Abstract
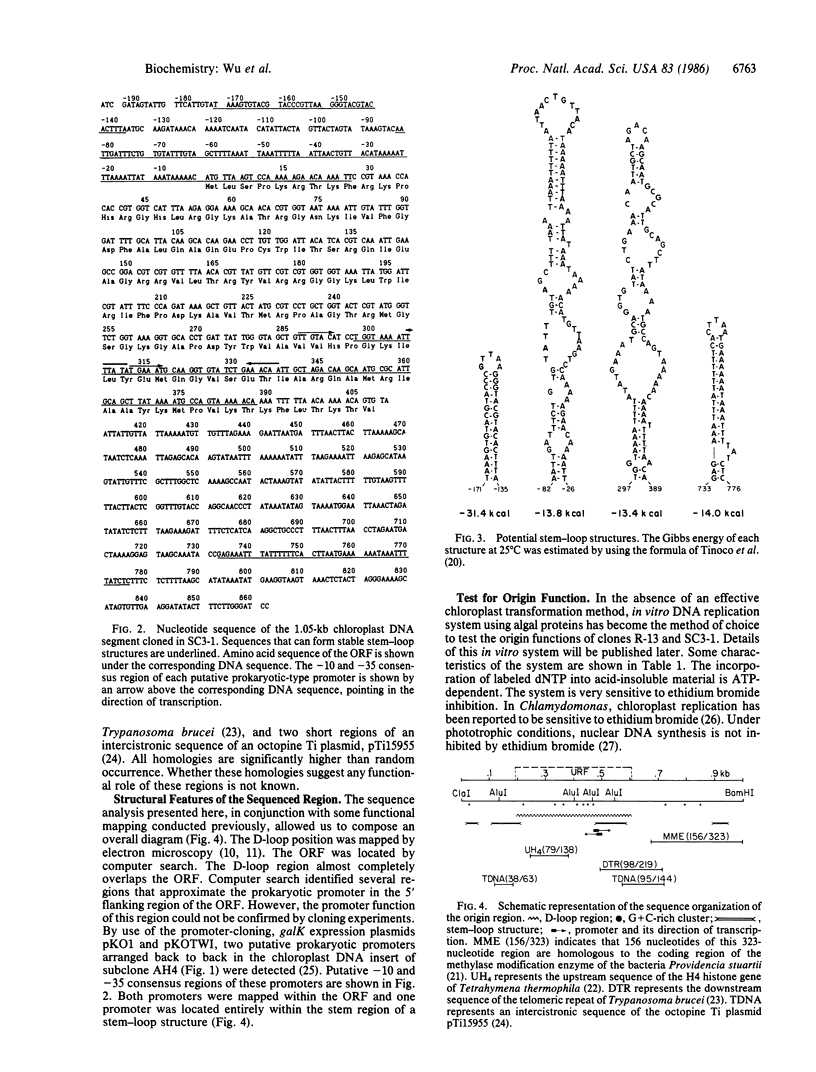
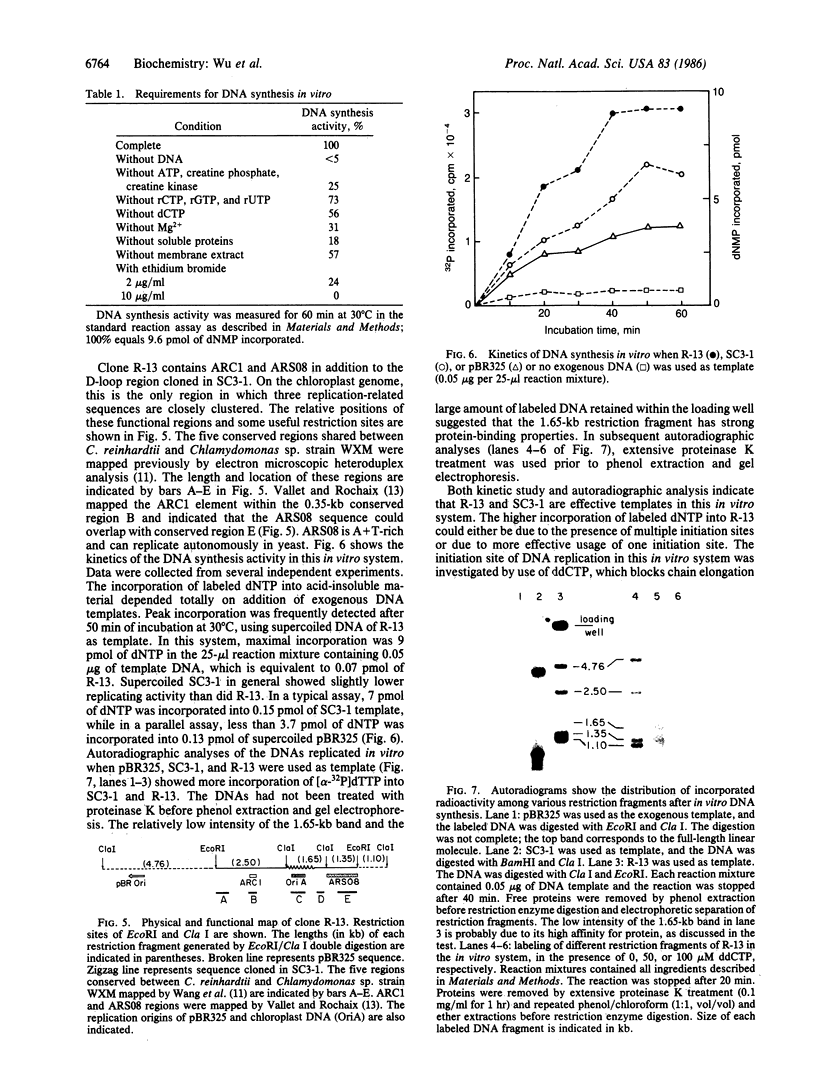
Chloroplast DNA replication in Chlamydomonas reinhardtii is initiated by the formation of a displacement loop (D-loop) at a specific site. One D-loop site with its flanking sequence was cloned in recombinant plasmids SC3-1 and R-13. The sequence of the chloroplast DNA insert in SC3-1, which includes the 0.42-kilobase (kb) D-loop region, as well as 0.2 kb to the 5′ end and 0.43 kb to the 3′ end of the D-loop region, was determined. The sequence is A+T-rich and contains four large stem-loop stuctures. An open reading frame potentially coding for a polypeptide of 136 amino acids was detected in the D-loop region. One stem-loop structure and two back-to-back prokaryotic-type promoters were mapped within the open reading frame. The 5.5-kb EcoRI fragment cloned in R-13 contains the 1.05-kb SC3-1 insert and its flanking regions. A yeast autonomously replicating (ARS) sequence and an ARC sequence, which promotes autonomous replication in Chlamydomonas, have been mapped within the flanking regions [Vallet, J.-M. & Rochaix, J.-D. (1985) Curr. Genet. 9, 321-324]. Both R-13 and SC3-1 were active as templates in a crude algal preparation that supports DNA synthesis. In this in vitro system, chloroplast DNA synthesis initiated near the D-loop site.
Keywords: nucleotide sequence, DNA secondary structure, D-loop
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky C. W., Jr Relaxed cellular controls and organelle heredity. Science. 1983 Nov 4;222(4623):468–475. doi: 10.1126/science.6353578. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Challoner P. B. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell. 1984 Feb;36(2):447–457. doi: 10.1016/0092-8674(84)90238-1. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Attachment of chloroplast polysomes to thylakoid membranes in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1973 May;70(5):1554–1558. doi: 10.1073/pnas.70.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Wold M., Campbell J. L. Origin and direction of DNA replication of plasmid RSF1030. Proc Natl Acad Sci U S A. 1979 Feb;76(2):736–740. doi: 10.1073/pnas.76.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D., Mets L. First DNA sequence of a chloroplast mutation: a missense alteration in the ribulosebisphosphate carboxylase large subunit gene. Plasmid. 1983 May;9(3):321–324. doi: 10.1016/0147-619x(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Sager R. Ethidium bromide induced selective and reversible loss of chloroplast DNA. Nat New Biol. 1973 Feb 28;241(113):277–279. doi: 10.1038/newbio241277a0. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Delius H. Origin of replication in chloroplast DNA of Euglena gracilis located close to the region of variable size. EMBO J. 1982;1(8):995–998. doi: 10.1002/j.1460-2075.1982.tb01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Chloroplast DNA from higher plants replicates by both the Cairns and the rolling circle mechanism. Nature. 1975 Aug 28;256(5520):708–711. doi: 10.1038/256708a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Tewari K. K. Denaturation mapping studies on the circular chloroplast deoxyribonucleic acid from pea leaves. J Biol Chem. 1975 Jul 10;250(13):4888–4895. [PubMed] [Google Scholar]
- Lother H., Kölling R., Kücherer C., Schauzu M. dnaA protein-regulated transcription: effects on the in vitro replication of Escherichia coli minichromosomes. EMBO J. 1985 Feb;4(2):555–560. doi: 10.1002/j.1460-2075.1985.tb03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Mullet J. E., Chua N. H. An in vitro system for accurate transcription initiation of chloroplast protein genes. Nucleic Acids Res. 1985 Feb 25;13(4):1283–1302. doi: 10.1093/nar/13.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., van Dillewijn J., Rahire M. Construction and characterization of autonomously replicating plasmids in the green unicellular alga Chlamydomonas reinhardii. Cell. 1984 Apr;36(4):925–931. doi: 10.1016/0092-8674(84)90042-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Hiraga S. Negative control of oriC plasmid replication by transcription of the oriC region. Mol Gen Genet. 1985;200(1):21–26. doi: 10.1007/BF00383307. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Waddell J., Wang X. M., Wu M. Electron microscopic localization of the chloroplast DNA replicative origins in Chlamydomonas reinhardii. Nucleic Acids Res. 1984 May 11;12(9):3843–3856. doi: 10.1093/nar/12.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y., Walder J. A., Donelson J. E. The organization and complete nucleotide sequence of the PstI restriction-modification system. J Biol Chem. 1984 Jun 25;259(12):8015–8026. [PubMed] [Google Scholar]
- Wang X. M., Chang C. H., Waddell J., Wu M. Cloning and delimiting one chloroplast DNA replicative origin of Chlamydomonas. Nucleic Acids Res. 1984 May 11;12(9):3857–3872. doi: 10.1093/nar/12.9.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]