1927–1930. doi: 10.1126/science.7661899. [DOI] [PubMed] [Google Scholar]
- Guthrie J. P. Short strong hydrogen bonds: can they explain enzymic catalysis? Chem Biol. 1996 Mar;3(3):163–170. doi: 10.1016/s1074-5521(96)90258-6. [DOI] [PubMed] [Google Scholar]
- Hawkinson D. C., Eames T. C., Pollack R. M. Kinetic competence of an externally generated dienol intermediate with steroid isomerase. Biochemistry. 1991 Jul 16;30(28):6956–6964. doi: 10.1021/bi00242a021. [DOI] [PubMed] [Google Scholar]
- Hawkinson D. C., Pollack R. M., Ambulos N. P., Jr Evaluation of the internal equilibrium constant for 3-oxo-delta 5-steroid isomerase using the D38E and D38N mutants: the energetic basis for catalysis. Biochemistry. 1994 Oct 11;33(40):12172–12183. doi: 10.1021/bi00206a021. [DOI] [PubMed] [Google Scholar]
- Kintanar A., Metzler C. M., Metzler D. E., Scott R. D. NMR observation of exchangeable protons of pyridoxal phosphate and histidine residues in cytosolic aspartate aminotransferase. J Biol Chem. 1991 Sep 15;266(26):17222–17229. [PubMed] [Google Scholar]
- Kuliopulos A., Mildvan A. S., Shortle D., Talalay P. Kinetic and ultraviolet spectroscopic studies of active-site mutants of delta 5-3-ketosteroid isomerase. Biochemistry. 1989 Jan 10;28(1):149–159. doi: 10.1021/bi00427a022. [DOI] [PubMed] [Google Scholar]
- Kuliopulos A., Mullen G. P., Xue L., Mildvan A. S. Stereochemistry of the concerted enolization catalyzed by delta 5-3-ketosteroid isomerase. Biochemistry. 1991 Apr 2;30(13):3169–3178. doi: 10.1021/bi00227a003. [DOI] [PubMed] [Google Scholar]
- Kuliopulos A., Talalay P., Mildvan A. S. Combined effects of two mutations of catalytic residues on the ketosteroid isomerase reaction. Biochemistry. 1990 Nov 6;29(44):10271–10280. doi: 10.1021/bi00496a017. [DOI] [PubMed] [Google Scholar]
- Li Y. K., Kuliopulos A., Mildvan A. S., Talalay P. Environments and mechanistic roles of the tyrosine residues of delta 5-3-ketosteroid isomerase. Biochemistry. 1993 Feb 23;32(7):1816–1824. doi: 10.1021/bi00058a016. [DOI] [PubMed] [Google Scholar]
- Liang T. C., Abeles R. H. Complex of alpha-chymotrypsin and N-acetyl-L-leucyl-L-phenylalanyl trifluoromethyl ketone: structural studies with NMR spectroscopy. Biochemistry. 1987 Dec 1;26(24):7603–7608. doi: 10.1021/bi00398a011. [DOI] [PubMed] [Google Scholar]
- Liepinsh E., Otting G., Wüthrich K. NMR spectroscopy of hydroxyl protons in aqueous solutions of peptides and proteins. J Biomol NMR. 1992 Sep;2(5):447–465. doi: 10.1007/BF02192808. [DOI] [PubMed] [Google Scholar]
- Loh S. N., Markley J. L. Hydrogen bonding in proteins as studied by amide hydrogen D/H fractionation factors: application to staphylococcal nuclease. Biochemistry. 1994 Feb 1;33(4):1029–1036. doi: 10.1021/bi00170a023. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ibañez I. B. Zymogen activation in serine proteinases. Proton magnetic resonance pH titration studies of the two histidines of bovine chymotrypsinogen A and chymotrypsin Aalpha. Biochemistry. 1978 Oct 31;17(22):4627–4640. doi: 10.1021/bi00615a008. [DOI] [PubMed] [Google Scholar]
- Robillard G., Shulman R. G. High resolution nuclear magnetic resonance studies of the active site of chymotrypsin. I. The hydrogen bonded protons of the "charge relay" system. J Mol Biol. 1974 Jul 5;86(3):519–540. doi: 10.1016/0022-2836(74)90178-8. [DOI] [PubMed] [Google Scholar]
- Shan S. O., Loh S., Herschlag D. The energetics of hydrogen bonds in model systems: implications for enzymatic catalysis. Science. 1996 Apr 5;272(5258):97–101. doi: 10.1126/science.272.5258.97. [DOI] [PubMed] [Google Scholar]
- Tobin J. B., Whitt S. A., Cassidy C. S., Frey P. A. Low-barrier hydrogen bonding in molecular complexes analogous to histidine and aspartate in the catalytic triad of serine proteases. Biochemistry. 1995 May 30;34(21):6919–6924. doi: 10.1021/bi00021a002. [DOI] [PubMed] [Google Scholar]
- WANG S. F., KAWAHARA F. S., TALALAY P. The mechanism of the delta5-3-ketosteroid isomerase reaction: absorption and fluorescence spectra of enzyme-steroid complexes. J Biol Chem. 1963 Feb;238:576–585. [PubMed] [Google Scholar]
- Warshel A., Papazyan A., Kollman P. A. On low-barrier hydrogen bonds and enzyme catalysis. Science. 1995 Jul 7;269(5220):102–106. doi: 10.1126/science.7661987. [DOI] [PubMed] [Google Scholar]
- Xue L. A., Talalay P., Mildvan A. S. Studies of the catalytic mechanism of an active-site mutant (Y14F) of delta 5-3-ketosteroid isomerase by kinetic deuterium isotope effects. Biochemistry. 1991 Nov 12;30(45):10858–10865. doi: 10.1021/bi00109a008. [DOI] [PubMed] [Google Scholar]
- Xue L. A., Talalay P., Mildvan A. S. Studies of the mechanism of the delta 5-3-ketosteroid isomerase reaction by substrate, solvent, and combined kinetic deuterium isotope effects on wild-type and mutant enzymes. Biochemistry. 1990 Aug 14;29(32):7491–7500. doi: 10.1021/bi00484a019. [DOI] [PubMed] [Google Scholar]
- Zeng B. F., Bounds P. L., Steiner R. F., Pollack R. M. Nature of the intermediate in the 3-oxo-delta 5-steroid isomerase reaction. Biochemistry. 1992 Feb 11;31(5):1521–1528. doi: 10.1021/bi00120a032. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Abeygunawardana C., Mildvan A. S. 13C NMR relaxation studies of backbone and side chain motion of the catalytic tyrosine residue in free and steroid-bound delta 5-3-ketosteroid isomerase. Biochemistry. 1996 Feb 6;35(5):1525–1532. doi: 10.1021/bi9525381. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Li Y. K., Mildvan A. S., Talalay P. Ultraviolet spectroscopic evidence for decreased motion of the active site tyrosine residue of delta 5-3-ketosteroid isomerase by steroid binding. Biochemistry. 1995 May 16;34(19):6562–6572. doi: 10.1021/bi00019a038. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Mildvan A. S., Talalay P. Enzymatic and nonenzymatic polarizations of alpha,beta-unsaturated ketosteroids and phenolic steroids. Implications for the roles of hydrogen bonding in the catalytic mechanism of delta 5-3-ketosteroid isomerase. Biochemistry. 1995 Jan 17;34(2):426–434. doi: 10.1021/bi00002a006. [DOI] [PubMed] [Google Scholar]


