Abstract
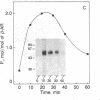
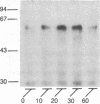
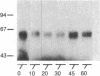
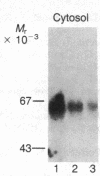
beta-Adrenergic receptor kinase (beta-AR kinase) is a cytosolic enzyme that phosphorylates the beta-adrenergic receptor only when it is occupied by an agonist [Benovic, J. Strasser, R. H., Caron, M. G. & Lefkowitz, R. J. (1986) Proc. Natl. Acad. Sci. USA 83, 2797-2801.] It may be crucially involved in the processes that lead to homologous or agonist-specific desensitization of the receptor. Stimulation of DDT1MF-2 hamster smooth muscle cells or S49 mouse lymphoma cells with a beta-agonist leads to translocation of 80-90% of the beta-AR kinase activity from the cytosol to the plasma membrane. The translocation process is quite rapid, is concurrent with receptor phosphorylation, and precedes receptor desensitization and sequestration. It is also transient, since much of the activity returns to the cytosol as the receptors become sequestered. Stimulation of beta-AR kinase translocation is a receptor-mediated event, since the beta-antagonist propranolol blocks the effect of agonist. In the kin- mutant of the S49 cells (lacks cAMP-dependent protein kinase), prostaglandin E1, which provokes homologous desensitization of its own receptor, is at least as effective as isoproterenol in promoting beta-AR kinase translocation to the plasma membrane. However, in the DDT1MF-2 cells, which contain alpha 1-adrenergic receptors coupled to phosphatidylinositol turnover, the alpha 1-agonist phenylephrine is ineffective. These results suggest that the first step in homologous desensitization of the beta-adrenergic receptor may be an agonist-promoted translocation of beta-AR kinase from cytosol to plasma membrane and that beta-AR kinase may represent a more general adenylate cyclase-coupled receptor kinase that participates in regulating the function of many such receptors.
Full text
PDF
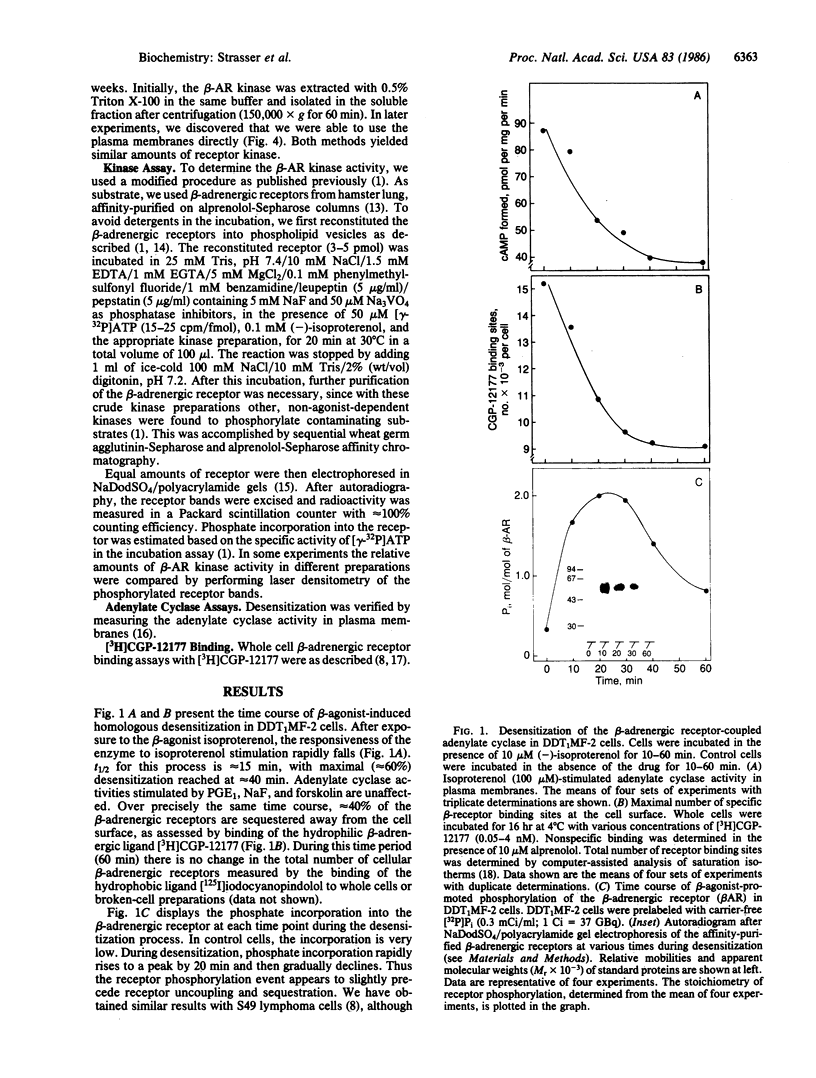
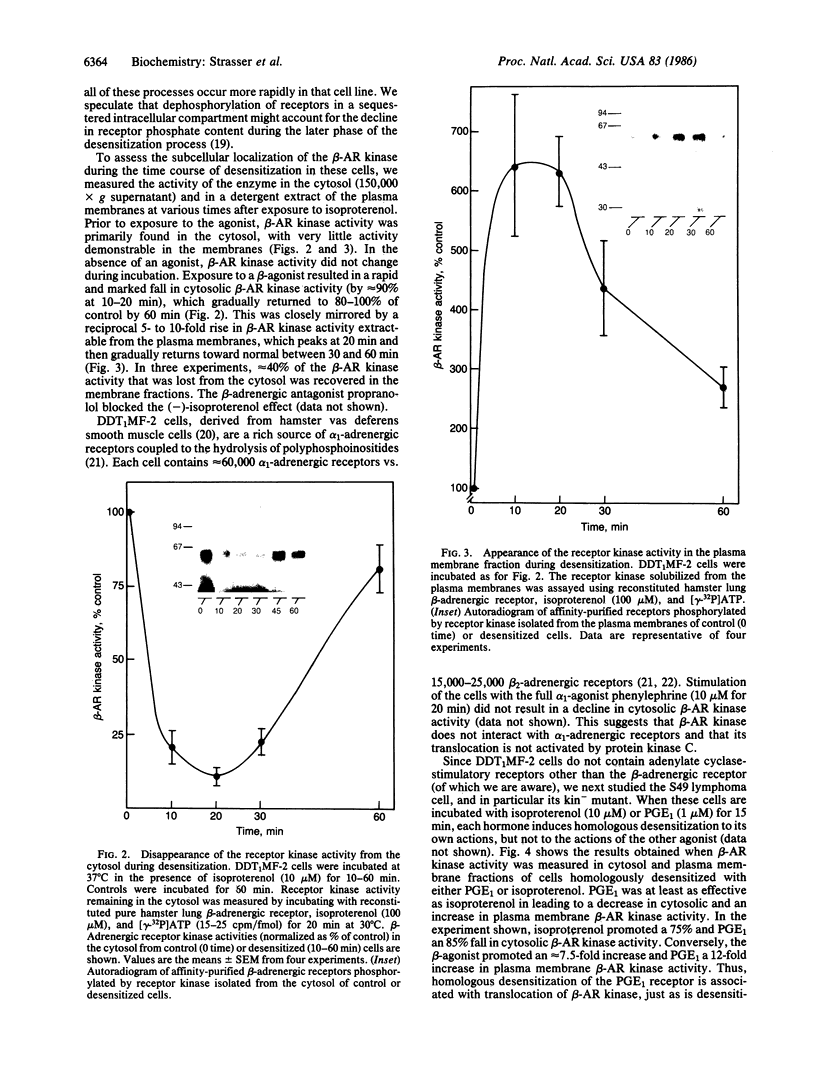
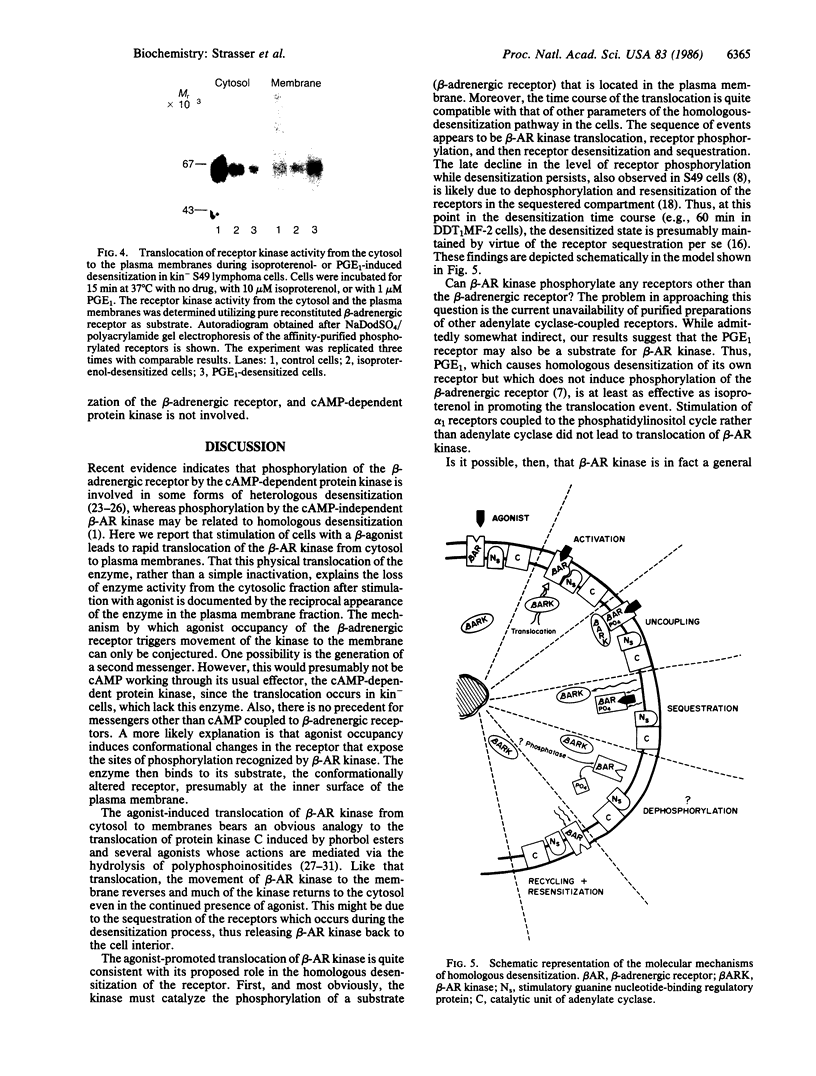

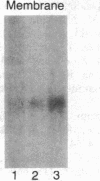
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Pike L. J., Cerione R. A., Staniszewski C., Yoshimasa T., Codina J., Caron M. G., Lefkowitz R. J. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985 Jun 10;260(11):7094–7101. [PubMed] [Google Scholar]
- Benovic J. L., Shorr R. G., Caron M. G., Lefkowitz R. J. The mammalian beta 2-adrenergic receptor: purification and characterization. Biochemistry. 1984 Sep 25;23(20):4510–4518. doi: 10.1021/bi00315a002. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: characterization of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):611–620. doi: 10.1002/jcp.1040850313. [DOI] [PubMed] [Google Scholar]
- Bownds D., Dawes J., Miller J., Stahlman M. Phosphorylation of frog photoreceptor membranes induced by light. Nat New Biol. 1972 May 24;237(73):125–127. doi: 10.1038/newbio237125a0. [DOI] [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Cornett L. E., Norris J. S. Characterization of the alpha 1-adrenergic receptor subtype in a smooth muscle cell line. J Biol Chem. 1982 Jan 25;257(2):694–697. [PubMed] [Google Scholar]
- De Lean A., Hancock A. A., Lefkowitz R. J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982 Jan;21(1):5–16. [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Protein kinase C translocates from cytosol to membrane upon hormone activation: effects of thyrotropin-releasing hormone in GH3 cells. Biochem Biophys Res Commun. 1985 Apr 30;128(2):531–537. doi: 10.1016/0006-291x(85)90079-8. [DOI] [PubMed] [Google Scholar]
- Frank R. N., Cavanagh H. D., Kenyon K. R. Light-stimulated phosphorylation of bovine visual pigments by adenosine triphosphate. J Biol Chem. 1973 Jan 25;248(2):596–609. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Adenylate cyclase coupling proteins are not essential for agonist-specific desensitization of lymphoma cells. J Biol Chem. 1981 Mar 10;256(5):2105–2108. [PubMed] [Google Scholar]
- Harden T. K. Agonist-induced desensitization of the beta-adrenergic receptor-linked adenylate cyclase. Pharmacol Rev. 1983 Mar;35(1):5–32. [PubMed] [Google Scholar]
- Hertel C., Müller P., Portenier M., Staehelin M. Determination of the desensitization of beta-adrenergic receptors by [3H]CGP-12177. Biochem J. 1983 Dec 15;216(3):669–674. doi: 10.1042/bj2160669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Hirota T., Aguilera G., Catt K. J. Hormone-induced redistribution of calcium-activated phospholipid-dependent protein kinase in pituitary gonadotrophs. J Biol Chem. 1985 Mar 25;260(6):3243–3246. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kühn H., Dreyer W. J. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 1972 Jan 15;20(1):1–6. doi: 10.1016/0014-5793(72)80002-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Nambi P., Sibley D. R., Stadel J. M., Michel T., Peters J. R., Lefkowitz R. J. Cell-free desensitization of catecholamine-sensitive adenylate cyclase. Agonist- and cAMP-promoted alterations in turkey erythrocyte beta-adrenergic receptors. J Biol Chem. 1984 Apr 10;259(7):4629–4633. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Scarpace P. J., Baresi L. A., Sanford D. A., Abrass I. B. Desensitization and resensitization of beta-adrenergic receptors in a smooth muscle cell line. Mol Pharmacol. 1985 Dec;28(6):495–501. [PubMed] [Google Scholar]
- Shichi H., Somers R. L. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J Biol Chem. 1978 Oct 10;253(19):7040–7046. [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Peters J. R., Nambi P., Caron M. G., Lefkowitz R. J. Desensitization of turkey erythrocyte adenylate cyclase. Beta-adrenergic receptor phosphorylation is correlated with attenuation of adenylate cyclase activity. J Biol Chem. 1984 Aug 10;259(15):9742–9749. [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. H., Lefkowitz R. J. Homologous desensitization of beta-adrenergic receptor coupled adenylate cyclase. Resensitization by polyethylene glycol treatment. J Biol Chem. 1985 Apr 25;260(8):4561–4564. [PubMed] [Google Scholar]
- Strasser R. H., Sibley D. R., Lefkowitz R. J. A novel catecholamine-activated adenosine cyclic 3',5'-phosphate independent pathway for beta-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986 Mar 25;25(6):1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- van Daalen Wetters T., Murtaugh M. P., Coffino P. Revertants of a trans-dominant S49 mouse lymphoma mutant that affects expression of cAMP-dependent protein kinase. Cell. 1983 Nov;35(1):311–320. doi: 10.1016/0092-8674(83)90234-9. [DOI] [PubMed] [Google Scholar]