Abstract
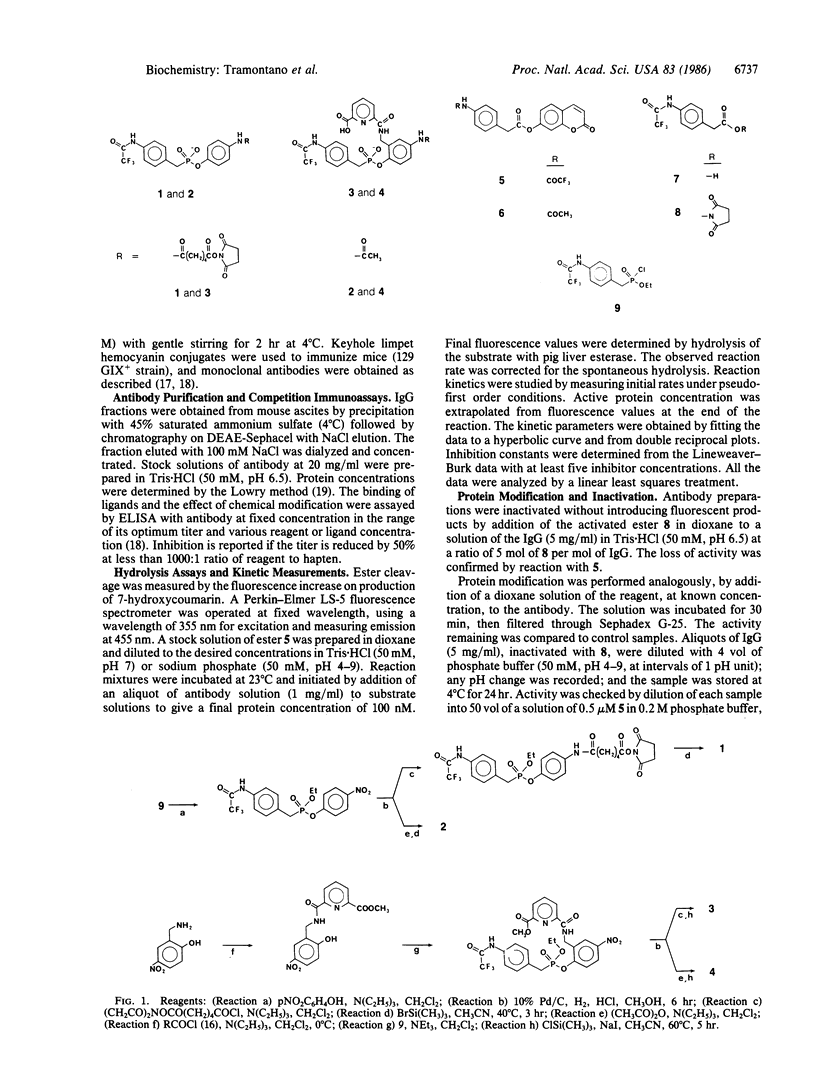
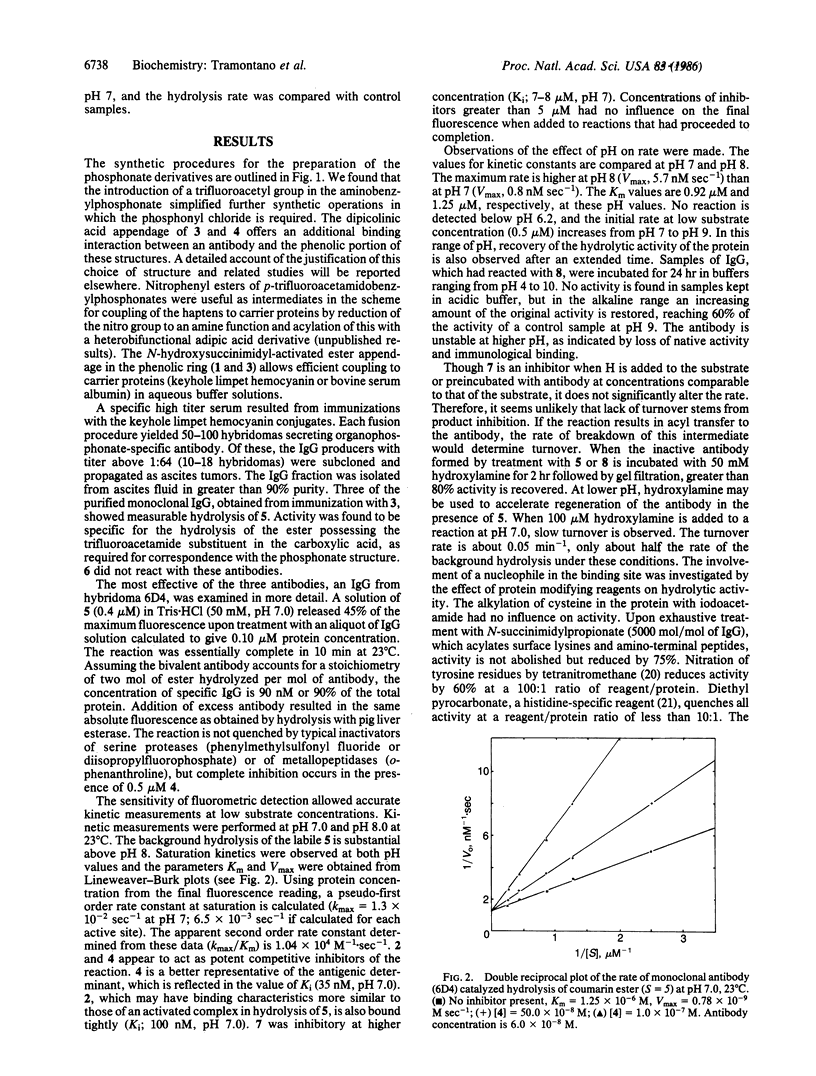
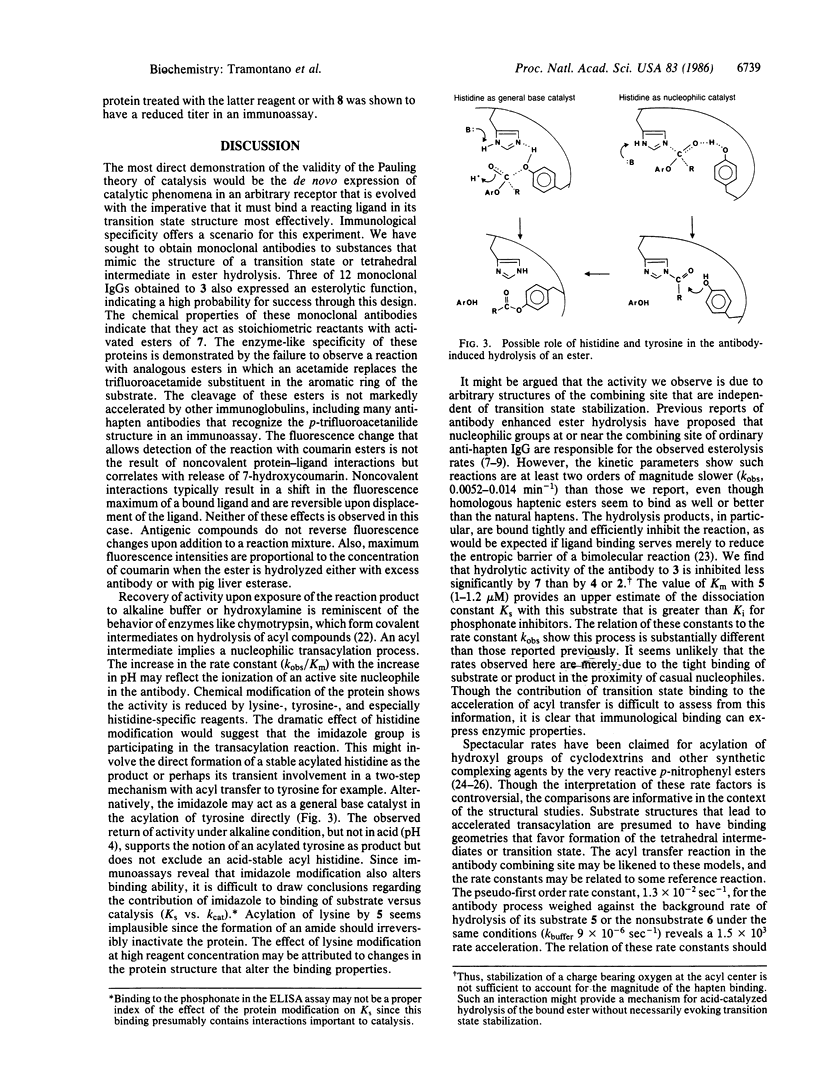
Monoaryl phosphonate esters, designated as analogs of the transition state in the hydrolysis of carboxylic esters, were synthesized and used as haptens to generate specific monoclonal antibodies. Some of these antibodies react with cognate aryl carboxylic esters to release a fluorescent alcohol. The reaction appears to be stoichiometric; however, the activity is slowly regenerated under alkaline conditions or by treatment with hydroxylamine. Specificity is rigorous for esters of p-trifluoroacetamidophenylacetic acid, demonstrating a structural correspondence with the phosphonate hapten. Saturation kinetics are observed and kinetic parameters (kmax, Vmax, and Km) are reported. The haptenic phosphonate is a competitive inhibitor of the reaction (Ki, 35 nM); whereas the carboxylate product of ester hydrolysis is a less effective inhibitor (Ki, ca. 7500 nM). Chemical modification of side chain groups in the protein show a partial reduction in activity on acylation of lysine or nitration of tyrosine and a dramatic quenching upon modification of histidine. The evidence is discussed in terms of a mechanism in which amino acids of the antibody combining site participate in nucleophilic and/or general base catalysis. The properties of this system suggest that it is an example of enzymic transacylation where a deacylation step is not catalyzed. The possibility of deriving enzymic function from immunological specificity through this approach is advanced.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHARD S. A., ORGEL L. E. Mechanism of enzyme inhibition by phosphate esters. Science. 1959 Sep 11;130(3376):625–626. doi: 10.1126/science.130.3376.625. [DOI] [PubMed] [Google Scholar]
- Bartlett P. A., Marlowe C. K. Phosphonamidates as transition-state analogue inhibitors of thermolysin. Biochemistry. 1983 Sep 27;22(20):4618–4624. doi: 10.1021/bi00289a002. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Holmquist B., Vallee B. L. Reversible inactivation and superactivation by covalent modification of thermolysin. Biochem Biophys Res Commun. 1973 Apr 16;51(4):987–992. doi: 10.1016/0006-291x(73)90024-7. [DOI] [PubMed] [Google Scholar]
- Breslow R. Artificial enzymes. Science. 1982 Nov 5;218(4572):532–537. doi: 10.1126/science.7123255. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Kontoyiannidou-Ostrem V., Kortylewicz Z. P. Inhibition of angiotensin converting enzyme by phosphonic amides and phosphonic acids. Biochemistry. 1983 Apr 12;22(8):1990–1995. doi: 10.1021/bi00277a039. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- Kam C. M., Nishino N., Powers J. C. Inhibition of thermolysin and carboxypeptidase A by phosphoramidates. Biochemistry. 1979 Jul 10;18(14):3032–3038. doi: 10.1021/bi00581a019. [DOI] [PubMed] [Google Scholar]
- Kohen F., Hollander Z., Burd J. F., Boguslaski R. C. A steroid immunoassay based on antibody-enhanced hydrolysis of a steroid-umbelliferone conjugate. FEBS Lett. 1979 Apr 1;100(1):137–140. doi: 10.1016/0014-5793(79)81149-7. [DOI] [PubMed] [Google Scholar]
- Kohen F., Kim J. B., Barnard G., Lindner H. R. Antibody-enhanced hydrolysis of steroid esters. Biochim Biophys Acta. 1980 May 7;629(2):328–337. doi: 10.1016/0304-4165(80)90105-1. [DOI] [PubMed] [Google Scholar]
- Kohen F., Kim J. B., Lindner H. R., Eshhar Z., Green B. Monoclonal immunoglobulin G augments hydrolysis of an ester of the homologous hapten: an esterase-like activity of the antibody-containing site? FEBS Lett. 1980 Mar 10;111(2):427–431. doi: 10.1016/0014-5793(80)80842-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamden L. A., Bartlett P. A. Aminoalkylphosphonofluoridate derivatives: rapid and potentially selective inactivators of serine peptidases. Biochem Biophys Res Commun. 1983 May 16;112(3):1085–1090. doi: 10.1016/0006-291x(83)91729-1. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E. Enzymatic catalysis and transition-state theory. Science. 1973 Apr 15;180(4082):149–154. doi: 10.1126/science.180.4082.149. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Elder J. H. Molecular dissection of Rauscher virus gp70 by using monoclonal antibodies: localization of acquired sequences of related envelope gene recombinants. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4524–4528. doi: 10.1073/pnas.77.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Thorsett E. D., Harris E. E., Peterson E. R., Greenlee W. J., Patchett A. A., Ulm E. H., Vassil T. C. Phosphorus-containing inhibitors of angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2176–2180. doi: 10.1073/pnas.79.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Matthews B. W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J Mol Biol. 1977 Jul;114(1):119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]


