Abstract
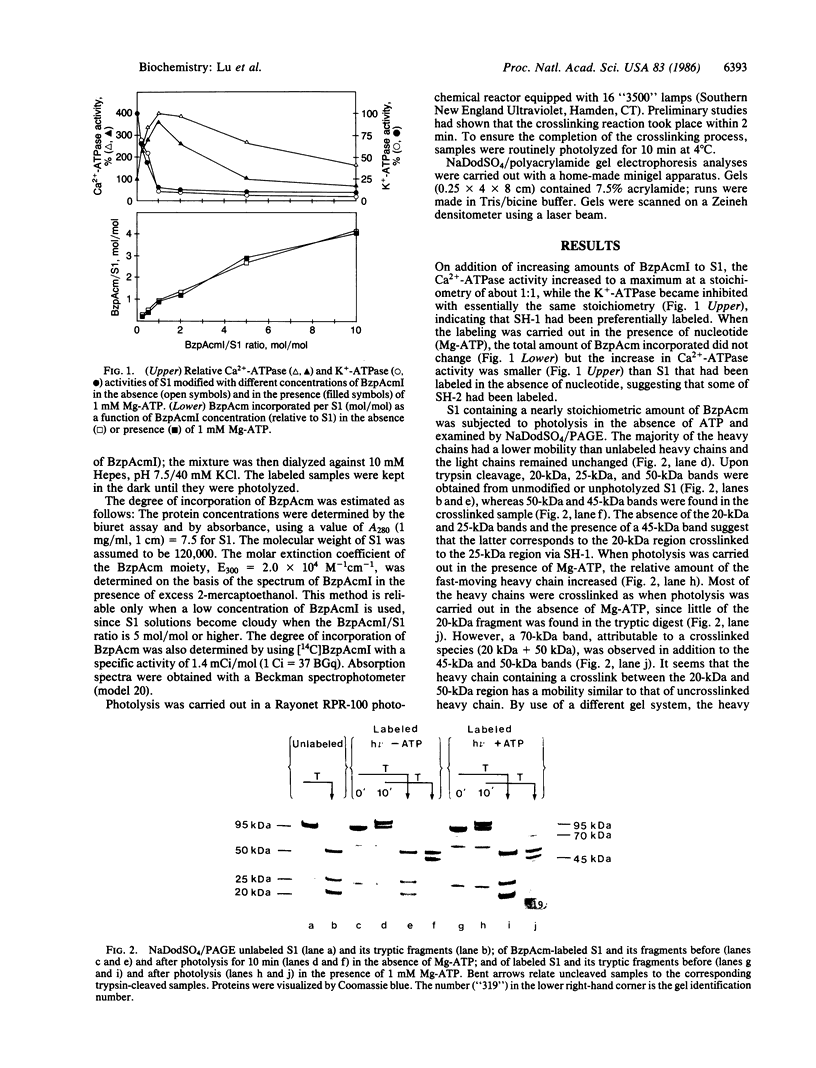
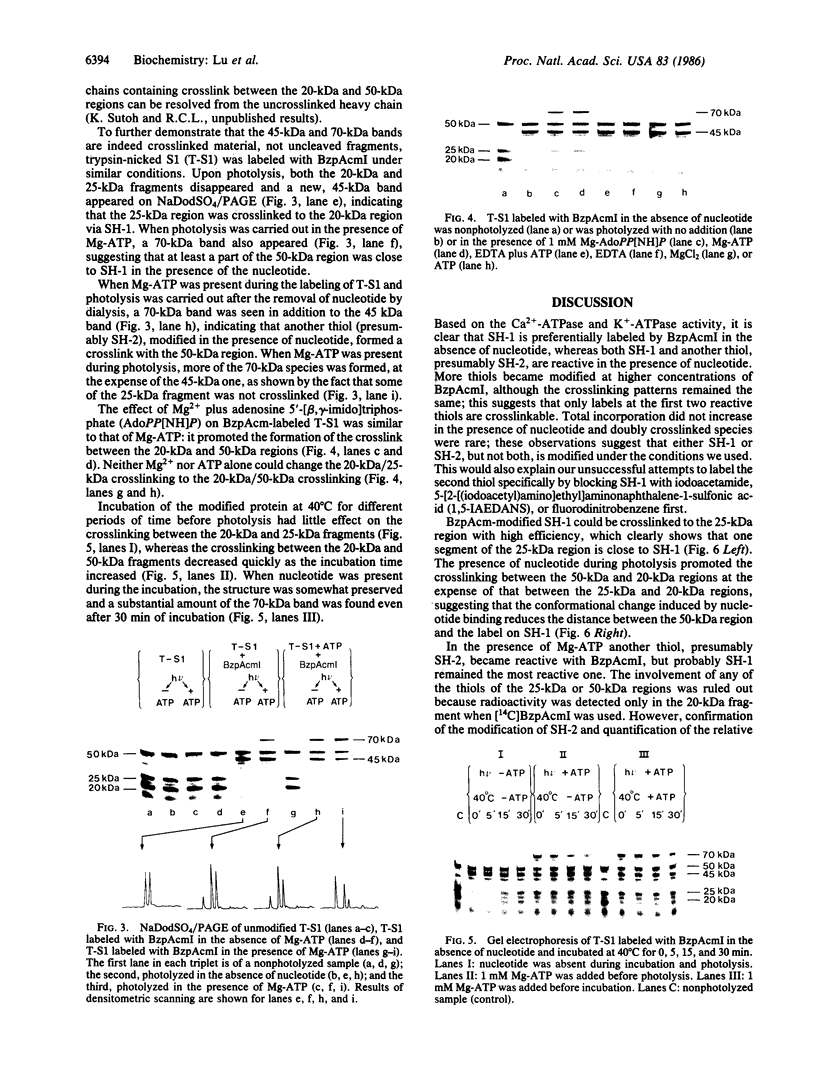
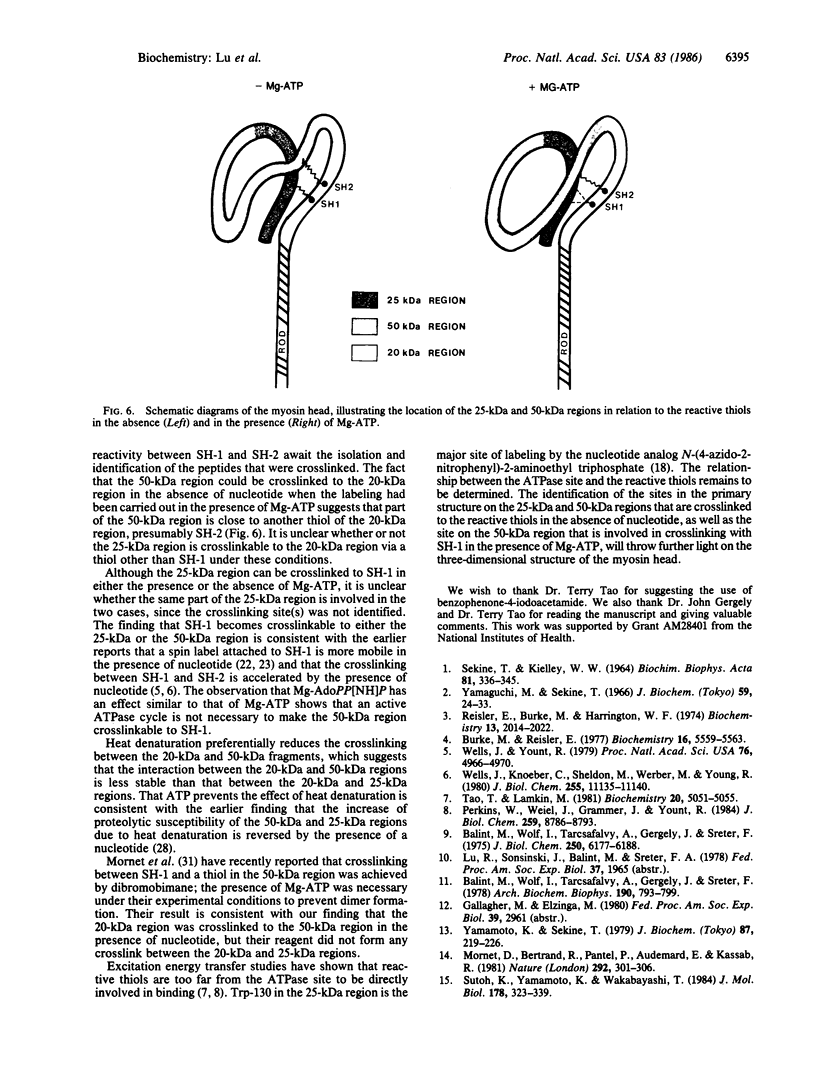
The thiol-specific photoactivatable reagent benzophenone-4-iodoacetamide can be incorporated into myosin subfragment 1 (S1), accompanied by an increase of Ca2+-ATPase and the loss of K+-ATPase activities, a characteristic property of S1 when reactive sulfhydryl 1 (SH-1) is modified. After trypsin cleavage, 25-kDa, 50-kDa, and 20-kDa fragments were found upon NaDodSO4/polyacrylamide gel electrophoresis of the unphotolyzed sample, whereas only the 50-kDa fragment and a 45-kDa fragment appeared in the photolyzed sample, indicating that the NH2-terminal 25-kDa fragment was crosslinked to the COOH-terminal 20-kDa fragment via SH-1. When photolysis was carried out in the presence of Mg2+ and ATP or Mg2+ and adenosine 5-[beta, gamma-imido]triphosphate (AdoPP[NH]P), a 70-kDa band, attributable to a crosslinked (50 kDa + 20 kDa) species, was also observed. This suggests that the conformational change induced by nucleotide binding reduces the distance between the 50-kDa region and the label on SH-1. Similar results were obtained when labeling and photolysis were carried out on trypsin-nicked S1, in which the 25-kDa, 50-kDa, and 20-kDa fragments are held together noncovalently. Further, when labeling with benzophenone-4-iodoacetamide was carried out in the presence of Mg-ATP, which increases the reactivity of another thiol, presumably SH-2, both 45-kDa and 70-kDa species were formed upon photolysis in the absence of ATP, suggesting that SH-2 is close to the 50-kDa region. More of the 70-kDa species was formed, at the expense of the 45-kDa species, when photolysis was carried out in the presence of Mg-ATP. Partial heat denaturation preferentially reduced the crosslinking between the reactive thiols and the 50-kDa region.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M., Reisler E. Effect of nucleotide binding on the proximity of the essential sulfhydryl groups of myosin. Chemical probing of movement of residues during conformational transitions. Biochemistry. 1977 Dec 13;16(25):5559–5563. doi: 10.1021/bi00644a026. [DOI] [PubMed] [Google Scholar]
- Bálint M., Sréter F. A., Wolf I., Nagy B., Gergely J. The substructure of heavy meromyosin. The effect of Ca2+ and Mg2+ on the tryptic fragmentation of heavy meromyosin. J Biol Chem. 1975 Aug 10;250(15):6168–6177. [PubMed] [Google Scholar]
- Bálint M., Wolf I., Tarcsafalvi A., Gergely J., Sréter F. A. Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin. Arch Biochem Biophys. 1978 Oct;190(2):793–799. doi: 10.1016/0003-9861(78)90339-9. [DOI] [PubMed] [Google Scholar]
- Chen T., Applegate D., Reisler E. Cross-linking of actin to myosin subfragment 1 in the presence of nucleotides. Biochemistry. 1985 Sep 24;24(20):5620–5625. doi: 10.1021/bi00341a050. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. Photosensitized direct cross-linking of fluorescent analogs of ATP to the adenine recognition domain in myosin ATPase. J Biochem. 1985 Jan;97(1):71–78. doi: 10.1093/oxfordjournals.jbchem.a135069. [DOI] [PubMed] [Google Scholar]
- Mahmood R., Yount R. G. Photochemical probes of the active site of myosin. Irradiation of trapped 3'-O-(4-benzoyl)benzoyladenosine 5'-triphosphate labels the 50-kilodalton heavy chain tryptic peptide. J Biol Chem. 1984 Nov 10;259(21):12956–12959. [PubMed] [Google Scholar]
- Mocz G., Szilagyi L., Chen Lu R., Fabian F., Balint M., Gergely J. Effect of nucleotides, divalent cations and temperature on the tryptic susceptibility of myosin subfragment 1. Eur J Biochem. 1984 Dec 3;145(2):221–229. doi: 10.1111/j.1432-1033.1984.tb08542.x. [DOI] [PubMed] [Google Scholar]
- Morita F. Interaction of heavy meromyosin with substrate. I. Difference in ultraviolet absorption spectrum between heavy meromyosin and its Michaelis-Menten complex. J Biol Chem. 1967 Oct 10;242(19):4501–4506. [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Derancourt J., Kassab R. Molecular movements promoted by metal nucleotides in the heavy-chain regions of myosin heads from skeletal muscle. J Mol Biol. 1985 Jun 5;183(3):479–489. doi: 10.1016/0022-2836(85)90015-4. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K., Morales M. F. Stabilization of a primary loop in myosin subfragment 1 with a fluorescent crosslinker. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1658–1662. doi: 10.1073/pnas.82.6.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad A., Hozumi T. Tryptic digestion as a probe of myosin S-1 conformation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):958–962. doi: 10.1073/pnas.79.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. J. Circular dichroism of the adenine and 6-mercaptopurine nucleotide complexes of heavy meromyosin. Arch Biochem Biophys. 1974 Jul;163(1):290–296. doi: 10.1016/0003-9861(74)90479-2. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Yount R. G. Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1575–1579. doi: 10.1073/pnas.82.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. J., Weiel J., Grammer J., Yount R. G. Introduction of a donor-acceptor pair by a single protein modification. Förster energy transfer distance measurements from trapped 1,N6-ethenoadenosine diphosphate to chromophoric cross-linking reagents on the critical thiols of myosin subfragment. J Biol Chem. 1984 Jul 25;259(14):8786–8793. [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974 May 7;13(10):2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. The conformation of myosin during the steady state of ATP hydrolysis: studies with myosin spin labeled at the S 1 thiol groups. Biochem Biophys Res Commun. 1971 Aug 20;44(4):826–830. doi: 10.1016/0006-291x(71)90785-6. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Yamamoto K., Wakabayashi T. Electron microscopic visualization of the SH1 thiol of myosin by the use of an avidin-biotin system. J Mol Biol. 1984 Sep 15;178(2):323–339. doi: 10.1016/0022-2836(84)90147-5. [DOI] [PubMed] [Google Scholar]
- Szilagyi L., Balint M., Sreter F. A., Gergely J. Photoaffinity labelling with an ATP analog of the N-terminal peptide of myosin. Biochem Biophys Res Commun. 1979 Apr 13;87(3):936–945. doi: 10.1016/0006-291x(79)92047-3. [DOI] [PubMed] [Google Scholar]
- Tao T., Lamkin M. Excitation energy transfer studies on the proximity between SH1 and the adenosinetriphosphatase site in myosin subfragment 1. Biochemistry. 1981 Aug 18;20(17):5051–5055. doi: 10.1021/bi00520a035. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Knoeber C., Sheldon M. C., Werber M. M., Yount R. G. Cross-linking of myosin subfragment 1. Nucleotide-enhanced modification by a variety of bifunctional reagents. J Biol Chem. 1980 Dec 10;255(23):11135–11140. [PubMed] [Google Scholar]
- Wells J. A., Yount R. G. Active site trapping of nucleotides by crosslinking two sulfhydryls in myosin subfragment 1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4966–4970. doi: 10.1073/pnas.76.10.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Sekine T. Substructure of myosin subfragment-1 as revealed by digestion with proteolytic enzymes. J Biochem. 1980 Jan;87(1):219–226. doi: 10.1093/oxfordjournals.jbchem.a132728. [DOI] [PubMed] [Google Scholar]