Abstract
Insufficient sleep and poor quality sleep are pervasive during adolescence and relate to impairments in cognitive control and increased risk taking. However, the neurobiology underlying the association between sleep and adolescent behavior remains elusive. In the current study, we examine how poor sleep quality relates to cognitive control and reward related brain function during risk taking. Forty-six adolescents participated in a functional magnetic imaging (fMRI) scan during which they completed a cognitive control and risk taking task. Behaviorally, adolescents who reported poorer sleep also exhibited greater risk-taking. This association was paralleled by less recruitment of the dorsolateral prefrontal cortex (DLPFC) during cognitive control, greater insula activation during reward processing, and reduced functional coupling between the DLPFC and affective regions including the insula and ventral striatum during reward processing. Collectively, these results suggest that poor sleep may exaggerate the normative imbalance between affective and cognitive control systems, leading to greater risk-taking in adolescents.
Keywords: Adolescence, Sleep, Risk taking, Cognitive control, fMRI
Introduction
Adolescence is a time of biological and social changes that greatly impact sleep and risk taking. Although good sleep is important at all stages of development, sleep may have particularly consequential effects on cognitive and affective functioning during adolescence, a developmental phase when insufficient and poor quality sleep is prevalent (Colrin and Baker, 2011; Dahl and Lewin, 2002). Poor sleep quality is related to a host of cognitive and emotional deficits, including a bias towards high risk behaviors, diminished attentional and behavioral control, and poor emotion regulation (Dahl, 1996; Harrison and Horne, 2000), making poor sleep a significant public health and developmental concern that may impact high risk behaviors during adolescence. Although neuroimaging research in adults has shown that sleep deprivation impacts brain function related to reward processing, risk-taking, and cognition (Chee et al., 2011; Gujar et al., 2011; Libedinsky et al., 2011; Venkatraman et al., 2011), only a few developmental studies have examined how sleep influences reward-related brain function in adolescents (Hasler et al., 2012; Holm et al., 2009) and none have examined how sleep impacts risk taking behavior, cognitive control, and related neural circuitry. Given the dramatic increase in risk taking behavior, coupled with the rise in sleep deprivation, it is important to understand the underlying neural mechanisms by which sleep increases risk taking in adolescence.
Sleep deprivation during adolescence occurs in tandem with normative developmental increases in risk-taking and poor decision making. For example, sleep-deprived adolescents and adolescents who report high levels of sleepiness show detriments in higher-level executive functioning, decreased cognitive modulation of drives, impulses, and emotions, and less effortful control of attention (Anderson et al., 2008; Beebe et al., 2008; Dahl, 1996). In terms of risky behavior, variability in weekend–weeknight sleep time and insufficient sleep (i.e., <8 h on school nights) are associated with tobacco, alcohol, and drug use, unsafe sex, poorer safety behaviors, and aggressive behaviors (McKnight-Eily et al., 2011; O'Brien and Mindell, 2005). Although the link between inadequate sleep, cognitive control, and risk taking in adolescents has been established, there is limited research indicating the neural mechanisms that may explain why sleep increases adolescent risk taking.
Dual system theories of adolescent neurodevelopment posit that risk taking increases during adolescence due to a competition between two neural systems — the affective system, which is involved in reward sensitivity, and the cognitive control system, which is involved in cognitive regulation (Somerville et al., 2010; Steinberg, 2010). Whereas reward sensitivity shows curvilinear developmental patterns, peaking in mid adolescence, impulse control gradually increases throughout adolescence, showing linear improvements into adulthood (Bunge et al., 2002; Galván et al., 2006; Geier et al., 2010). The heightened reward seeking and immature impulse control, coupled with poor sleep, may hinder appropriate evaluation of risk and bias youth towards risky decisions. Indeed, functional connectivity analyses in adults reveal that poor sleep not only impairs brain function in affective and cognitive control regions but also disrupts cross-talk between these neural networks (Gujar et al., 2011; Venkatraman et al., 2011; Yoo et al., 2007). This adult work linking sleep problems to impairments in affective and regulatory brain function highlights the importance of focusing on these neural networks, which are still maturing during adolescence. Inadequate sleep may amplify the neural imbalance present during adolescence by diminishing adolescents' ability to control their impulses while increasing their reactivity to rewarding stimuli. This exacerbated neural imbalance may push adolescents towards riskier decisions and hinder their ability to regulate their behaviors.
In the current study, we sought to examine the links between normative levels of sleep and brain function during risk taking in adolescence. We had three primary research questions. First, we examined whether poorer sleep quality was associated with cognitive control and risk taking. We predicted that poorer sleep quality would be associated with impairments in impulse control and greater risk taking behavior. Second, we examined whether poor sleep quality was associated with dampened activation in the lateral PFC during cognitive control and heightened activation in affective regions during risk taking, consistent with adult work showing that sleep deprivation is associated with amplified insula and ventral striatum reactivity in response to positive and rewarding stimuli (Gujar et al., 2011) and dampened PFC activation during cognitive control (Chuah, Venkatraman, Dinges, & Chee, 2006; Drummond et al., 1999). Second, we examined whether poorer sleep quality disrupts functional coupling between affective regions (e.g., insula and ventral striatum) and cognitive regulation (e.g., lateral prefrontal cortex) during risk taking. If poor sleep exacerbates the cortical–subcortical imbalance, poor sleep should be directly associated with a failure of prefrontal, top-down regulation during affective arousal.
Methods
Participants
Participants included forty-six adolescents from a larger sample of adolescents who participated in an fMRI scan during which they completed a risk taking task and a cognitive control task. The main effects for these tasks from the larger sample have been published previously (Telzer et al., 2013). Participants ranged in age from 14 to 16 years (Mage =15.23; 19 males, 27 females). Participants completed written consent and assent in accordance with UCLA's Institutional Review Board. Participants were not currently taking any medications and did not report being diagnosed with any mood or sleep disorder.
Questionnaire measures
Sleep quality
Subjective sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). Adolescents answered 19 questions describing their subjective sleep quality and sleep disturbances over the past 30 days. The 19 questions generated 7 clinically derived component scores: daytime dysfunction, sleep duration, sleep disturbances, sleep latency, sleep efficiency, use of sleep medications, and sleep quality. The 7 component scores were summed to obtain a global score ranging from 0 to 21, with higher scores indicating poorer sleep quality and scores less than 6 indicating good sleep quality as recommended by the scale developers (Buysse et al., 1989). Average sleep quality in the current study was 5.3, with substantial variation, ranging from 2 to 16.
Risk taking perceptions
A modified version of the Cognitive Appraisal of Risk Activities (CARE; Fromme et al., 1997) was used to assess evaluation of risks and perception of consequences. Participants provided ratings to 34 questions measuring diverse aspects of risk taking, including risky sexual behavior, heavy drinking, illicit drug use, aggressive and illegal behaviors, irresponsible academic/work behaviors, and high risk sports. Adolescents provide three ratings for each question on a scale from 1 to 7 (1=not likely at all to 7 = extremely likely): (1) the likelihood of engaging in this activity in the next 6 months; (2) the likelihood of a negative consequence and (3) the likelihood of a positive consequence. The mean likelihood for each subscale was calculated, resulting in 3 measures, Risk Taking Likelihood, which measures the extent to which adolescents perceive they will engage in risk taking in the next 6 months, Risk Taking Positive Consequences, which measures the extent to which adolescents perceive risk taking will result in positive consequences, and Risk Taking Negative Consequences, which measures the extent to which adolescents perceive risk taking will result in negative consequences. These measures have been related to brain function during reward processing in adolescents (Galvan et al., 2007).
Decision making
The Flinders Adolescent Decision Making Questionnaire (Mann et al., 1989) was used to examine adolescents' decision-making strategies. Adolescents responded to 30 questions using a four-point scale (1 = “not at all true of me” to 4 = “almost always true of me”). There are 5 subscales that measure competent and maladaptive decision-making styles: (1) the decision making self-esteem subscale measures adolescents' confidence in making decisions (e.g., “The decisions I make turn out well”); (2) the vigilance subscale assesses the reported use of considering goals, generating options, gathering facts, and implementing the decision (e.g., “I try to be clear about my objectives before choosing”); (3) the panic subscale measures self-reported tendency towards hasty and impulsive choices (e.g., “When I get upset by having to make a decision, I choose on the spur of the moment”), (4) the avoidance subscale measures tendencies towards decision avoidance, including leaving decisions to others, procrastinating, shifting responsibilities, or rationalizing (e.g., “I prefer to leave decisions to others”); and (5) the complacency subscale measures tendencies to apathy, noninvolvement, and unconflicting change or unconflicting adherence when confronted with a decision (e.g., “I tend to drift into decisions without thinking about them”). High scores on the self-esteem and vigilance subscales represent competent decision making, whereas high scores on the panic, avoidance, and complacency subscale represent maladaptive and poor decision making.
fMRI paradigms
Cognitive control task
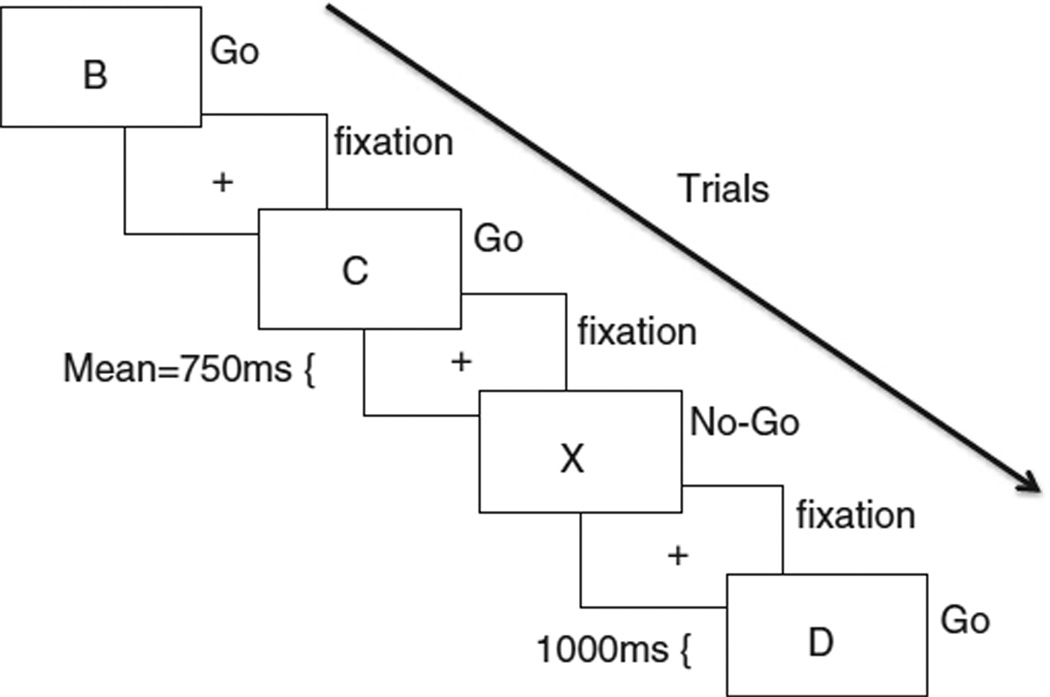
Participants completed a standard Go–No-Go (GNG) task to target cognitive control related brain function. Participants were presented with a series of rapid trials, each displaying a single letter, and were instructed to respond with a button press as quickly as possible to all letters except for X (see Fig. 1). The X occurred on 25% of trials. Thus, participants developed a pre-potent response to press (go) upon stimulus onset, and must inhibit the go response on X trials (no-go). Response inhibition was operationalized as successful no-go trials (overriding the pre-potent “go” response) compared to go trials. Participants completed 5 blocks during one functional run. Each block contained 10 no-go trials and 30 go trials. Each trial was presented for 1000 ms with a fixation between each trial that was jittered according to a random gamma distribution (M = 750 ms). Each block (40 trials total) lasted 70 s, and each block was separated by a twelve-second rest period.
Fig. 1.
The Go-No-go Task. Participants are shown a letter, presented rapidly with a jittered fixation between each letter trial. Participants push a button as quickly as possible to all letters (Go) except for X (No-go), during which they must withhold the button response.
Risk taking task
To examine neural sensitivity to risk, participants completed the Balloon Analogue Risk Task (BART; Lejuez et al., 2002). Prior research has used the BART in adults to examine risk taking and sleep and has found an association between sleep deprivation and risk taking on the task (Killgore et al., 2011). Moreover, behavioral performance on the BART correlates with real-life risk behaviors such as adolescent smoking, sexual promiscuity, addiction, and drug use (Bornovalova et al., 2009; Lejuez et al., 2003) suggesting that this task provides a scanner-compatible proxy for measuring real-world risky behaviors.
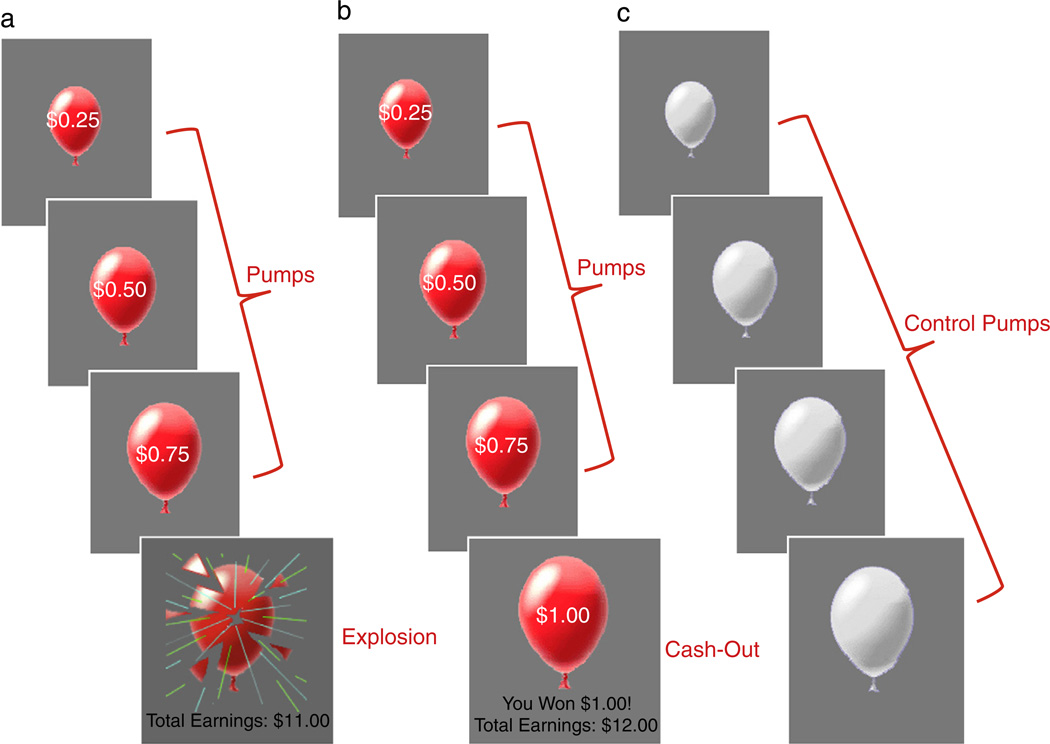
On each trial of the task, participants were presented with either a computerized red-colored balloon or a white-colored balloon. The red balloons represented “risk-taking” trials. When presented with a red balloon, participants had to choose between pumping up the balloon or not pumping up the balloon. With each pump, there was the possibility that the balloon would either grow larger or explode. The larger the balloon was inflated, the greater the monetary reward but the higher the probability of explosion. Participants were instructed to press one of two buttons to either pump the balloon or to cash-out. Each trial began with the presentation of a balloon and ended when the balloon either exploded or the participant chose to cash out (see Fig. 2). The task was self-paced, so the balloon stayed on the screen until the participant made a decision. For each pump on which the balloon was successfully pumped, the participant received a payment (25 cents) and could stop pumping the balloon at any point to keep the accumulated payoff. If the balloon exploded before cashing out, the participant received no payoff for that trial. The number of pumps before explosion was varied probabilistically according to a Poisson distribution. This pattern models the unpredictable rewards and punishments that characterize real-world risky behaviors. As pumping progresses during a trial, explosion probability increases exponentially. The explosion point of each balloon was drawn from a uniform distribution from 1 to 12 pumps. In addition to the red balloons, 25% of the balloons were white and were not associated with a reward or possible explosion. White balloons provided a control for the visual and motor aspects of pumping. Participants were instructed to pump the white balloons until they disappeared. White balloons did not explode but inflated according to the same distribution as the red balloons. The task was self-paced, such that participants could make decisions to pump or cash out at their own pace. After each pump, the balloon image disappeared (1–3 s, variable duration) until the outcome was displayed: a larger balloon or an exploded one. At the end of each trial, the screen was blank for a varying duration (1–12 s, average 4 s). The task was performed during a 9 minute run. Participants received their total earnings at the end of the task.
Fig. 2.
The Balloon Analogue Risk Task. Examples of the three trial types: a) risk-taking trial with an explosion outcome, b) risk-taking trial with a cash-out outcome, and c) control trial in which no money was earned or loss. The trial types used in analyses are depicted in red writing. Note. Pumps on explosion trials and cash-out trials were modeled separately.
fMRI data acquisition and analysis
fMRI data acquisition
Imaging data were collected using a 3 T Siemens Trio MRI scanner. The tasks were presented on a computer screen through scanner-compatible goggles. The BART consisted of 270 functional T2*-weighted echoplanar images (EPI) and the GNG task consisted of 200 images [slice thickness, 4 mm; 34 slices; TR=2 s; TE=30 ms; flip angle=90°; ma-trix=64×64; FOV=200 mm; voxel size 3×3×4 mm3]. A T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan and magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan were acquired for registration purposes (TR: 2.3; TE: 2.1; FOV: 256; matrix: 192×192; sagittal plane; slice thickness: 1 mm; 160 slices). The orientation for the MBW and EPI scans was oblique axial to maximize brain coverage.
fMRI data preprocessing and analysis
Analyses were performed using FSL 4.1.6 (www.fmrib.ox.ac.uk/fsl). All images were skull-stripped using FSL BET. The images were realigned to compensate for small head movements (Jenkinson et al., 2002). No participants exceeded >2 mm in movement. Data were smoothed using a 5-mm FWHM Gaussian kernel to increase the signal-to-noise ratio, and filtered in the temporal domain using a nonlinear high-pass filter (100-s cutoff). EPI images were registered to the MBW, then to the MPRAGE, and finally into standard MNI space (MNI152, T1 2 mm) using linear registration with FSL FLIRT.
For the GNG task, one general linear model (GLM) was defined, which included multiple regressors for each event type: (1) successful go trials (i.e., pushing button on go trials), (2) successful no-go trials (i.e., withholding button press on no-go trials), (3) and false alarms (i.e., pushing button on no-go trials). Events were modeled with a 1 s duration. The rest periods and jittered inter-trial intervals were not explicitly modeled and therefore served as an implicit baseline. Individual-level models were defined, with the contrast of interest being No-go>Go trials.
For the BART, one GLM was defined, which included multiple regressors for each event type: (1) pumps, (2) cash-outs, (3) explosions, and (4) control pumps (i.e., pumps to white balloons). For the pumps, we analyzed the adjusted pumps, which represent the number of pumps on balloons that did not explode. This is preferable to examining pumps on balloons that did explode, because the number of pumps is necessarily constrained on balloons that explode (Lejuez et al., 2002). Pumps, cash outs, explosions, and control pumps were modeled with a parametric regressor that tested for the linear relationship between brain activation and the magnitude of pumps, reward, or loss. We used pump number as a parametric modulator, with each pump in a trial assigned a weight that increased linearly across pumps within a trial. On cash-out trials and explosions, this number represented how many pumps occurred before the cash-out or explosion. The number of pumps was demeaned by subtracting the mean number of pumps from each pump number within the trial. Because the task was self-paced, the duration of each trial represented the RT for that trial. Null events, consisting of the jittered inter-trial intervals, were not explicitly modeled and therefore constituted an implicit baseline. Individual-level models were defined, with the following contrasts: pumps>control pumps, cash-outs>baseline, and explosions>baseline. For both tasks, temporal derivatives and motion parameters were included as covariates of no interest for all regressors.
The FSL FEAT package was used for statistical analysis. Regressors of interest were created using a stick function of the event duration at the onset time of each trial with a canonical (double-gamma) HRF. A group-level analysis was performed using the FMRIB Local Analysis of Mixed Effects module in FSL (Beckmann et al., 2003). Sleep quality was demeaned and entered as a regressor in whole brain regression analyses to examine activation during the BART and GNG tasks.
We conducted psychophysiological interaction (PPI) analyses (Friston et al., 1997) to examine whether functional coupling between affective regions and cognitive control regions was disrupted in adolescents with poorer quality sleep. We focused the PPI analyses on the BART task, given that both affective and cognitive control regions are involved during the task (Schonberg et al., 2012), whereas the GNG task targets primarily cognitive control-related brain function. The seed region for the PPI analysis was defined as the area in the DLPFC that correlated with sleep quality during the GNG task. The seed region was first transformed into the functional space of each participant using FLIRT in FSL, and the deconvolved time-series was extracted for each ROI. The first-level design matrix of each participant consisted of three regressors: 1) the time course of the seed region, 2) the psychological variable, and 3) their product. The physiological regressor comprised the time-series for the ventral striatum or insula. The psychological (task condition) variable modeled the parametric regressor to pumps and cash-outs, convolved with a double-gamma hemodynamic response function (HRF). A third regressor modeled the interaction of the psychological regressor and the physiological regressor, with the psychological regressor zero-centered about the minimum and maximum values and the physiological regressor demeaned. This interaction term identified regions that covaried in a task-dependent manner with the seed region. The remaining task conditions were also included as regressors of no interest, convolved with a double-gamma HRF. Significant, group-level clusters were obtained using the same approach as the whole-brain analyses indicated above, whereby sleep quality was demeaned and entered as a regressor in whole-brain regression analyses.
Thresholded Z statistic images were prepared to show clusters determined by a corrected, cluster-forming threshold of Z>2.3 and an extent threshold of p<.05 familywise error corrected using the theory of Gaussian random fields (Poline et al., 1997). Outliers were de-weighted in the multi-subject statistics using mixture modeling (Woolrich, 2008). For visualization, statistical maps of all analyses were projected onto a study-specific average brain of the participants. For descriptive purposes only, the percent signal change from significant clusters was extracted and displayed in scatterplots.
Results
Behavioral results
Behavioral performance on the Go–No-go
On average, participants made false alarms on 19.58% (SD=14.64, range=2–66%) of the no-go trials, and correctly responded to 98.8% of go trials (SD=.03, range=86%–100%). Participants' mean reaction time was significantly faster to false alarms (M=355 ms, SD=70 ms) than to correct go trials (M=427 ms, SD=48 ms), t(45)=7.67, p<.001.
Behavioral performance on the BART
Participants pumped each balloon 3.82 (SD=1.07) pumps on average. Participants exploded 33.58% (SD=9.8) and successfully cashed out on 64.53% (SD=10.52) of balloons. Participants took significantly longer to cash out (M=90 ms, SD=32 ms) than to inflate balloons (M=74 ms, SD=26 ms, t(45)=3.96, p<.001), and earned a total of $15.82 (SD=4.01) on average (range=$8.25–$26.75).
Sleep and decision making
Regression analyses were conducted to examine whether adolescents' sleep quality related to their self-reported decision making skills, controlling for gender and age. Adolescents who reported poorer sleep quality reported more decision making complacency (β=.54, p<.001) and lower decision making self-esteem (β=−.30, p<.05). Next we examined how sleep quality was related to behavioral performance on the GNG task. We examined associations with false alarm percentage, mean response times to go trials, and mean response times to false alarms. Poorer sleep quality was marginally associated with slower reaction time on go trials (β=.26, p=.09).
Sleep and risk taking
To examine how adolescents' overall sleep quality related to their self-reported evaluation of risk taking, we ran regression analyses, controlling for gender and age for each subscale of the CARE. Adolescents who reported greater sleep problems reported a greater likelihood of engaging in risk taking behaviors (β=.40, p<.01) and a greater perception of positive consequences for engaging in risk taking behaviors (β= .44, p<.005). Next, we examined how sleep quality was associated with total adjusted pumps, percent explosions, and percent cashed out on the BART. Adolescents who reported poorer sleep quality had greater mean adjusted pumps (β=.40, p<.01). In other words, poorer sleep was associated with inflating the balloons more, which is an index of riskier behavior and an orientation towards greater rewards.
fMRI results
Main effects on the GNG
In whole brain analyses,we examined neural activation to successful response inhibitions compared to go trials (No-go>Go).Similar to findings from a larger sample from this study (Telzer et al., 2013), successful response inhibitions were associated with activation in brain regions involved in cognitive control, including the bilateral DLPFC, and the dorsal anterior cingulate cortex dACC, as well as the bilateral anterior insula, inferior parietal lobule, visual cortex, precuneus, and cerebellum (Table 1; Supplementary Fig. 1).
Table 1.
Neural regions activated during (a) response inhibition and (b) risk taking.
| Contrast | Anatomical region |
x | y | z | Max Z |
k |
|---|---|---|---|---|---|---|
| (a) Response inhibition, No-go > Go |
R anterior insula | 28 | 20 | −10 | 5.91 | 11,142d |
| R DLPFC | 36 | 48 | 22 | 3.89 | d | |
| Dacc | 6 | 30 | 28 | 4.37 | d | |
| R inferior parietal Cortex |
58 | −44 | 34 | 6.60 | 7544 | |
| L inferior parietal Cortex |
−60 | −38 | 28 | 6.18 | 3384 | |
| L anterior insula | −34 | 18 | 6 | 5.17 | 1265 | |
| L DLPFC | −32 | 52 | 16 | 4.02 | 944 | |
| R visual cortex | 28 | −94 | −6 | 6.31 | 793 | |
| R precuneus | 10 | −68 | 40 | 4.67 | 623 | |
| L visual cortex | −30 | −96 | −6 | 5.23 | 609 | |
| L cerebellum | −34 | −60 | −28 | 3.84 | 536 | |
| (b) Risk taking, pumps > control pumps |
R anterior insula | 38 | 18 | 2 | 7.64 | 36,995a |
| L anterior insula | −36 | 18 | 6 | 6.20 | a | |
| dACC | −2 | 20 | 38 | 7.41 | a | |
| R VS | 19 | 8 | −6 | 4.50 | a | |
| L VS | −14 | 6 | −4 | 4.07 | a | |
| R DS | 16 | 4 | 14 | 4.06 | a | |
| L DS | −18 | 6 | 12 | 4.02 | a | |
| R DLPFC | 30 | 52 | 28 | 5.29 | a | |
| L DLPFC | −30 | 48 | 20 | 4.81 | a | |
| R cerebellum | 26 | −50 | −26 | 5.52 | a | |
| L cerebellum | −36 | −56 | −34 | 5.69 | a | |
| Ventral midbrain | 2 | −18 | −16 | 5.48 | a | |
| R inferior parietal cortex |
42 | −48 | 40 | 4.81 | 2195 |
Note. L and R refer to left and right hemispheres; x y and z refer to MNI coordinates; Max Z refers to the z-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. Anatomical regions that share functional clusters are denoted with the same superscript letter. All regions are listed at cluster-forming threshold of Z>2.3 and an extent threshold of p<.05 corrected using the theory of Gaussian random fields. The following abbreviations were used for the specific brain regions: dACC=dorsal anterior cingulate cortex; VS=ventral striatum; DS=dorsal striatum; DLPFC=dorsolateral prefrontal cortex.
Main effects on the BART
In whole brain analyses, we examined neural activation to pumps, cash outs, and explosions. Similar to findings previously reported from a larger sample from this study (Telzer et al., 2013), the contrast used to examine activation associated with increasing pumps (pumps>control pumps) revealed activation in the bilateral ventral striatum, bilateral caudate nucleus, ventral midbrain, bilateral anterior insula, bilateral DLPFC, dACC, bilateral temporal parietal junction (TPJ), and the cerebellum (Table 1; Supplementary Fig. 2). No brain regions were significantly activated to cash outs>baseline or explosions>baseline.
Correlations between sleep and cognitive control
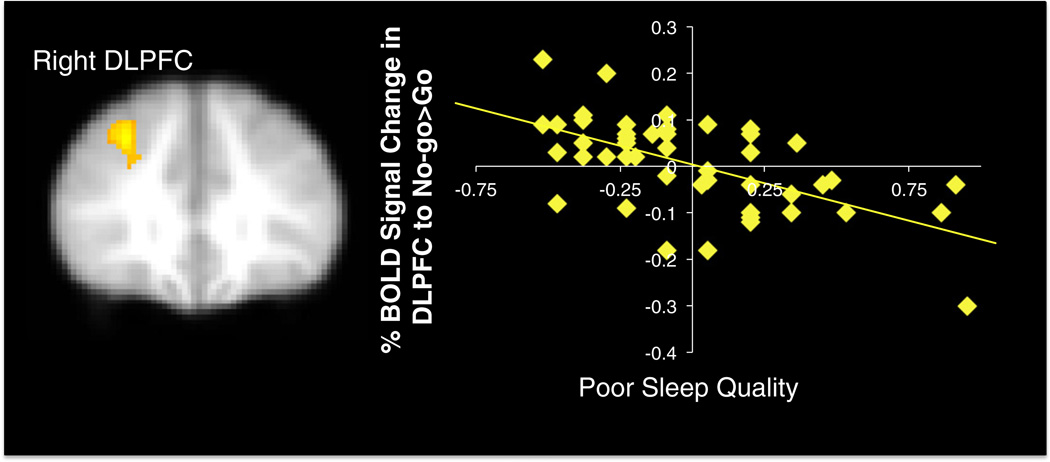
Next, we examined how sleep related to neural activation during response inhibition. In whole brain regression analyses, poorer sleep quality was correlated with decreased activation in the right dorsolateral prefrontal cortex (DLPFC) during No-go>Go trials (see Fig. 3). We examined how this decreased DLPFC activation during behavioral inhibitions relates to adolescents' behavioral performance and self-report behaviors. We extracted the percent BOLD signal change in the DLPFC to No-go>Go trials from the cluster that correlated with sleep and regressed it onto each behavioral measure separately in SPSS. Results indicate that decreased BOLD response in the right DLPFC during response inhibitions was significantly associated with more pumps on the BART (β=.58, p<.001) more decision making complacency (β=−.50, p<.001), and less decision making vigilance (β=.43, p<.005).
Fig. 3.
Percent signal change in right DLPFC to No-go>Go trials that correlated negatively with sleep quality. xyz=30 34 38, Z=3.81, k=441, p<.05 corrected. Note. Right=left. Greater values on the x-axis represent poorer sleep quality. Scatterplots are provided for descriptive purposes. All reported statistics are obtained from independent tests in FSL regressing sleep on brain activation.
Correlations between sleep and risk taking
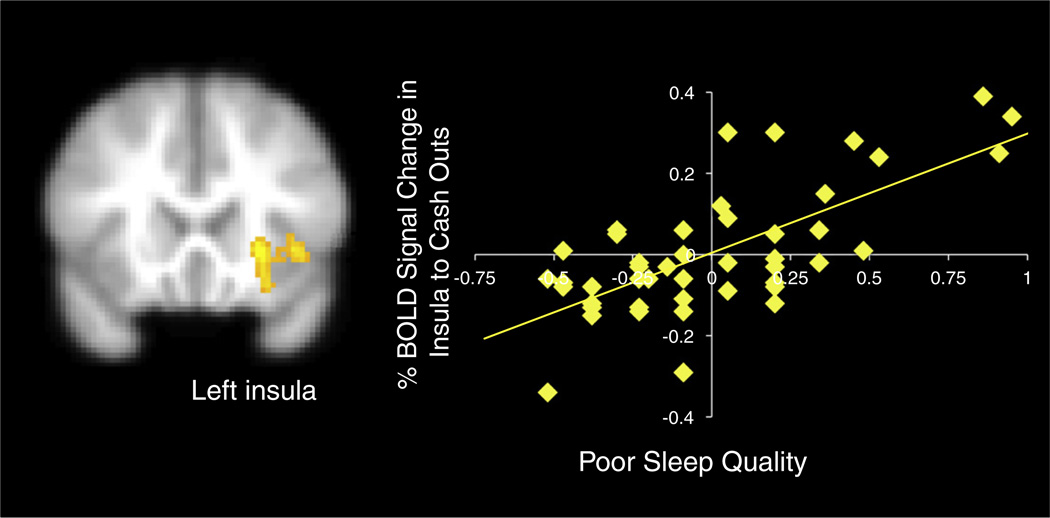
Next, we examined how sleep quality was related to neural activation during risk taking and reward processing. In whole brain regression analyses, we correlated sleep quality with neural activation during pumps>control pumps, cash outs>baseline, and explosions>baseline. We found a significant association during the cash out trials, such that when adolescents gained increasing monetary rewards, poorer sleep quality was associated with increased activation in the left insula (see Fig. 4). Sleep quality was not associated with neural activation during pumps>control pumps or explosions>baseline. Next, we examined how activation in the insula to cash outs relates to adolescents' behavioral performance and self-report behaviors. We extracted the percent BOLD signal change in the insula to cash out trials from the cluster that correlated with sleep and regressed it onto each behavioral measure separately in SPSS. Results indicate that increased BOLD response in the left insula during cash outs was significantly associated with greater pumps on the BART (β=.41, p<.005), greater risk taking likelihood (β=.37, p<.05), greater positive consequences for risk taking (β=.32, p<.05), more decision making complacency (β=.55, p<.001), and more decision making panic (β=.29, p<.05). Finally, percent BOLD response in the insula and the DLPFC was negatively correlated, such that reduced DLPFC activation during response inhibition was related to enhanced activation in the insula during cash outs (β=−.46, p<.001), suggesting a possible cortical–subcortical dysregulation.
Fig. 4.
Percent signal change in the insula during the BART to cash-outs that correlated with poorer sleep quality. xyz=−24 20 −2, Z=3.57, k=711, p<.05, corrected. Note. Right=left. Greater values on the x-axis represent poorer sleep quality. Scatterplots are provided for descriptive purposes. All reported statistics are obtained from independent tests in FSL regressing sleep on brain activation.
Mediating sleep quality and insula activation with DLPFC activation
Our next set of analyses examined whether adolescents with poorer sleep quality show heightened insula activation during the BART due to reduced DLPFC activation during cognitive control. In other words, does impaired cognitive control account for the association between poor sleep and heightened neural arousal to rewards? Mediation analyses were conducted in SPSS by regressing sleep quality on percent BOLD signal change in the insula to cash outs, and entering percent BOLD signal change in the DLPFC during No-go>Go trials as the mediator. As shown in Table 2, the original effect (i.e., total effect) of poor sleep quality on insula activation is reduced and becomes non-significant when DLPFC activation is entered into the model (i.e., direct effect). To test for the significance of the indirect effect, we used a Sobel test. Reduced DLPFC activation accounted for 53.86% of the original effect of poor sleep quality on insula activation. We conducted post hoc tests of the significance of the mediation analyses using MacKinnon et al.'s (2007) PRODCLIN program, which computes asymmetric confidence limits based on the distribution of products. As shown in Table 2, the confidence interval of the indirect effect does not include zero, consistent with a statistically significant mediation.
Table 2.
Mediating poor sleep quality and insula activation with DLPFC activation.
| Total effect (C) |
Direct effect (C′) |
Indirect effect of DLPFC |
||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | Z | % of total effect | 95% CI |
| .14** | .05 | .06 | .05 | .07* | .02 | 4.19*** | 53.86% | .02–.14 |
All analyses control for gender and age. B refers to the unstandardized coefficient. C refers to total effect of sleep quality on insula activation. C′ refers to the direct effect of sleep quality on insula activation, with DLPFC in the model. Indirect effect refers to the effects of sleep quality on insula activation through DLPFC activation. Z refers to the tests of the statistical significance of the indirect effect, and the percentage of total effect refers to the proportion of the total effect that was accounted for by the indirect effect. 95% CI represents the asymmetric confidence interval based on the distribution of the product calculated using PRODCLIN.
p<.05.
p<.01.
p<.001.
Psychophysiological interaction analyses
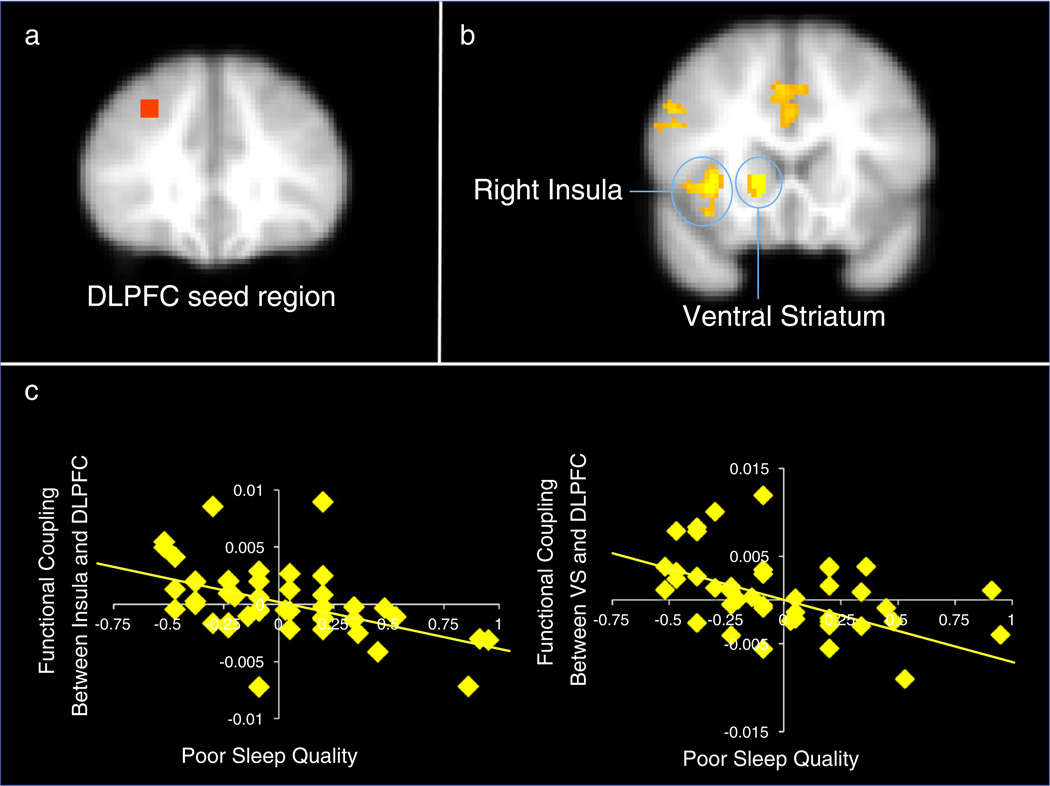
Our final set of analyses examined whether poor sleep quality was associated with altered functional coupling between affective regions and the lateral PFC. Given that sleep may alter DLPFC activation during cognitive control (as reported in the “Correlations between sleep and cognitive control” section above), we sought to examine how this specific cluster of activation may relate to affective processing during risk taking. Thus, we defined the seed region for the PPI analyses based on the analysis in the “Correlations between sleep and cognitive control” section, therefore representing an independent seed for the risk taking task. The seed was defined as a 6 mm sphere around the peak voxel of activation (xyz=30 34 38; see Fig. 5a).
Fig. 5.
(a) the seed region was defined as a 6 mm sphere centered in the DLPFC from the cluster that correlated with sleep quality during the GNG. (b) Psychophysiological interaction analyses reveal less functional coupling between the DLPFC and neural regions for adolescents with poorer sleep quality. (c) Percent signal change in the insula and ventral striatum that showed decreased functional coupling with the DLPFC that correlated negatively with sleep quality. Note, greater values on the x-axis represent poorer sleep quality. Note. Right=left. Scatterplots are provided for descriptive purposes. All reported statistics are obtained from independent tests in FSL regressing sleep on brain activation.
We ran psychophysiological interaction analyses regressing sleep quality on neural activation during the BART. As shown in Figs. 5b–c, during cash-outs, poor sleep quality was related to reduced functional coupling between the DLPFC and the right insula (xyz=36 20 0, Z=3.78, p<.05) and the ventral striatum (xyz=14 18 −2, Z=4.21, p<.05). The DLPFC was also associated with reduced functional coupling with the anterior cingulate cortex (xyz=0 12 40, Z=2.89, p<.05). Sleep quality was not associated with enhanced functional coupling between the DLPFC and other neural regions during cash-outs.
Next, we examined how the extent of functional coupling relates to adolescents' behavioral performance and self-reports. Reduced functional coupling between the insula and DLPFC was associated with greater pumps on the BART (β=−.49, p<.001), more decision making complacency (β=−.34, p<.05), less decision making self esteem (β=.43, p<.005), and less decision making vigilance (β=.41, p=.005). Reduced functional coupling between the VS and DLPFC was associated with greater pumps on the BART (β=−.36, p<.05), greater reported likelihood of engaging in risk taking (β=−.37, p<.05), and more decision making complacency (β=−.29, p=.05).
Discussion
Insufficient sleep and poor quality sleep are pervasive during adolescence (Carskadon, 2011; Dahl and Lewin, 2002) and relate to impairments in cognitive regulation and increases in health compromising behaviors such as substance use (Hasler et al., 2012; Kenney et al., 2012; McKnight-Eily et al., 2011; O'Brien and Mindell, 2005). It is therefore important to understand the effects of sleep on risk taking and brain function during this developmental window in order to better understand the underlying mechanisms that may push adolescents towards suboptimal decisions. Our findings suggest that the normative imbalance between affective and cognitive control systems may be exaggerated by poor sleep, such that adolescents show less DLPFC activation during cognitive control, greater insula activation during reward processing, and reduced functional coupling between the DLPFC and affective regions. Each of these neural activations was paralleled by poorer self-reported decision making skills and greater risk taking and reward sensitivity. Thus, adolescents with poorer sleep quality may have both a greater orientation towards risk and compromised decision making abilities.
Behaviorally, we found that poorer sleep quality was associated with more apathy and less self-esteem when making decisions as well as marginally slower reaction time during go trials on the GNG. These associations suggest that adolescents with poorer sleep may be more apathetic, less confident, and take less care during decision making thus demonstrating lower motivation to engage in the cognitive control task. Although we did not find differences in false alarm rates as a function of sleep, this may be due to adolescents with poorer sleep taking more time on go trials and thus having to inhibit less on the no-go trials. In addition, we found that poor sleep quality was associated with greater self-reported likelihood of engaging in risk taking, greater positive consequences for risky behaviors, and riskier behavior on the BART. These behavioral findings suggest that adolescents who obtain poorer quality sleep are more oriented towards rewards, which may account for their riskier behavior.
At the neural level, poorer sleep quality was related to dampened activation in the DLPFC during response inhibition. The lateral PFC is involved in cognitive control, goal directed behavior, and impulse control, and is one of the last brain regions to develop both structurally and functionally (Gogtay et al., 2004). Prior work has found that adults recruit the lateral PFC to a greater extent than adolescents during risk taking (Chein et al., 2011; Eshel et al., 2007), and children show decreased activation in the lateral PFC compared to adults when matched on performance during a cognitive control task (Bunge et al., 2002). Therefore, dampened DLPFC activation following poor sleep may be indicative of the relatively immature use of this region. In addition, researchers have recently suggested that rather than being immature during adolescence, cognitive control capacities may be highly flexible and depend on the social and motivational context (Crone and Dahl, 2012). Thus, adolescents with poorer sleep quality may demonstrate decreased motivation to engage in cognitive control and therefore recruit the DLPFC to a lesser extent during cognitive control. Indeed, adolescents with poorer sleep were marginally slower to react on go trials during the cognitive control task despite instructions to press the button as fast as they can. Moreover, the dampened DLPFC activation during response inhibition was associated with greater decision-making complacency (i.e., more apathy and less care in decision-making) and less decision making vigilance (i.e., not taking care when making decisions), suggesting that the dampened DLPFC response may represent a decreased motivation to put effort into decisions.
During risk taking, adolescents who reported poorer sleep quality showed greater activation in the insula during cash out trials as the reward increased, findings that are consistent with research on sleep deprived adults who show hyperactivation in the insula when processing positive stimuli (Gujar et al., 2011). Given that we performed a parametric analysis examining how the brain responds to increasing monetary rewards, the insula appears to be tracking the amount of reward during the BART. Indeed, the insula is a brain region involved in tracking risk in the environment (Singer et al., 2009) and has shown heightened activation among adults during the BART (Schonberg et al., 2012). Moreover, the insula integrates emotional information to and from limbic and cortical areas and is involved in the representation of interoceptive responses to emotionally salient and rewarding stimuli, such as drug craving, urgency, and impulsivity (Bonson et al., 2002; Naqvi and Bechara, 2009; Villafuerte et al., 2011). The insula also plays a role in reactivity to and encoding of positive stimuli, supporting motivated behaviors (Camara et al., 2008; Gujar et al., 2011). Together, these data highlight the role of the insula in craving and reward processing. Indeed, we found that the insula response was associated with the increased likelihood of engaging in risk taking, greater positive consequences for risk taking, and riskier behavior on the BART. Thus, sleep may increase the relative salience of rewards pushing adolescents towards riskier behavior.
Interestingly, we did not find that sleep quality was associated with ventral striatum activation during reward processing even though studies of sleep deprived adults have shown increased striatal reactivity during reward receipt or when observing pleasant images (Gujar et al., 2011; Venkatraman et al., 2007, 2011), and studies of adolescents have shown irregular sleep to relate to reduced striatal activation in anticipation of rewards (Hasler et al., 2012; Holm et al., 2009). Instead, we found that poor sleep among our adolescent sample was associated with decreased functional coupling between the ventral striatum and the DLPFC. Therefore, adolescents showed similar levels of ventral striatum activation when receiving monetary rewards, but those with poorer sleep quality were evidencing less coupling with the DLPFC, a neural region involved in cognitive control. These findings are consistent with adult work showing that sleep deprivation is related to reduced functional connectivity between affective and regulatory regions (Gujar et al., 2011; Yoo et al., 2007). Thus, trials resulting in greater rewards and heightened striatal reactivity are associated with less PFC activation in adolescents with poorer sleep. Poor sleep quality was also associated with reduced functional coupling between the DLPFC and the insula. This reduced PFC coupling may result in an inability to refrain from riskier behavior during high reward trials. Indeed, we found that the extent of decreased coupling between the DLPFC and the insula and ventral striatum were paralleled by increased risk taking behavior and poorer decision making skills. Thus, adolescents with poorer sleep may engage in more risk taking because of a failure of prefrontal, top-down regulation during affective arousal. These findings are consistent with the dual system model of neural development, which suggests that risk taking arises, in part, due to a cortical–subcortical imbalance, in which a relatively immature PFC is less effective at regulating a sensitive and more rapidly developing ventral striatum (Somerville et al., 2010; Steinberg, 2010).
Our findings suggest that sleep is associated with greater arousal to rewards and a potential lack of motivation to engage in cognitive control. The interaction between poor sleep, heightened arousal, and dampened cognitive control may create a negative spiral of events whereby sleep increases arousal and decreases regulation which then pushes adolescents towards more arousing stimuli and thereby are less likely to obtain sleep at night. Our study cannot delineate the direction of the effects. Perhaps adolescents who already have an exacerbated neural imbalance obtain poor sleep because they are more oriented towards rewards and less likely to engage in cognitive control and therefore stay up late to engage in late-night arousing and rewarding activities. Future research should use experimental designs and longitudinal data to determine whether sleep itself heightens neural sensitivity to risk taking or whether those who are more sensitive to risk taking obtain poorer sleep. In addition, future research should examine how sleep impacts risk-related behavior and neural functioning across development. We cannot be certain that our findings are specific to adolescence, or whether similar effects would be found at other developmental periods. There is some initial evidence that sleep and reward-related brain function differ in early versus late pubertal adolescents (Hasler et al., 2012). Therefore, longitudinal research that maps changes in sleep and risk taking is essential.
It is striking that normative levels of poor sleep quality among a healthy sample of adolescents are related to risk-related behavior and brain function. This highlights how impactful sleep can be during adolescence and how likely it is that sleep affects a large population of adolescents. Most prior work has examined more extreme levels of sleep problems by using sleep deprivation in a sleep lab or by examining sleep disorders (Beebe et al., 2009; Dagys et al., 2012). Altered brain function following high levels of sleep deprivation or among adolescents with sleep disorders may not translate to less extreme forms of sleep problems. Because insufficient sleep is so pervasive during adolescence, it is important to capture variability along this normative continuum. Our findings underscore how important sleep is during adolescence, and that even relatively small, normative levels of poor sleep can disrupt brain function and make adolescents even more vulnerable to suboptimal decision making.
Supplementary Material
Acknowledgments
Support for this study was provided by the NICHD (R01HD057164-S and R01HD057164; Fuligni), a UCLA Center for Culture, Brain and Development Research Grant (Fuligni and Galvan), an NSF Doctoral Dissertation Improvement Grant (Telzer), an SRCD Dissertation Fund Award (Telzer), an APF and COGDOP Graduate Research Grant (Telzer), and a University of California Institute for Mexico and the United States Dissertation Research Grant (Telzer). Preparation of this manuscript was supported in part by a National Research Service Award Graduate Fellowship (Telzer).
Footnotes
The authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2013.01.025.
References
- Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2008;123:e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith S. General multilevel linear modeling for group analysis in fMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Fallone G, Godiwala N, et al. Feasibility and behavioral effects of an at-home multinight sleep restriction protocol for adolescents. J. Child Psychol Psychiatry. 2008;49:915–923. doi: 10.1111/j.1469-7610.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Beebe D, Difrancesco M, Tlustos S, McNally K, Holland S. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav. Brain Funct. 2009;5:9–13. doi: 10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Cashman-Rolls A, O'Donnell JM, Ettinger K, Richards JB, Dewit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacol. Biochem. Behav. 2009;34:685–692. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Münte TF. Functional connectivity of reward processing in the brain. Front. Hum. Neurosci. 2008;2:1–19. doi: 10.3389/neuro.09.019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon M. Sleep in adolescents: the perfect storm. Pediatr. Clin. N. Am. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M, Goh G, Namburi P, Parimal S, Seidl K, Kastner S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. NeuroImage. 2011;58:595–604. doi: 10.1016/j.neuroimage.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev. Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YML, Venkatraman V, Dinges DF, Chee MWL. The neural basis on inter individual variability in inhibitory efficiency after sleep deprivation. J. Neurosci. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrin IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol. Rev. 2011;21:5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dagys N, McGlinchey E, Talbot L, Kaplan K, Dahl R, Harvey A. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J. Child Psychol. Psychiatry. 2012;53:660–667. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R. The regulation of sleep and arousal: development and psychopathology. Dev. Psychopathol. 1996;8:3–27. [Google Scholar]
- Dahl R, Lewin D. Pathways to adolescent health sleep regulation and behavior. J. Adolesc. Heal. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. NeuroReport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fromme K, Katz E, Rivet K. Outcome expectancies and risk-taking behavior. Cogn. Ther. Res. 1997;21:421–442. [Google Scholar]
- Galván A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo S, Hu P, Walker M. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J. Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J. Exp. Psychol. Appl. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Holm SM, Jakubcak JL, Silk JS, Ryan ND, Phillips ML, Dahl RE, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol. Psychol. 2012;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, Forbes E, Ryan N, Phillips M, Tarr J, Dahl R. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J. Adolesc. Heal. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kenney S, LaBrie J, Hummer J, Pham A. Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addict. Behav. 2012;37:507–512. doi: 10.1016/j.addbeh.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W, Kamimori G, Balkin T. Caffeine protects against increased risk-taking propensity during severe sleep deprivation. J. Sleep Res. 2011;20:395–403. doi: 10.1111/j.1365-2869.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavior measure of risk taking: the Balloon Analogue Risk Task BART. J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2003;26:320–345. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Libedinsky C, Smith D, Teng C, Namburi P, Chen V, Huettel SA, et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front. Behav. Neurosci. 2011;5:70–78. doi: 10.3389/fnbeh.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav. Res. Methods. 2007;39:1–12. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann L, Harmoni R, Power C. Adolescent decision-making: the development of competence. J. Adolesc. 1989;12:265–278. doi: 10.1016/0140-1971(89)90077-8. [DOI] [PubMed] [Google Scholar]
- McKnight-Eily L, Eaton D, Lowry R, Croft J, Presley-Cantrell L, Perry G. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev. Med. 2011;53:271–273. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E, Mindell J. Sleep and risk-taking behavior in adolescents. Behav. Sleep Med. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Poline J-B, Worsley KJ, Evans A, Friston K. Combining spatial extent and peak intensity to test for activations in functional imaging. NeuroImage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an fMRI investigation of the balloon analog risk task. Front. Neurosci. 2012;6:1–11. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy, and uncertainty. Trends Cogn. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Gálvan A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. J. Cogn. Neurosci. 2013;25:374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Chuah Y, Huettel SA, Chee M. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah L, Payne J, Chee M. Sleep deprivation biases the neural mechanisms underlying economic preferences. J. Neurosci. 2011;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte S, Heitzeg MM, Foley S, Yau WW, Majczenko K, Zubieta J, et al. Impulsiveness and insula activation during reward anticipation are associated with genetic variants in GABRA2 in a family sample enriched for alcoholism. Mol. Psychiatry. 2011:1–9. doi: 10.1038/mp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Yoo S, Gujar N, Hu P, Jolesz F, Walker M. The human emotional brain without sleep: a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.