Abstract
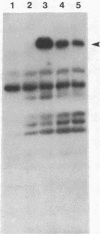
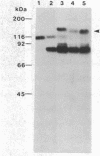
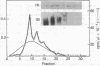
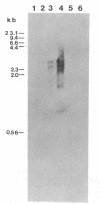
A cloned complementary DNA sequence has been isolated from a human placental cDNA library in the bacteriophage expression vector lambda gt11 after screening with polyclonal antibodies against human placental aromatase-system cytochrome P-450 (P-450Arom). A single recombinant clone, lambda hAROM1, was characterized by its ability to generate a beta-galactosidase fusion protein that reacted independently with polyclonal antibodies raised against beta-galactosidase and cytochrome P-450Arom and with monoclonal antibodies specific for cytochrome P-450Arom. The cDNA insert, which was found to be 1.8 kilobases in length, was radiolabeled and used to analyze poly(A)+ RNA isolated from human placenta and total RNA isolated from human adipose stromal cells cultured in the absence or presence of regulatory factors. The radiolabeled cDNA hybridized to several size species of mRNA in both placental and adipose stromal cell RNA fractions. Changes in the levels of adipose stromal cell RNA that hybridized to the cDNA insert were associated with comparable changes in the levels of translatable cytochrome P-450Arom mRNA and aromatase system activity. These findings are indicative that lambda hAROM1 contains DNA sequences complementary to human cytochrome P-450Arom mRNA and are suggestive that regulatory factors affect aromatase activity by altering the transcriptional activity of the cytochrome P-450Arom gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman G. E., Smith M. E., Mendelson C. R., MacDonald P. C., Simpson E. R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab. 1981 Aug;53(2):412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen S., Shively J. E., Nakajin S., Shinoda M., Hall P. F. Amino terminal sequence analysis of human placenta aromatase. Biochem Biophys Res Commun. 1986 Mar 28;135(3):713–719. doi: 10.1016/0006-291x(86)90987-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Durham C. R., Zhu H., Masters B. S., Simpson E. R., Mendelson C. R. Regulation of aromatase activity of rat granulosa cells: induction of synthesis of NADPH-cytochrome P-450 reductase by FSH and dibutyryl cyclic AMP. Mol Cell Endocrinol. 1985 May;40(2-3):211–219. doi: 10.1016/0303-7207(85)90177-7. [DOI] [PubMed] [Google Scholar]
- Fishman J., Goto J. Mechanism of estrogen biosynthesis. Participation of multiple enzyme sites in placental aromatase hydroxylations. J Biol Chem. 1981 May 10;256(9):4466–4471. [PubMed] [Google Scholar]
- Fishman J., Raju M. S. Mechanism of estrogen biosynthesis. Stereochemistry of C-1 hydrogen elimination in the aromatization of 2 beta-hydroxy-19-oxoandrostenedione. J Biol Chem. 1981 May 10;256(9):4472–4477. [PubMed] [Google Scholar]
- Fritz I. B., Griswold M. D., Louis B. G., Dorrington J. H. Similarity of responses of cultured Sertoli cells to cholera toxin and FSH. Mol Cell Endocrinol. 1976 Aug-Sep;5(3-4):289–294. doi: 10.1016/0303-7207(76)90090-3. [DOI] [PubMed] [Google Scholar]
- Goto J., Fishman J. Participation of a nonenzymatic transformation in the biosynthesis of estrogens from androgens. Science. 1977 Jan 7;195(4273):80–81. doi: 10.1126/science.831259. [DOI] [PubMed] [Google Scholar]
- Grodin J. M., Siiteri P. K., MacDonald P. C. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973 Feb;36(2):207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hahn E. F., Miyairi S., Fishman J. 19-Hydroxylation of androgens in the rat brain. Proc Natl Acad Sci U S A. 1985 May;82(9):2728–2730. doi: 10.1073/pnas.82.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsell D. L., Grodin J. M., Brenner P. F., Siiteri P. K., MacDonald P. C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J Clin Endocrinol Metab. 1974 Mar;38(3):476–479. doi: 10.1210/jcem-38-3-476. [DOI] [PubMed] [Google Scholar]
- Ilaria R., Wines D., Pardue S., Jamison S., Ojeda S. R., Snider J., Morrison M. R. A rapid microprocedure for isolating RNA from multiple samples of human and rat brain. J Neurosci Methods. 1985 Oct-Nov;15(2):165–174. doi: 10.1016/0165-0270(85)90053-6. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Simpson E. R., Waterman M. R. Regulation of cytochrome P-45011 beta gene expression by adrenocorticotropin. J Biol Chem. 1985 May 10;260(9):5760–5767. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F., Goldman-Rakic P. S. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci U S A. 1986 Jan;83(2):513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson C. R., Cleland W. H., Smith M. E., Simpson E. R. Regulation of aromatase activity of stromal cells derived from human adipose tissue. Endocrinology. 1982 Oct;111(4):1077–1085. doi: 10.1210/endo-111-4-1077. [DOI] [PubMed] [Google Scholar]
- Mendelson C. R., Corbin C. J., Smith M. E., Smith J., Simpson E. R. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3',5'-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology. 1986 Mar;118(3):968–973. doi: 10.1210/endo-118-3-968. [DOI] [PubMed] [Google Scholar]
- Mendelson C. R., Wright E. E., Evans C. T., Porter J. C., Simpson E. R. Preparation and characterization of polyclonal and monoclonal antibodies against human aromatase cytochrome P-450 (P-450AROM), and their use in its purification. Arch Biochem Biophys. 1985 Dec;243(2):480–491. doi: 10.1016/0003-9861(85)90525-9. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Davies I. J., Reddy V. V., Flores F., Petro Z., Kuhn M., White R. J., Takaoka Y., Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Simmen F. A., Gope M. L., Schulz T. Z., Wright D. A., Carpenter G., O'Malley B. W. Isolation of an evolutionarily conserved epidermal growth factor receptor cDNA from human A431 carcinoma cells. Biochem Biophys Res Commun. 1984 Oct 15;124(1):125–132. doi: 10.1016/0006-291x(84)90926-4. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Ackerman G. E., Smith M. E., Mendelson C. R. Estrogen formation in stromal cells of adipose tissue of women: induction by glucocorticosteroids. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5690–5694. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Cleland W. H., Mendelson C. R. Aromatization of androgens by human adipose tissue in vitro. J Steroid Biochem. 1983 Jul;19(1B):707–713. doi: 10.1016/0022-4731(83)90239-x. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Jr, Siiteri P. K. The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem. 1974 Sep 10;249(17):5373–5378. [PubMed] [Google Scholar]
- Thompson E. A., Jr, Siiteri P. K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem. 1974 Sep 10;249(17):5364–5372. [PubMed] [Google Scholar]
- Tsai-Morris C. H., Aquilano D. R., Dufau M. L. Cellular localization of rat testicular aromatase activity during development. Endocrinology. 1985 Jan;116(1):38–46. doi: 10.1210/endo-116-1-38. [DOI] [PubMed] [Google Scholar]
- Valladares L. E., Payne A. H. Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinology. 1979 Aug;105(2):431–436. doi: 10.1210/endo-105-2-431. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]