Abstract
Vesicular stomatitis virus (VSV) is a prototypic nonsegmented negative-strand RNA virus. VSV’s broad cell tropism makes it a popular model virus for many basic research applications. In addition, a lack of preexisting human immunity against VSV, inherent oncotropism and other features make VSV a widely used platform for vaccine and oncolytic vectors. However, VSV’s neurotropism that can result in viral encephalitis in experimental animals needs to be addressed for the use of the virus as a safe vector. Therefore, it is very important to understand the determinants of VSV tropism and develop strategies to alter it. VSV glycoprotein (G) and matrix (M) protein play major roles in its cell tropism. VSV G protein is responsible for VSV broad cell tropism and is often used for pseudotyping other viruses. VSV M affects cell tropism via evasion of antiviral responses, and M mutants can be used to limit cell tropism to cell types defective in interferon signaling. In addition, other VSV proteins and host proteins may function as determinants of VSV cell tropism. Various approaches have been successfully used to alter VSV tropism to benefit basic research and clinically relevant applications.
Keywords: Vesicular stomatitis virus, VSV, Tropism, Host factors, Oncolytic, Neurotropism, Neurotoxicity
1. Introduction
Viral tropism commonly refers to the specificity of a virus for a particular cell type, tissue, organ or species. Here, we define cell tropism as the specificity of VSV for cell types supporting virus infection, replication and production of infectious progeny. Understanding viral tropism is important for basic virology, but also for development of effective gene therapy, vaccines and oncolytic virus therapies. This review focuses on the cellular tropism of vesicular stomatitis virus (VSV), one of the best-studied RNA viruses, which is extensively exploited in various applications.
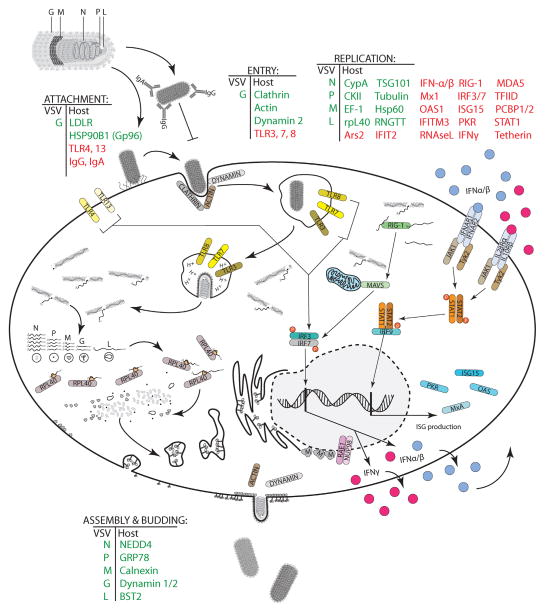
Studies of virus cell tropism reveal not only viral but also host components, which if present or absent may provide a hospitable environment for the virus life cycle (Fig. 1). Permissive cells usually contain required factors for virus attachment, entry, biosynthesis and exit, but lack effective antiviral mechanisms. The complex interaction of viral and host components determines the rates of infection, replication, and production of progeny. Understanding these interactions helps to develop various strategies to manipulate VSV tropism.
Fig. 1.
Known and putative determinants of cell tropism of VSV. Host and viral proteins are involved (positively or negatively) in VSV infection and replication. Different viral and host proteins are involved in VSV attachment, entry, replication, assembly, or release. Proteins known to be involved in the VSV life cycle are shown at each step: green indicates viral or host proteins known to assist VSV while red indicates putative host proteins as well as host proteins responsible for an antiviral response.
VSV is a prototypic, non-segmented negative sense RNA virus (order Mononegavirales, family Rhabdoviridae). Five genes are encoded by the 11-kb VSV genome: nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and large polymerase (L). All 5 gene products assemble to create an enveloped, bullet-shaped virion that measures approximately 185 nm × 75 nm (Ge et al., 2010). In addition to G, an attachment protein, that enables infection of very wide range of cells, other VSV proteins may also play roles in directing VSV tropism, including evasion of the innate immune response and interaction with host factors (discussed later). VSV’s broad cell tropism, relative independence on cell cycle, rapid replication, high virus yields, and small, easily manipulated genome make it a popular model virus for many basic research applications. In addition, a lack of preexisting human immunity against VSV and its inability to transform host cells make VSV a widely used experimental platform for vaccine vectors (Bukreyev et al., 2006). Moreover, VSV is a promising oncolytic virus (OV), preferentially infecting and killing cancer cells while leaving nonmalignant “normal” cells unharmed. Numerous preclinical studies demonstrated effectiveness of VSV against various malignancies (Hastie and Grdzelishvili, 2012) and a VSV recombinant encoding the IFN-β gene is currently in a phase I clinical trial against hepatocellular carcinoma (clinicaltrials.gov, 2012, Trial ID: NCT01628640).
The major VSV serotypes, Indiana (IN) and New Jersey (NJ), are endemic to parts of the United States as well as much of Central and South America. Among VSV’s natural hosts are cattle, pigs, horses and other mammals and their insect vectors (Drolet et al., 2005; Hansen et al., 1985). Instances of VSV infection of humans are rare, but cases have been documented for agricultural and laboratory workers (Reif et al., 1987). VSV infection can cause mild flu-like symptoms in adults. However, viral encephalitis has been reported in a 3-year-old child in Panama (Quiroz et al., 1988). In nature, VSV can be transmitted to mammalian hosts by arthropod vectors that include sandflies, houseflies, and mosquitos, or direct contact with infected animals (Bergold et al., 1968; Cupp et al., 1992; Drolet et al., 2005, 2009; Letchworth et al., 1999; Mead et al., 2004; Tesh et al., 1971). Experimental studies of VSV pathology have reported varied outcomes dependent on the route of administration. In the laboratory, due mainly to VSV’s ability to cause experimental encephalitis, studies generally focus on intranasal (i.n.) injection in murine and non-human primate models. Non-human primate models were also used with no evidence of VSV in the brain, spinal cord, or plasma found following i.n. injection (Johnson et al., 2007). However, intrathalmic (i.t.) injection resulted in neurological disease and animal sacrifice (Johnson et al., 2007; Mire et al., 2012). VSV spread within the CNS (mouse and primate models) will be discussed in detail in a later section of this review. Interestingly, inoculation of immunocompetent mice with VSV by various routes of administration: intramuscular (i.m.), subcutaneous (s.c.), or intraperitoneal (i.p.), generally results in no apparent disease and limited virus replication (Trottier et al., 2007). Limited studies have examined early infection of VSV throughout the body. Murine models are the only known vertebrates to consistently develop detectable viremia following infection (Cornish et al., 2001). In the study with deer mice examined over a seven-day period, intradermal infection resulted in detectable viremia as well as infection in the spinal cord (Cornish et al., 2001). Soon after viremia VSV was also detectable in cardiac muscles and in macrophages in lymph nodes. Another study reported that following intravenous (i.v.) injection VSV demonstrates a rapid evasion to the nervous system and that transfer of VSV-immune serum 16 h after infection fails to protect against mortality even though the mice produced high titers of neutralizing antibodies (Steinhoff et al., 1995). Interestingly, this was also shown when mice developed similar levels of neutralizing antibodies to VSV injected i.n. or i.v., but mice injected i.n. still died (Thomsen et al., 1997). Mice injected s.c. or i.v. showed no virus in any organs and only low titers of virus in the brain at 8 days post infection (Stojdl et al., 2000a). In general, these results suggest a strong tropism of VSV toward the CNS and clearance of virus from the periphery.
In the next sections of this review, we will describe VSV biology, specifically focusing on steps and molecules that determine VSV cell tropism at points of attachment, endocytosis, viral gene trancription and translation, genome replication, and budding of progeny. We will review various approaches that have been successfully used to alter VSV tropism to benefit basic research and clinically relevant applications. The neurotropism of VSV is a particularly important issue to address for development of safe VSV-based vectors, and will be reviewed in detail. While the format of this review does allow us to describe all the details of VSV biology, we would like to refer readers to an excellent review by Lyles and Rupprecht in Fields Virology, Rhabdoviridae chapter (Lyles and Rupprecht, 2007).
2. Entry
2.1. Biology of VSV entry
VSV enters the cell via the endocytic pathway and subsequently fuses with a cellular membrane within the acidic environment of the endosome. This penetration process is relatively inefficient for VSV. Many virions that attach to the cell surface are not internalized, and many of the internalized virions appear to be degraded by proteases and other enzymes (Matlin et al., 1982). Differences among cell types in the efficiency of each step may lead to altered infection efficacy, thereby, affecting the cellular tropism. A siRNA screen of the human kinome (the genomic collection of human protein, lipid and carbohydrate kinases) in HeLa cells showed that the productive entry of VSV involves a large cohort of kinases from different families, suggesting that multiple cellular proteins involved with endocytosis may determine the VSV tropism at the level of entry (Pelkmans et al., 2005).
The VSV G protein is the main viral determinant of the entry (Roche et al., 2008), and is involved in two of the initial steps of the infectious process: virus attachment to the host cell surface and viral-induced pH-dependent endosomal membrane fusion. VSV G enables infection of most, if not all, human cell types, and of organism as distant as zebrafish and Drosophila (Gillies and Stollar, 1980; Mudd et al., 1973; Seganti et al., 1986).
The membrane lipid phosphatidylserine (PS) has long been considered to play an important role in VSV entry and may be involved in attachment or triggering endocytosis, although this possible role is controversial. A PS-binding segment was mapped in the G protein (134–161 aa) from several rhabdoviruses (Coll, 1995, 1997), and nuclear magnetic resonance, as well as fluorescence studies showed interactions of another fragment of VSV G (118–136 aa) with PS (Hall et al., 1998). This interaction was modulated by both ionic and hydrophobic factors and appeared to be dependent on the fluidity and lipid packing of the target bilayer (Hall et al., 1998). Also, of various purified lipids, only PS was able to inhibit VSV attachment and infectivity (Schlegel et al., 1983). However, Coil and Miller (2004) demonstrated no correlation between the cell surface PS levels and VSV infection, and also demonstrated that an excess of annexin A5, which binds PS, does not inhibit infection or binding by VSV. The authors concluded that the VSV binding to PS is not a determinant event in attachment, but it may be involved in a post-binding step of virus entry (discussed below).
The endoplasmic reticulum (ER) chaperone Gp96 (HSP90B1) appears important for VSV entry. Recent work demonstrated that VSV infection requires Gp96, which is essential for toll-like receptor (TLR) maturation in the ER (Bloor et al., 2010). As VSV does not attach to cells with mutated Gp96, the authors proposed that Gp96 is essential for the presence of functional VSV G receptor at the cell surface, most likely because it facilitates the correct folding of either a protein receptor or an enzyme required for the synthesis of a glycolipid receptor. Currently, low density lipoprotein receptor (LDLR) and its family members have been proposed to be the cell surface receptors for VSV (Finkelshtein et al., 2013). The study suggests that LDLR serves as the major entry port of VSV and VSV G-pseudotyped lentiviral vectors in human and mouse cells, whereas other LDLR family members serve as alternative receptors. The widespread expression of LDLR family members would account for the pantropism of VSV and for the broad application of VSV G-pseudotyped viral vectors for gene transduction.
After binding to the cell surface, VSV particles enter the cell through endocytosis, in a clathrin-based, dynamin-2-dependent manner (Superti et al., 1987). The virus can enter either through a preformed clathrin-coated pit (CCP) or by de novo induction of pit formation (Johannsdottir et al., 2009). Interestingly, because VSV is significantly larger than the dimensions of a typical clathrin-coated vesicle, the vesicles used by the virus for entry are only partially clathrin-coated, and require actin polymerization for efficient uptake (Cureton et al., 2009). However, endocytosis of shorter defective interfering (DI) VSV particles does not depend on actin assembly (Cureton et al., 2010).
After internalization membrane fusion occurs rapidly in early endosomes (Johannsdottir et al., 2009). Several findings indicate that PS is essential for VSV–membrane interactions triggering fusion, rather than as a receptor. In most cell types, either a vast majority or all of the PS is located on the inner leaflet of the plasma membrane, making it inaccessible as a receptor for virus (Boon and Smith, 2002). Furthermore, membrane fusion mediated by VSV G reconstituted in lipid vesicles showed a large preference for target membranes containing PS or phosphatidic acid (Eidelman et al., 1984). The extent of pH-induced VSV G protein conformational changes depends on the presence of PS on the target membrane, and increasing the PS content remarkably increased the rate of the fusion reaction (Carneiro et al., 2002, 2006). In contrast, it was shown in erythrocytes that the susceptibility to VSV fusion is not dependent on any particular phospholipid but rather the packing characteristics (symmetric vs. asymmetric bilayer distributions of phospholipids) of the target membrane and, unlike virus binding, is not modulated electrostatically (Herrmann et al., 1990; Yamada and Ohnishi, 1986).
It has long been assumed that the viral envelope fuses directly to the limiting endosomal membrane, but it has also been suggested that VSV G targets the membrane of intraendosomal vesicles first (Le Blanc et al., 2005). The authors propose a two-step process, with the initial fusion event occurring with internal vesicles followed by release of the viral NC into the cytosol by back-fusion of the internal vesicle with the limiting membrane of late endosomes. This alternative mechanism is supported by in vitro experiments demonstrating the presence of lipid bis(monoacylglycero)phosphate (BMP) on the endosomal internal vesicles selectively promotes VSV G-mediated membrane fusion (Roth and Whittaker, 2011). The authors concluded the two-step model remains controversial but that differential composition of endosomal domains across cell types may allow the virus to either fuse directly from the early endosome or enter via back-fusion in late endosomes.
2.2. Altering VSV tropism by targeting entry
While the broad tropism of VSV is beneficial in many applications, others could be improved by specific targeting. VSV G protein modification by molecular and genetic engineering is an effective strategy for targeting of VSV particles. A site on VSV G exposed on the protein surface and tolerant to foreign epitope insertion was identified (Schlehuber and Rose, 2004). The feasibility of this approach was demonstrated by (Dreja and Piechaczyk, 2006), who constructed a chimeric VSV G protein by linking a large (253 aa) cell-directing single-chain variable fragment (scFv) antibody to the N-terminus of VSV G. HIV-1 particles pseudotyped with VSV G linked to a scFv against human major histocompatibility complex class I (MHC-I) bound strongly and specifically to human cells. However, the fusogenicity of the novel protein was diminished, resulting in a reduced infectivity.
Another approach is to replace VSV G with a heterologous glycoprotein from another virus. Detargeting of VSV from neurons was accomplished by pseudotyping the virus with the non-neurotropic envelope glycoprotein of the lymphocytic choriomeningitis virus (LCMV) (Muik et al., 2011). In a combination of approaches, VSV G was replaced with Sindbis virus G protein fused to a single-chain antibody against the Her2 receptor, commonly overexpressed on breast cancer cells (Bergman et al., 2007; Gao et al., 2006). Several serial passages generated an adapted recombinant VSV that successfully targeted and eliminated Her2-expressing tumors in mice in vivo. Using a similar strategy, replication defective VSV particles were pseudotyped with measles virus envelope glycoproteins displaying single chain antibodies meant to target cancer cells expressing epidermal growth factor receptor, folate receptor or prostate membrane-specific antigen. These retargeted VSV infected only cells expressing the targeted receptor in vitro and in vivo in s.c. tumors established in mice (Ayala-Breton et al., 2012).
A different strategy comprises the modification of the cellular and/or viral environment to non-specifically alter the viral tropism. For instance, repetitive administration of viral vectors (e.g., in OV therapy) provokes the generation of neutralizing antibodies that can diminish virus efficacy by depleting the amount of virions free to infect the host. One approach extends circulation time by conjugating polyethylene glycol (PEG) to a VSV G in pseudotyped lentivirus particles, preventing virion inactivation in serum (Croyle et al., 2004). Also, DNA aptamers against the antigen-binding fragment (Fab) of polyclonal antibodies against VSV are used to shield the virus from neutralizing antibodies and enhances in vivo survival of VSV (Labib et al., 2012; Muharemagic et al., 2012). In a novel approach, nanotechnology was used to generate an encapsulated VSV G-pseudotyped lentiviral vector by crosslinking a polymer shell to reduce non-specific targeting. Acrylamide-tailored cyclic RGD (cRGD) peptide was also introduced to the shell to target this “nanovirus” specifically to HeLa cells (Liang et al., 2013). The resulting targeting nanovirus had similar titer to non-crosslinked pseudotypes, specifically transduced HeLa cells with high transduction efficiency, and did not change the viral entry pathway. Importantly, the polymer shell provided the targeting nanovirus with enhanced stability in the presence of human serum, protecting nanovirus from human serum complement inactivation. Alternatively, VSV could be passaged in the presence of polyclonal antiserum resulting in the selection of antibody-escape mutants. Recently, directed evolution was used to select for VSV G mutants displaying increased resistance to human serum neutralization (Hwang and Schaffer, 2013). Numerous common mutations were found which exhibited higher in vitro resistance to human serum as well as thermostability when introduced to pseudotyped lentiviral vectors.
Finally, VSV can also infect human lymphocytes, dendritic cells, and natural killer cells and these cells have been exploited as delivery vehicles to prevent premature virus clearance prior to therapeutic effect (Boudreau et al., 2009; Waibler et al., 2007).
3. Replication
3.1. Biology of VSV replication
The ability of VSV to enter a cell does not guarantee successful virus replication. Permissive cells must provide an optimal environment (including host factors) for viral genome transcription, replication, and viral mRNA translation. Importantly, VSV also needs to evade the host cell innate antiviral responses.
Following release from the endosome, the VSV ribonucleoprotein (RNP) complex, composed of the VSV nucleocapsid and associated VSV L and P proteins, is released into the cytoplasm. VSV L protein, a multifunctional RNA dependent RNA polymerase (RdRp), forms a complex with VSV P, a multifunctional polymerase co-factor, to begin primary transcription of viral mRNAs (Gao and Lenard, 1995; Green and Luo, 2009). Viral transcripts are synthesized, capped, methylated, and polyadenylated by the L protein (Emerson and Wagner, 1973; Grdzelishvili et al., 2005, 2006; Hercyk et al., 1988; Hunt, 1983, 1989; Li et al., 2005, 2008; Ogino and Banerjee, 2007; Sleat and Banerjee, 1993). VSV mRNAs are virtually indistinguishable from host mRNAs and are translated preferentially by host cell ribosomes (Connor and Lyles, 2002; Mire and Whitt, 2011; Whitlow et al., 2006, 2008). Unlike viral mRNA synthesis, VSV genome replication requires N protein, which is used to encapsidate newly produced antigenomic or genomic RNA but also a component of the polymerase complex specifically involved in genome replication rather than mRNA synthesis (Masters and Banerjee, 1988; Peluso and Moyer, 1988; Qanungo et al., 2004). A previous study suggested that phosphorylation of the N-terminal domain of the P protein is required for VSV transcription but not for replication (Pattnaik et al., 1997), although another study demonstrated that P mutants lacking N-terminal phosphorylation cannot support either transcription or replication (Chen et al., 2013). While transcription of viral mRNAs appears to occur throughout the cytoplasm, there is evidence to suggest that genome replication occurs in cytoplasmic inclusions. It remains unclear if these are virus developed inclusions or stress granules induced by cellular response to infection (Dinh et al., 2011; Heinrich et al., 2010). At this step in the life cycle, primary transcription still occurs, but it appears that mRNA transcripts are transported away from the inclusion sites in a microtubule dependent manner (Heinrich et al., 2010).
As with other RNA viruses, the VSV polymerase lacks proofreading activities and makes an error every 103 to 104 nucleotides (Holland et al., 1990; Steinhauer et al., 1989; Steinhauer and Holland, 1986). These genetically differing viruses, so called quasispecies, make VSV extremely adaptable to changing environments, such as a change in the host cells it is grown on (Novella et al., 2010). This feature of VSV can be exploited to experimentally adapt virus to a particular cell type (Novella et al., 1995, 1999; Turner and Elena, 2000). For example, Gao et al. (2006) conducted a serial passage experiment of VSV on a mouse mammary carcinoma cell line, which the virus infected poorly at first. In the course of this experiment a VSV variant was selected that grew better on that cell line (Gao et al., 2006). Despite error-prone VSV replication and unlike most positive-strand RNA viruses, VSV is able to stably maintain expression of an additionally inserted gene, especially when it is inserted between the G and L genes (Schnell et al., 1996; Wertz et al., 2002). Such stability is highly beneficial for VSV as a vector and vaccine delivery agent. Currently, a large number of VSV recombinants expressing heterogeneous genes were generated and characterized, and many studies demonstrated stable expression of these genes (Table 1).
Table 1.
VSV recombinants with altered tropism.
| VSV | Description and how tropism is affected | References |
|---|---|---|
| Parental recombinant “WT” VSV | ||
| VSV-WT (“Rose Lab”) | The parental rWT VSV. The L gene and the N-terminal 49 residues of the N gene are derived from the Mudd-Summers strain, the rest is from the San Juan strain (both Indiana serotype). | Lawson et al. (1995) |
| VSV-WT (“Wertz Lab”) | An alternative rWT VSV. The N, P, M, and L genes originated from the San Juan strain; G gene from the Orsay strain (both Indiana serotype). | Whelan et al. (1995) |
| VSV-WT-XN2 (or XN1) | A derivative of VSV-WT (“Rose Lab”) commonly used to make recombinant VSVs. Generated using pVSV-XN2 (or pVSV-XN1), a full-length VSV plasmid containing unique XhoI and NheI sites flanked by VSV transcription start and stop signals between G and L genes. | Schnell et al. (1996) |
| Attachment Modification | ||
| VSV-DV/F(L289A) (same as rVSV-F) | VSV expressing the Newcastle disease virus (NDV) fusion protein gene between G and L. The L289A mutation in this protein targets VSV to cells with sialic acid-containing receptors and allows it to induce syncytia alone (without NDV HN protein). | Ebert et al. (2004) |
| VSV-S-GP | Pseudotyped VSV with a Sindbis virus (SV) glycoprotein. Targets VSV to cells with the Her2 receptor. | Bergman et al. (2007) |
| VSV-FAST, VSV-(ΔM51)-FAST | VSV or VSV-MΔ51 expressing the p14 FAST protein of reptilian reovirus (between VSV G and L) demonstrates enhanced fusogenic ability and induces extensive neuropathology. | Brown et al. (2009) |
| VSV-CT9-M51 | Cytoplasmic tail of VSV-G was reduced from 29 to 9 amino acids in combination with the ΔM51 mutation. Attenuated neurotoxicity. | Ozduman et al. (2009), Wollmann et al. (2010) |
| VSV-CT1 | Cytoplasmic tail of the G protein was truncated from 29 amino acids to 1 amino acid. Attenuated neurotoxicity. | Ozduman et al. (2009), Wollmann et al. (2010) |
| VSV-ΔG-SV5-F | Pseudotyped VSV with the fusogenic simian parainfluenza virus 5 fusion protein (SV5-F). Shows increased syncytial formation and apoptosis. | Chang et al. (2010) |
| VSV-LCMV-GP (Replication-defective) | Pseudotyped VSV with the lymphocytic choriomeningitis virus (LCMV) glycoprotein. Allows for infection of brain cancer cells while decreasing neurotoxicity. | Muik et al. (2011) |
| VSV-G5, -G5R, -G6, -G6R | VSV with a mutant G protein (aa substitutions at various positions between residues 100 and 471). Triggers type I IFN secretion that promotes infection/replication in IFN defective cells and provides alternate antigen epitopes. | Janelle et al. (2011) |
| VSV-H/F, - αEGFR, -αFR, -αPSMA (Replication-defective) | Pseudotyped VSV with the measles virus (MV) F and H displaying single-chain antibodies (scFv). Targets VSV to cells that express epidermal growth factor receptor, folate receptor, or prostate membrane-specific antigen. | Ayala-Breton et al. (2012) |
| VSV G protein (S162 T, T230 N and T368A mutations enhanced serum resistance) | VSV could be passaged in the presence of polyclonal antiserum resulting in the selection of antibody-escape mutants. | Hwang and Schaffer (2013) |
| Replication Modification | ||
| VSV N1G4(WT), G1N2, G3N4, or G1N4 | Changes to the gene order of VSV can attenuate the virus. | Flanagan et al. (2001) |
| VSV-12′GFP | Placement of two reporter GFP genes at position 1 and 2 attenuates VSV replication by moving viral genes downward, to positions 3 to 7. | van den Pol and Davis (2013) |
| VSV-G/GFP | Fusing a GFP sequence to the VSV G gene, inserted between the WT G and L genes, in addition to WT G. Phenotype is similar to WT VSV. | Dalton and Rose (2001) |
| VSV-p1-GFP, VSV-p1-RFP | Placement of a GFP or red fluorescent protein (RFP or dsRed) reporter gene at position 1 attenuates VSV replication by moving viral genes downward, to positions 2 to 6. | Wollmann et al. (2010) |
| VSV-WT-GFP, -RFP, -Luc, -LacZ | Insertion of reporter genes between G and L. Mild attenuation. | Fernandez et al. (2002), Wu et al. (2008) |
| VSV-ΔM51, VSV-ΔM51-GFP, - RFP, -FLuc, -Luc, - LacZ | Deletion of VSV M methionine at position 51 (ΔM51) mutation prevents WT VSV M from inhibiting the host cell antiviral response. Additionally, some variants encode a reporter gene between the G and L. | Stojdl (2003), Power and Bell (2007), Wu et al. (2008) |
| VSV-M51R | The M51R mutation was introduced into M. This mutation prevents WT VSV M from inhibiting the host cell antiviral response. | Kopecky et al. (2001) |
| VSV-M6PY > A4-R34E and other M mutants | Mutation of aa M51R or the PSAP motif (residues 37–40) of VSV M prevents the protein from inhibiting nuclear export of cellular mRNA to reduce cellular antiviral response. | Irie et al. (2007) |
| VSV-*Mmut | Single mutations to VSV M or combination of mutations at VSV M aa positions M33A, M51R, V221F and S226R reduce the ability of VSV to prevent an antiviral response. | Hoffmann et al. (2010) |
| VSV-M(mut) | Mutation to VSV M residues 52 to 54 from DTY to AAA M(mut) prevents the ability of WT M to block nuclear mRNA export. | Heiber and Barber (2011) |
| VSV-mIFNβ, VSV-hIFNβ, VSV-rIFNβ | Addition of the mouse, rat, or human IFN-β gene in VSV enhances the antiviral state of normal cells, but retains oncolytic abilities against cancer cells with defective IFN responses. | Obuchi et al. (2003), Jenks et al. (2010) |
| VSV-let-7wt | Addition of let-7 microRNA target into the 3′-UTR of VSV M of the VSV genome limits replication only to cancer cells. | Edge et al. (2008) |
| VSV-124, -125, -128, -134 (M or L mRNA) | Addition of neuron-specific microRNA (miR-124, 125, 128, or 134) targets inserted in the 3′-UTR of VSV M or L mRNA result in reduced neurotoxicity. | Kelly et al. (2010) |
| VSV-IRESFMDV-GFP and VSV-IRESHRV-GFP | Internal ribosomal entry sites (IRES) from human rhinovirus 2 and foot and mouth disease virus were incorporated to control the translation of VSV M and attenuate neurovirulence. | Ammayappan et al. (2013) |
| VSV-rp30 | Positive selection of VSV-G/GFP (see above) on glioblastoma cells results in a virus with two silent mutations and two missense mutations, one in P and one in L (rp30 = 30 times repeated passaging). Better growth on glioblastoma cells. | Wollmann et al. (2005) |
| VSV-ΔP, -ΔL, -ΔG, (Semireplication-competent) | Three replication defective VSV variants, upon coinfection, show good replication, safety, and oncolysis (especially the combination of VSVΔG/VSVΔL). | Muik et al. (2012) |
| VSV-dG-GFP (or RFP) (Replication-defective) | Similar to VSV-p1-GFP or VSV-p1-RFP, above, but with a deleted VSV G that prevents a second round of infection. | Wollmann et al. (2010) |
Success of VSV replication can be influenced by the formation of DI particles. DIs arise as a result of one or more RNA recombination events at a variety of genomic sites (Colonno et al., 1977; Perrault and Leavitt, 1978). These truncated genomes can still be encapsidated and form virus-like particles. Although these particles can not sustain an infection by themselves, they are able to replicate in cells coinfected with a helper VSV, resulting in substantial reductions in virus titer (Colonno et al., 1977). Different ratios of “normal” VSV and DI particles are made when VSV is grown either in bovine kidney cells or Chinese hamster ovary cells in identical medium and culture conditions, suggesting that cell type affects the synthesis of DI particles (Huang and Baltimore, 1970). In HeLa cells, detectable DI particles are not generated (Holland et al., 1976). Bovine kidney cells fail to produce DI particles through 10 serial undiluted passages, while BHK-21 cells are efficient producers of DI particles (Youngner et al., 1981). Importantly, in vivo DI particles can also play a role in viral pathogenesis by modulating the host immune response (Cave et al., 1985; Plakhov et al., 1995a). By suppressing the synthesis of viral proteins in dually infected cells, DI particles may prevent the proper presentation of endogenously synthesized antigens (Browning et al., 1991).
The broad tropism of VSV suggests viral proteins may be responsible for the bulk of enzymatic activity required for viral gene transcription and genome replication, or that conserved host proteins assist virus replication. Variable levels of VSV replication in different cell types suggests that at least some host proteins may play a role in VSV’s cell tropism (Fig. 1 and Table 2). Cyclophilin A (CypA), a chaperone protein, binds the VSV N protein of both VSV IN and NJ serotypes. However, CypA only appears to be required for VSV NJ primary transcription, as inhibition of CypA did not significantly effect VSV IN replication (Bose et al., 2003). Poly (C) binding proteins 1 and 2, regulators of host transcription, translation, and mRNA stability, interact with VSV P and function as negative regulators of virus gene expression (Dinh et al., 2011). VSV P is phosphorylated by casein kinase II (CKII) prior to interacting with RdRp (Barik and Banerjee, 1992; Gupta et al., 1995). Moreover, CKII remains associated with the RNP complex throughout the replication cycle and is incorporated into progeny virions (Gupta et al., 1995). Direct association of eukaryotic elongation factor-1 (EF-1) with VSV L contributes to enhanced replicative success (Das et al., 1998). It is important to note that host proteins may play different roles in viral gene transcription versus genome replication. For example, heat shock protein 60 (Hsp60), EF-1 and guanylyltransferase associate with the RdRp transcriptase complex, while the RdRp replicase complex lacks these host factors (Qanungo et al., 2004). Another study suggested that cellular protein La binds VSV leader RNAs to facilitate genome replication (Wilusz et al., 1983). Additionally, tubulin was shown to facilitate transcription and may directly associate with VSV L (Moyer et al., 1986). Interferon induced transmembrane protein 3 (IFITM3), tetherin (BST2, CD137) (Weidner et al., 2010), and arsenate resistance protein (Ars2) (Sabin et al., 2009), are not known to interact directly with any specific viral protein, but impact VSV’s replicative success.
Table 2.
Host proteins potentially determining VSV tropism.
| Common Protein name | Uniprot gene Name | Potential role in VSV life cycle | Selected References |
|---|---|---|---|
| Attachment | |||
| Low Density Lipoprotein Receptor | LDLR | Proposed VSV cell surface receptor. | Finkelshtein et al. (2013) |
| Toll-like receptor 4 | TLR4 | Detects VSV G protein. | Georgel et al. (2007) |
| Toll-like receptor 13 | TLR13 | Detects VSV, but ligand is unknown. | Shi et al. (2011) |
| Heat Shock Protein 90 kDa Beta | HSP90B1 (Gp96) | Facilitates the correct folding of either a protein receptor or an enzyme required for the synthesis of a VSV receptor(s). May promote the stable configuration of VSV L multimers and facilitate the L-P interaction. | Bloor et al. (2010) |
| Entry | |||
| Clathrin heavy chain | CLTC | Required for VSV endocytosis. | Sun et al. (2005) |
| Dynamin-1/2 | DNM2-1/2 | Binds VSV M to facilitate for virus assembly and budding. | Cureton et al. (2009), Raux et al. (2010) |
| Toll-like receptor 3 | TLR3 | Detects double-stranded RNA in endosomes. | Alexopoulou et al. (2001) |
| Toll-like receptor 7 | TLR7 | Detects single-stranded RNA in endosomes. | Diebold et al. (2004), Lund et al. (2004) |
| Toll-like receptor 8 | TLR8 | Detects single stranded RNA. | Heil et al. (2004) |
| Replication | |||
| Peptidylprolyl Isomerase A (Cyclophilin A) | CYPA | Interacts with VSV N and is found bound to viral RNP in progeny. Required for VSV NJ replication, but not VSV IN. | Bose et al. (2003) |
| Casein kinase II | CSNK2A1 | Phosphorylates VSV P to facilitate transcription. | Barik and Banerjee (1992) |
| Elongation factor 1-alpha 1 | EEF1A1 | Associates with VSV L to facilitate transcription. | Das et al. (1998) |
| Ubiquitin-60S ribosomal protein L40 | UBA52 | Required for VSV cap-dependent translation. | Lee et al. (2013) |
| Serrate RNA effector molecule homolog | SRRT/ARS2 | Modulates antiviral responses to inhibit VSV infection. | Sabin et al. (2009) |
| Poly (RC) binding protein 1/2 | PCBP-1/2 | Interact with VSV P to inhibit viral mRNA synthesis. | Dinh et al. (2011) |
| Tubulin beta chain | TUBB | Facilitates transcription and may associate directly with VSV L. | Moyer et al. (1986) |
| 60 kDa heat shock protein | HSPD1 | Found to be bound to the transcriptase complex and in purified virions. | Qanungo et al. (2004) |
| mRNA-capping enzyme | RNGTT | Found to be bound to the transcriptase complex and in purified virions. | Qanungo et al. (2004) |
| Interferon-induced protein with tetratricopeptide repeats 2 | IFIT2 | Inhibits VSV replication as VSV titers rose several hundred folds higher in knockout mice compared to wt mice. | Fensterl et al. (2012) |
| Interferon induced transmembrane protein 3 | IFITM3 | Inhibits a post endocytosis event of VSV entry. | Weidner et al. (2010) |
| Interferon-induced GTP-binding protein Mx1 | MX1 | Inhibits VSV RNA synthesis. | Schwemmle et al. (1995) |
| 2′-5′-Oligoadenylate Synthetase 1 | OAS1 | Detects single and double-stranded RNA with secondary structure and activates RNAseL, which degrades cellular mRNA. | Kumar et al. (1988) |
| Ubiquitin-like protein ISG15 | ISG15 | Regulator of antiviral proteins. | Zhao et al. (2005) |
| Probable ATP-dependent RNA helicase DDX58 | DDX58/RIG-I | Detects uncapped 5′-triphosphate RNA and signals type I IFN production. | Hornung et al. (2006), Kato et al. (2006) |
| Interferon-induced, double-stranded RNA-activated protein kinase | EIF2AK2/PKR | Detects double-stranded RNA and inhibits translation of viral mRNAs through phosphorylation of eukaryotic translation initiation factor 2α. | Balachandran et al. (2000) |
| Interferon-induced helicase C domain-containing protein 1 | IFIH1/MDA5 | Recognizes dsRNA to facilitate antiviral signaling. | Kato et al. (2006) |
| TATA-box-binding protein | TBP/TFIID | VSV inhibits cellular host transcription by inactivation of TFIID. | Yuan et al. (1998) |
| tumor susceptibility gene 101 | TSG101 | Plays a role in nucleocapsid release from endosomes to the cytoplasm | Luyet et al. (2008) |
| Interferon alpha-1/13/Interferon beta | IFNA1/IFNB1 | Key cytokine responsible for promoting upregulation of antiviral genes. | Gresser et al. (1979) |
| Interferon regulatory factor 3 and 7 | IRF3, IRF7 | VSV infection causes results in activation and upregulations of this transcription factor that upregulates type I IFN production for antiviral response. | Stojdl (2003) |
| Signal transducer and activator of transcription 1-alpha/beta | STAT1 | Activation of STAT1 via IFN signaling results in this transcription factor causing upregulation of antiviral genes. | Wong et al. (2001) |
| Lupus La protein | SSB/La | Binds to VSV leader RNAs and may influence transcription of full-length genome. | Wilusz et al. (1983) |
| Assembly and Exit | |||
| Actin | Cureton et al. (2009) | ||
| E3 ubiquitin-protein ligase NEDD4 | NEDD4 | A role in VSV budding. | Irie et al. (2004) |
| 78 kDa glucose-regulated protein | HSPA5/GRP78 | A role in folding of VSV G. | Hammond and Helenius (1994) |
| Calnexin | CANX | A role in folding of VSV G. | Hammond and Helenius (1994) |
| Dynamin-1/2 | DNM2-1/2 | Binds VSV M to facilitate for virus assembly and budding. | Cureton et al. (2009), Raux et al. (2010) |
| Bone marrow stromal antigen 2/Tetherin | BST2 | May impair VSV release. | Sarojini et al. (2011), Weidner et al. (2010) |
Regarding translation of viral mRNA, studies suggest dephosphorylation of the cap-binding subunit eIF4E in infected cells (Connor and Lyles, 2002) may lead to preferential translation of newly synthesized, viral mRNAs (Whitlow et al., 2006, 2008). Additionally, ribosomal subunit protein rpL40 was shown to be associated with VSV mRNA translation (Lee et al., 2013). Interestingly, host mRNAs were also shown to be sensitive to rpL40 depletion, suggesting VSV hijacks an endogenous translation pathway (Lee et al., 2013). It was also shown that VSV M protein contributes to regulation of protein translation in the infected cell (Mire and Whitt, 2011).
VSV tropism is greatly determined by cellular innate antiviral responses, especially those associated with the type I IFN (IFN-α and IFN-β) signaling. VSV can be detected by both cell surface and endosomal pattern recognition receptors (PRR). VSV detection can occur at the cell surface with the toll-like receptor 4 (TLR4)-CD14 complex (Georgel et al., 2007). Presence of TLR13, a novel PRR, has also been implicated in virus detection at the cell surface (Shi et al., 2011). Interestingly TLR13 appears to be specific to cell types such dendritic cells, macrophages or cells of the spleen and TLR13 knockdown increased cell susceptibility to VSV (Shi et al., 2011). VSV can also be detected by endosomal PRRs, TLRs 7 and 8 (detect single stranded RNA) (Diebold et al., 2004; Heil et al., 2004; Lund et al., 2004). TLR 3 is expected to detect VSV-associated dsRNA, however studies disagree on the extent of TLR3 involvement in detection of VSV and antiviral signaling, though this may be cell type dependent (Alexopoulou et al., 2001; Edelmann et al., 2004; Ostertag et al., 2007). In addition to TLRs, two cytoplasmic PRRs detect VSV: (1) the retinoic-acid-inducible gene I (RIG-I), which detects single-stranded, 5-triphosphate RNA; and (2) the melanoma differentiation-associated protein 5 (MDA5), which detects dsRNA intermediates (Hornung et al., 2006; Kato et al., 2006; Rehwinkel et al., 2010). VSV detection initiates signaling cascades that result in IFN-α/β production and often the induction of a proinflammatory state through NF-κB. Secreted IFN-α/β act in an autocrine or paracrine manner to induce ISG production through IFN receptor (IFNAR1/2) binding and subsequent tyrosine-protein kinase JAK1 (JAK1) and signal transducer and activator of transcription (STAT) 1 activation (Dunn et al., 2005; Sun et al., 1998; Zhang et al., 2010). ISGs with known antiviral effects include: myxovirus (influenza virus) resistance 1 (MxA) (Schwemmle et al., 1995; Staeheli and Pavlovic, 1991), 2′–5′ oligoadenylate synthetase (OAS) and ribonuclease L (RNAse L) (Clemens and Vaquero, 1978; Monsurro et al., 2010; Saloura et al., 2010; Verheijen et al., 1999), and double-stranded RNA-activated protein kinase (PKR) (Saloura et al., 2010). ISG15, an ubiquitin homologue, is an important regulator of these proteins, though infection of ISG15 knockout mice showed no change in virus kinetics or increase in virus-induced death over control animals (Osiak et al., 2005; Zhao et al., 2005). In vivo studies with IFNAR, STAT1, or PKR knockout mice demonstrate that mice succumb to infection rapidly without functional innate immune pathways (Durbin et al., 1996; Muller et al., 1994; Stojdl et al., 2000a). Interestingly, RIG-I and mitochondrial antiviral signaling protein (MAVS) knockout mice succumb to infection whereas as MDA5 knockout mice do not (Kato et al., 2006). Fas-associated protein with death domain (FADD), an adapter molecule that bridges the Fas receptor to capase-8 has also been implicated in resistance to VSV as knockout mice succumb to infection and appear defective in type I IFN expression.
The type I IFN pathway displays pleiotropic activities and is involved not only in innate immune responses, but also in acquired immune responses, cell growth and apoptosis (Stetson and Medzhitov, 2006). Not surprisingly, different cell types were shown to differentially express various components of this pathway, which may affect their susceptibility to VSV infection. For example, human genomes contain at least 12 genes encoding closely related IFN-a subtypes, and, in addition contain genes for IFN-β, IFN-κ, IFN-ε/τ, and others (Hardy et al., 2004; Oritani et al., 2001). While the biological significance of such redundancy in type I IFNs (all bind to the same heterodimeric receptor) in mammalian cells is not clear, the situation is further complicated by the fact that this system coexists with a recently discovered type III IFN system. The triggers for expression of type III IFN (also called IFN-I or IL-28/29), and its activities are very similar to those of type I IFNs, but type I and III IFNs bind to unrelated heterodimeric receptors (Levy et al., 2011). A recent study showed organ and tissue-dependent differential expression of type I and type III IFNs in mice (Sommereyns et al., 2008), possibly determining cell, tissue and organ tropism of VSV in the infected host. In the CNS, glial cells and/or infiltrating leukocytes are recognized as expressing an array of PRRs capable of initiating type I IFN production either constitutively or following activation. The expression of viral sensors by neurons and the role of IFN production by this cell type has been more controversial (see the VSV neurotropism section of this review). The role of innate antiviral responses in VSV cell tropism was also studied in regard to VSV as an oncolytic agent against various cancers. The oncoselectivity of VSV is mainly based on defective or reduced type I IFN responses in cancer cells (Barber, 2004; Lichty et al., 2004; Stojdl et al., 2000b, 2003) as these responses are generally anti-proliferative, anti-angiogenic, and pro-apoptotic (Wang et al., 2011), and therefore unfavorable for tumor formation. Downregulation or inactivation of specific genes associated with type I IFN responses (such as PKR, IRF3, or IFN receptor) were shown in some cancer types (Balachandran and Barber, 2004; Marozin et al., 2008, 2010; Moussavi et al., 2010; Zhang et al., 2010). IFN signaling can also be inhibited by MEK/ERK signaling (Noser et al., 2007) or by epigenetic silencing of IFN responsive transcription factors IRF7 or IRF5 (Li and Tainsky, 2011). Importantly, many cancers display potent IFN signaling and resistance to VSV (Linge et al., 1995; Matin et al., 2001; Naik and Russell, 2009; Pfeffer et al., 1996; Saloura et al., 2010; Stojdl et al., 2000b, 2003; Sun et al., 1998; Wong et al., 1997). Our own studies showed a good correlation between susceptibility of human pancreatic ductal adenocarcinoma cell lines to VSV and their type I IFN signaling status (Moerdyk-Schauwecker et al., 2013; Murphy et al., 2012).
One factor that may greatly affect susceptibility of cells to VSV is their prior infection with another virus. The presence of another virus prior to VSV infection or during superinfection can inhibit VSV replication (de la Torre et al., 1985; Doyle and Holland, 1972; Kornbluth et al., 1990; Nicholson et al., 1981; Otto and Lucas-Lenard, 1980), have no effect on VSV (Nicholson et al., 1981; Whitaker-Dowling et al., 1983), or even help VSV replication. The latter example are cervical carcinoma cancer cells infected with human papilloma viruses (HPV) that show improved VSV infection and killing compared to cervical carcinomas not infected with HPV (Le Boeuf et al., 2012). HPV can inhibit IFN signaling, possibly creating a more hospitable environment for VSV. Vaccinia virus coinfection demonstrated a similar, positive effect on VSV replication. This was due to the ability of vaccinia virus to inhibit IFN-β signaling, resulting in enhanced VSV replication (Le Boeuf et al., 2010).
VSV infection induces cell death via the mitochondrial (intrinsic) or death receptor (extrinsic) pathway or both (Cary et al., 2011; Gaddy and Lyles, 2005, 2007; Sharif-Askari et al., 2007). Interestingly, while wild type (WT) VSV induces apoptosis primarily via the intrinsic pathway, VSV M51 mutants induce apoptosis primarily via the extrinsic pathway (Cary et al., 2011; Gaddy and Lyles, 2005). Also, it was reported that VSV M has a cryptic mitochondrial targeting motif, and expression led to loss of mitochondrial membrane permeability and altered mitochondrial organization in a manner similar to viral infection (Lichty et al., 2006). As the M protein itself can induce apoptosis (Kopecky and Lyles, 2003; Kopecky et al., 2001), the authors speculated that the mitochondrial targeting of the M protein may contribute to VSV mediated apoptosis or, alternatively to inhibit this response (Lichty et al., 2006). Correlation between apoptotic status of cells and levels of VSV replication in these cells is not clear, although it was shown that VSV infection induces p53 gene transcriptionally via type I IFN signaling (Takaoka et al., 2003). This activation had an antiviral effect likely due to p53-mediated apoptosis, and p53 knockout mouse succumb to VSV infection and 100-fold increase in VSV was found in sera when compared to WT mice.
3.2. Altering VSV tropism by targeting replication
VSV tropism can be effectively changed by modifying the viral genome and/or the cellular environment to make it more or less hospitable for viral replication. While experimental adaptation of VSV to a particular cell type in vitro is still used in some studies (Wollmann et al., 2005), most approaches are based on rational designs of VSV-based recombinants generated using a reverse genetic system (Lawson et al., 1995; Whelan et al., 1995). A hallmark of VSV is its rapid replication. Any strategy that attenuates VSV replication (e.g., inhibiting viral polymerase activities, VSV mRNA stability and/or translatability, virus abilities to evade antiviral responses) has the potential to alter VSV cell tropism. Non-specific attenuation via rearrangement of the highly conserved VSV gene order (Flanagan et al., 2001; Novella et al., 2004) may change the cell tropism of VSV as cell types less permissive to wild type WT VSV may become resistant to the attenuated VSV-recombinant. Similar results can be achieved by mutation of individual proteins. For example, single amino acid changes in VSV L can abolish its mRNA cap methylation ability, resulting in a so-called host range phenotype characterized by the ability of the VSV mutants to replicate only in some permissive cell lines (Grdzelishvili et al., 2005, 2006).
More rational strategies employ VSV recombinants with mutations diminishing VSV’s abilities to evade cellular antiviral responses. VSV M protein localizes to the nuclear membrane where it interacts with cellular Rae1 complexes to thwart antiviral response by inhibition of cellular mRNA trafficking and possibly mRNA synthesis through interaction with transcription factor II D (Faria et al., 2005; Rajani et al., 2012; von Kobbe et al., 2000; Yuan et al., 1998). The well-studied VSV M51 mutation, either a mutation or deletion of the methionine codon at position 51 of the M protein, abrogates M protein’s ability to inhibit nuclear exit of host mRNAs (Black et al., 1993; Coulon et al., 1990; Stojdl et al., 2003). As a result, VSV-M51 recombinants are more attenuated in normal cell types, but are still very effective in cells with defective antiviral responses (Hastie and Grdzelishvili, 2012; Wang et al., 2011).
VSV recombinants are also designed to express host molecules modulating the cellular environment to make it more or less hospitable to virus. VSV encoding IFN-β is being used in a clinical trial against hepatocellular carcinoma (clinicaltrials.gov, 2012, Trial ID: NCT01628640). VSV-IFN-β is highly attenuated in normal tissues, as increased secretion of IFN-β stimulates a protective antiviral response in surrounding cells (Obuchi et al., 2003). Instead, VSV-IFN-β specifically targets cancer cells, which are frequently defective in type I IFN signaling (Wang et al., 2011). Cell-specific micro-RNA expression can also be used to target VSV to s particular cell type. A VSV with a let-7 micro-RNA target sequence demonstrated increased tropism for cells, like cancer cells, that express lower levels of the let-7 micro-RNA (Kelly et al., 2010). Beyond direct virus modification, VSV tropism can be altered through drug treatments that modulate the cellular environment, for example by inhibiting the type I IFN response. Treatment with Jak inhibitor 1 was shown to dramatically improve VSV cancer killing in resistant cancer cells with intact IFN response (Basu et al., 2006; Moerdyk-Schauwecker et al., 2013; Paglino and van den Pol, 2011). A recombinant VSV encoding miRNA-4661 was able to repress IFN-β expression to inhibit host antiviral response (Li et al., 2012). As mentioned above, co-infection of VSV with vaccinia virus can be used to evade type I IFN response, thus enhancing VSV infection and replication (Le Boeuf et al., 2010).
4. Exit
Even if cells provide a hospitable environment for virus infection and replication, there is no guarantee that progeny virions will be produced or that produced virions will be highly infectious. VSV virions acquire an envelope by budding through sites in the host plasma membrane enriched in VSV G protein (Swinteck and Lyles, 2008). Viral RNPs are transported to the site of budding in a microtubule dependent manner (Das et al., 2006; Heinrich et al., 2010; Lyles and Rupprecht, 2007). Association of the RNP with VSV M results in RNP condensation and facilitates budding (Swinteck and Lyles, 2008). VSV budding depends on the interactions of M protein with host factors (Lyles and Rupprecht, 2007). The N terminus of VSV M interacts with dynamins 1 and 2 (Harty et al., 1999; Raux et al., 2010) and is thought to affect endocytic vesicle trafficking as blocking the M-dynamin interaction inhibited budding and resulted in accumulation of nucleocapsids at the plasma membrane (Raux et al., 2010). The direct interaction of a host membrane localized E3 ubiquitin ligase, NEDD4, with the PPxY motif of M enhances budding (Harty et al., 1999, 2001). Ubiquitination of M also appears to be required, possibly for recruitment of host factors, as a decrease in availability of cytoplasmic ubiquitin reduces VSV titers (Harty et al., 2001). The PSAP region of VSV M is thought to regulate cytopathogenesis in a species-dependent manner (Irie et al., 2012). Disruption of the PSAP region alters apoptotic outcomes, resulting in virus attenuation in mice but enhanced cytopathic effect in insect cells. The authors speculated that the PSAP motif regulating cytopathogenicity in a species-dependent manner, and possibly important for maintaining persistence of VSV in an insect host (Irie et al., 2012).
In addition to all five VSV proteins, host factors from the cytoplasm and plasma membrane can be incorporated into progeny virions. Our proteomic analysis using mass spectrometry showed a large number of host proteins associated with virions of VSV, and this profile was dependent on the cell type (BHK-21, A549 or 4T-1) used to generate virions (Moerdyk-Schauwecker et al., 2009). Virions purified from these cell lines also differed by more than an order of magnitude in the number of infectious particles per μg of total protein, suggesting properties of the host cell may influence the infectivity of the resulting virions.
Differential post-translational modification of VSV G by host proteins may prevent its cell membrane localization or incorporation into progeny (Wyers et al., 1989). Following translation, VSV G travels through the endoplasmic reticulum and Golgi apparatus where it undergoes post-translation modification and interacts sequentially with chaperone proteins BiP (GRP78) and calnexin (Hammond and Helenius, 1994) that assist with folding G into its native conformation. The VSV G luminal domain may mediate its localization to the plasma membrane but host proteins responsible for this localization have yet to be identified (Compton et al., 1989). Interestingly, virions resulting from infection of Drosophila melanogaster cells contained 4–5 times less G protein than virions from chicken embryo cells (Wyers et al., 1980). The authors proposed that changes to the carbohydrate chains or sialic acid additions would impair the ability of G to incorporate into the plasma membrane or viral envelope (Wyers et al., 1980). The impact of cell type on virion infectivity was recently demonstrated for another nonsegmented negative-strand virus, respiratory syncytial virus (RSV) (Kwilas et al., 2009). It was found that different cell lines produced RSV G proteins with varying sizes and that cleavage of RSV G protein’s C terminus as well as varying glycosylation patterns was responsible for the differential infectivity profiles of produced virions (Kwilas et al., 2009).
5. Neurotropism of VSV
5.1. VSV neurotropism
While VSV is an attractive candidate for use as a vaccine, gene therapy or oncolytic vector, its inherent neurovirulence needs to be addressed for use as a safe, clinically relevant vector. VSV IN has been described as the causative agent of severe encephalitis in human subjects (Quiroz et al., 1988) and has been shown to result in potentially lethal CNS infection in a variety of rodent and non-human primate models (Johnson et al., 2007). In non-human primate models of WT VSV infection, i.n. injection showed no evidence of virus in the brain or spinal cord (Johnson et al., 2007), however i.t. injection resulted in neurological disease and animal sacrifice in two independent studies (Johnson et al., 2007; Mire et al., 2012).
VSV neurotropism was studied in detail in mice, where s.c. administration commonly fails to result in CNS infection, but between 50% and 90% of WT VSV infected rodents have been reported to die following i.n. administration (Muik et al., 2012; Reiss et al., 1998) from acute encephalitis characterized by neuronal necrosis and efficient viral replication in both the brain and spinal cord (Preble et al., 1980). The i.n. administration of VSV in rodents first leads to viral replication in the olfactory receptor neurons present in the nasal cavity followed by rapid transmission to the lungs and the olfactory bulb where focal cytopathology occurs (Forger et al., 1991; Reiss et al., 1998). Following invasion of the olfactory bulb, VSV continues to replicate invasively and by day 4–5 the virus reaches the olfactory ventricle (Reiss et al., 1998). It appears likely that the virus can then spread to other brain regions via non-neuronal routes such as ependymal cells and/or cerebrospinal fluid (Bi et al., 1995a,b; Christian et al., 1996; Plakhov et al., 1995b). While earlier studies have indicated that VSV can access other brain regions trans-synapically using both anterograde and retrograde transport (Bi et al., 1995a,b; Christian et al., 1996; Plakhov et al., 1995b) such dissemination routes remain controversial and the absence of virus outside of the olfactory bulb argues against the involvement of such axonal transport pathways (van den Pol et al., 2002). VSV then spreads caudally from the olfactory bulbs throughout the CNS infecting many cell types including ependymal cells (Ireland and Reiss, 2006), astrocytes, and even microglia (Chauhan et al., 2010). The entry of VSV into the ventricles precipitates encephalitis, and breakdown of the blood-brain barrier (BBB) begins at day 6 following infection (Bi et al., 1995a,b). Here, CD4+ and CD8+ T-cells can first be detected within the CNS of infected animals (Ireland and Reiss, 2006). Breakdown of the BBB and the reactive astrogliosis peak in infected tissues at day 8 following viral administration (Bi et al., 1995a,b; Christian et al., 1996; Plakhov et al., 1995b), by which time the virus has reached the hindbrain (Reiss et al., 1998). Necrosis occurs around the ventricles and VSV-associated necrotic lesions can be recognized in the lumbosacral region of the spinal cord (Bi et al., 1995a,b; Christian et al., 1996; Forger et al., 1991; Plakhov et al., 1995b). Mortality associated with WT VSV occurs during the period of 7–10 days post-infection correlating with peak viral titers (Forger et al., 1991). Interestingly, B-cells do not infiltrate the CNS until 14 days post infection and do so only in surviving animals after viral clearance and behavioral signs of recovery (Bi et al., 1995a,b).
The mechanisms underlying VSV neurotropism is not clear. The ability of VSV to replicate in the CNS may be due to increased trafficking of the virus to the CNS compared to non-neuronal tissues. Alternatively, or in addition to the preferential homing of the virus to this site, neurotropism may result from differences in resistance mechanism in the CNS compared to peripheral organs that favor VSV persistence and/or replication in the brain (Trottier et al., 2005) as originally proposed by Stanners and Goldberg (1975). Indeed, VSV is capable of causing CNS infection in mice even in the presence of peripheral neutralizing antibody responses.
A role for interferon in protective host responses within the brain is demonstrated by the observation that conditional knockout animals that possess neuroectodermal cells that lack type I IFNAR expression die following even low dose i.n. VSV administration and show much greater viral loads in the brain than WT mice (Detje et al., 2009). Such a finding is underscored by the ability of IFN-α or IFN-β pretreatment to strongly inhibit VSV replication in the NB41A3 neuroblastoma cell line or primary neuron cultures (Trottier et al., 2005). Interestingly, such protection does not occur in a PKR-mediated manner as determined in neuronal cells expressing the PKR-inhibiting influenza viral product NS1, and occurs in a NO and superoxide independent manner (Trottier et al., 2005). In addition, several ISGs have been shown to protect CNS against VSV. BST2 is an ISG encoded type 2 transmembrane protein that functions as an antiviral restriction factor. Importantly, recent studies have indicated that BST2 inhibits VSV release by neuroblastoma cells (Sarojini et al., 2011). In addition, the IFN-induced with tetratricopeptide repeats 2 (Ifit2) gene has been implicated in VSV neurotropism as Ifit2 deficient animals show increased susceptibility to VSV infection delivered via the i.n. route of administration but not when this virus is delivered directly into the CNS (Fensterl et al., 2012).
Recent evidence suggests that neurons, both in vitro and in situ, can be induced to express IFN-α or IFN-β following RNA virus challenge (Daffis et al., 2008; Delhaye et al., 2006; Nazmi et al., 2011; Peltier et al., 2010; Prehaud et al., 2005; Suthar et al., 2010; Wang et al., 2010). However, it should be noted that in some of these studies only a small proportion (3%) of neurons appear to express these cytokines (Delhaye et al., 2006). Similarly, while VSV infection is associated with a rapid induction of an array of cytokines and chemokines within the mouse CNS following i.n. infection, IFN-α or IFN-β levels are not elevated within the brain (Ireland and Reiss, 2006). As such, the susceptibility of brain tissue to VSV infection appears to stem primarily from insufficient levels of IFN production at this site. Such a conclusion is supported by studies in rats demonstrating that prophylactic IFN-α treatment alleviates neurotoxicity associated with the administration of high doses of a recombinant VSV (Shinozaki et al., 2005), and the high tolerance of animals to VSV engineered to express IFN-β(Jenks et al., 2010). An explanation for insufficient levels of IFN production in the brain could lie in the reported absence of TLR7, TLR8 and TLR9 expression in resting and virally challenged neurons (McKimmie and Fazakerley, 2005; Mishra et al., 2006). But the absence of such PRRs does not preclude the utilization of other viral sensors that signal via IRF-3 rather than IRF-7 to induce IFN production. Indeed, several studies have demonstrated the ability of neuronal cells to functionally respond to RNA viruses via TLR3 (Daffis et al., 2008; Delhaye et al., 2006; Peltier et al., 2010; Prehaud et al., 2005) and RIG-I (Nazmi et al., 2011; Peltier et al., 2010; Suthar et al., 2010; Wang et al., 2010). Taken together, the available data indicate that CNS cells are responsive to the antiviral actions of type I IFNs to limit VSV replication and/or release following infection. However, it is presently unclear why neurons are not a significant source of protective IFNs following VSV infection despite the apparent means to detect this pathogen.
5.2. Role of T-cells and glial cells in VSV neurotoxicity
Host response plays a pivotal role in the progressive inflammatory neurological damage following VSV infection of the CNS. As described in the previous section, neutrophils, natural killer cells, macrophages, and T cells are sequentially recruited into the CNS and such infiltration is associated with the robust expression of proinflammatory cytokines such as IL-1β and IFN-γ and the production of an array of chemokines including CCL1, CXCL10, CCL5, CCL4, CXCL1, CCL2, and CCL11 (Ireland and Reiss, 2006). Furthermore, lipid mediators also appear to play an important role in VSV-induced CNS pathology as inhibition of leukotriene production or their actions significantly reduces early neutrophil recruitment to the CNS and increases BBB disruption (Chen et al., 2001). Similarly, cyclooxygenase (COX) inhibitors prevent virally mediated BBB compromise and can even limit VSV propagation both in vitro and in vivo, perhaps indirectly via release of NO production from COX-mediated antagonism (Chen et al., 2000).
Interestingly, athymic mice show ten times greater recoverable viral loads than age and sex matched euthymic mice, and succumb to VSV neuropathology 1–2 days earlier (Huneycutt et al., 1993). Importantly, adoptive T-cell transfer to T-cell deficient mice can significant alter disease progression and confers protection against VSV infection. Furthermore, depletion of either CD4+ or CD8+ T-lymphocyte populations results in the wider distribution of viral antigen within the CNS, increased tissue necrosis and higher levels of inflammation (Huneycutt et al., 1993). Taken together, these studies indicate that T-cells play a minor role in histopathology and instead contribute to protective host responses to limit viral replication and dissemination. Further evidence for such T-cell-mediated protection comes from the demonstration that prophylactic or therapeutic administration of IL-12, a pivotal cytokine in the generation of cell-mediated immunity, increases T-cell infiltration into the olfactory bulb and augments MHC molecule and IFN-γ expression in the brain and peripherally, respectively, of infected animals (Bi et al., 1995; Ireland et al., 1999; Komatsu et al., 1997). Importantly, IL-12-mediated augmentation of host immune responses is associated with a marked decrease in VSV loads within the CNS, increased survival and enhanced recovery of infected animals (Bi et al., 1995; Ireland et al., 1999; Komatsu et al., 1997). Finally, such T-cell mediated protection may underlie the sexual dimorphism noted in mice in which female animals exhibit less severe VSV-induced neurological disease associated with earlier and higher levels of MHC molecule expression and greater T-cell infiltration (Barna et al., 1996).
As such, the CNS histopathology associated with VSV infection, and especially the neurological damage seen during acute infection, are likely to be due to the inflammatory responses of either innate immune cells such as neutrophils and NK cells that are recruited into the brain within 1 to 3 days following i.n. administration or by resident CNS cells. While the brain has traditionally been viewed as a “victim organ” of infiltrating leukocytes, it has become apparent that specialized resident glial cells, such as microglia and astrocytes, play an essential role in regulating BBB permeability, promoting the recruitment of leukocytes, and the activation of such cells following infiltration during CNS disease states (Bauer et al., 1995; Dong and Benveniste, 2001; Fischer and Reichmann, 2001; Stoll and Jander, 1999). Microglia are resident myeloid immune cells of the CNS that function as facultative phagocytes and express antigen presenting MHC class II molecules (Hickey and Kimura, 1988). Importantly, these cells produce key pro-inflammatory cytokines including IL-1β(Martin et al., 1993), TNF-α(Streit et al., 1998), and IL-6 (Kiefer et al., 1993) following activation. Astrocytes are the major glial cell type in the brain and can also express an array of cytokines and chemokines that can either promote protective or damaging inflammation (Dong and Benveniste, 2001). As such, these cells are ideally situated to detect and respond to invading pathogens including neurotropic viruses. Importantly, we have documented that microglia and astrocytes can be productively infected by VSV and respond by producing IL-6, TNF-α, and IL-1β(Chauhan et al., 2010; Furr et al., 2011, 2010), while earlier studies have demonstrated that these glial cells respond to VSV by proliferating, producing inducible NO synthase, and increasing the cell surface expression of MHC class II molecules (Bi et al., 1995), responses that are likely to set the stage for subsequent inflammatory damage.
Studies from our group and others have demonstrated that microglia and astrocytes constitutively or inducibly express these receptors, and that TLR activation is a significant contributor to sustained glial activation, inflammatory mediator production, and neuronal loss during viral CNS infections (Furr and Marriott, 2012). While cell-surface receptors like TLRs appear to play an important role in glial responses to extracellular pathogens or those present in intracellular compartments, the ability of such cells to respond to pathogens in the absence of TLR expression suggests that additional mechanisms are present. Our recent studies demonstrate that VSV replication is required to elicit robust immune responses by infected microglia and astrocytes (Chauhan et al., 2010), indicating that mechanisms exist to sense the presence of replicative viral products within infected cells. We have shown that heat inactivated WT VSV or a highly attenuated VSV mutant (Grdzelishvili et al., 2005) elicit glial immune responses that are an order of magnitude smaller than those induced by WT VSV (Chauhan et al., 2010). These results suggest that VSV-induced glial responses are not predominantly mediated by cell surface and/or endosomal PRRs, and active viral replication is a critical requirement for such responses. We have also demonstrated the expression of RIG-I in uninfected mouse brain tissue and isolated murine microglia and astrocytes constitutively express RIG-I (Furr et al., 2008). Furthermore, the expression of RIG-I has been confirmed in human glial cells (Furr et al., 2010; Yoshida et al., 2007). More direct evidence for the functional nature of RIG-I expression in glia comes from our observation that this molecule associates with its adaptor molecule IPS-1 following VSV infection and from the finding that the spe-cific RIG-I ligand, 5′ppp-ssRNA, elicits human astrocyte immune responses (Furr et al., 2010). Importantly, we also showed that RIG-I knockdown significantly reduces inflammatory cytokine production by VSV-infected human astrocytes and inhibits the production of soluble neurotoxic mediators by virally challenged cells (Furr et al., 2010). These findings directly implicate RIG-I in the initiation of glial inflammatory immune responses and suggest a potential mechanism underlying the neuronal cell death associated with acute viral CNS infections.
5.3. Altering VSV neurotropism
Replication defective VSV vectors have been developed and tested to overcome the inherent neurovirulence of replication-competent WT VSV. These approaches have centered on the replacement of VSV G, which seems to be required for its neurotoxic effects, to produce a pseudotyped VSV possessing envelope proteins of heterologous viruses (Tani et al., 2011). For example, VSV pseudotyped with the envelope glycoprotein of non-neurotropic LCMV are capable of infecting and killing cancer cells. However, such an engineered virus, VSV-LCMV-GP, spares human neurons in vitro and rat neurons in vivo (Muik et al., 2011). Semi-replication competent approaches have included the development of a system that uses recombinant VSVs lacking either the VSV G gene VSV L gene. Upon co-infection oncoloysis occurs as does spread of non-replicative progeny. This system was as potent at WT VSV in vivo in rodents but lacked neurotoxicity (Muik et al., 2012).
The neurotoxicity of WT VSV can be abolished by insertion of IFN-β gene. One of such recombinants expressing human IFN-β, VSV-IFN-β virus, showed no neurological signs or other abnormalities when injected into the liver of Buffalo rats or macaques (Jenks et al., 2010). Based on these studies, VSV-IFN-β entered a phase I clinical trial against hepatocellular carcinoma (clinicaltrials.gov, 2012, Trial ID: NCT01628640).
A proof of concept recombinant VSV was highly attenuated by the addition of two GFP reporter genes to the 3′ end of the VSV genome and this engineered virus demonstrated a significantly reduced neurotoxicity (van den Pol and Davis, 2013). Finally, VSV has been engineered to express picornovirus internal ribosome entry site (IRES) elements that possess restricted activity in neuronal tissues and this IRES-containing recombinant VSV shows attenuated neuropathogenesis (Ammayappan et al., 2013).
Although the major effort has been on the development of safe VSV recombinants that are unable to cause CNS infection and damage, certain application would benefit from VSV vectors specifically targeting cancer cells within CNS. Thus, a new VSV recombinant was selected after 30 repeated passages on glioblastoma cells (Wollmann et al., 2005). This replication-competent VSV, VSV-rp30, contains two silent mutations and two missense mutations, one in VSV P and one in VSV L. VSV-rp30 was able to selectively infect and kill implanted olfactory bulb tumors after i.n. inoculation (Ozduman et al., 2008).
6. Concluding remarks
With applications ranging from gene therapy to cancer targeting, a better understanding of the biological basis for VSV tropism is paramount. Recent identification of LDLR as a potential receptor for VSV is exciting (Finkelshtein et al., 2013), but additional studies confirming these results are necessary to explain the host range of VSV and also provide a means to predict and/or direct its tropism via receptor manipulation. Although the wide range tropism of VSV is a plus in many applications, other applications would benefit from more specific cell targeting. It is likely that more rationally designed VSV-based recombinants expressing foreign attachment genes will be generated to limit VSV tropism. Biosafety of such chimeras is an important issue as VSV expressing the p14 FAST reptilian reovirus virus fusion-associated protein demonstrated enhance neurotoxicity in mice (Brown et al., 2009). Tropism and safety of VSV are greatly controlled by cellular IFN responses, which can be exploited to target and kill IFN defective cancer cells without damaging healthy tissues. However, new approaches are needed to specifically target cancer cells retaining functional IFN signaling. While WT VSV is not acceptable as a clinical vector, there are some conflicting reports even in regard to the safety of VSV recombinants depending on the route of administration (Johnson et al., 2007). This is particularly important for oncolytic applications of VSV in immunocompromised cancer patients. Potential evolution of VSV used in clinical applications should be more seriously studied. With more VSV recombinants likely to begin clinical trials, we expect to see an increased focus on preventing premature clearance of therapeutic VSV by host immune responses. VSV will continue to be one of the most popular viruses and because of its unique qualities it remains an essential tool for discovery of basic biological research and will play an important role in vaccine development, gene and oncolytic therapies.
Acknowledgments
We are grateful to Megan Moerdyk-Schauwecker for critical comments on the manuscript. This work was supported by NIH grant 1R15CA167517-01 (to V.Z.G.).
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Ammayappan A, Nace R, Peng KW, Russell SJ. Neuroattenuation of vesicular stomatitis virus through picornaviral internal ribosome entry sites. J Virol. 2013;87:3217–3228. doi: 10.1128/JVI.02984-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Breton C, Barber GN, Russell SJ, Peng KW. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum Gene Ther. 2012;23:484–491. doi: 10.1089/hum.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Barik S, Banerjee AK. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992;89:6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- Basu M, Maitra RK, Xiang Y, Meng X, Banerjee AK, Bose S. Inhibition of vesicular stomatitis virus infection in epithelial cells by alpha interferon-induced soluble secreted proteins. J Gen Virol. 2006;87:2653–2662. doi: 10.1099/vir.0.82039-0. [DOI] [PubMed] [Google Scholar]
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- Bergman I, Griffin J, Gao Y, Whitaker-Dowling P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int J Cancer. 2007;121:425–430. doi: 10.1002/ijc.22680. [DOI] [PubMed] [Google Scholar]
- Bergold GH, Suarez OM, Munz K. Multiplication in and transmission by Aedes aegypti of vesicular stomatitis virus. J Invertebr Pathol. 1968;11:406–428. doi: 10.1016/0022-2011(68)90190-0. [DOI] [PubMed] [Google Scholar]
- Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995a;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z, Quandt P, Komatsu T, Barna M, Reiss CS. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol. 1995b;155:5684–5689. [PubMed] [Google Scholar]
- Black BL, Rhodes RB, McKenzie M, Lyles DS. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor S, Maelfait J, Krumbach R, Beyaert R, Randow F. Endoplasmic reticulum chaperone gp96 is essential for infection with vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2010;107:6970–6975. doi: 10.1073/pnas.0908536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon JM, Smith BD. Chemical control of phospholipid distribution across bilayer membranes. Med Res Rev. 2002;22:251–281. doi: 10.1002/med.10009. [DOI] [PubMed] [Google Scholar]
- Bose S, Mathur M, Bates P, Joshi N, Banerjee AK. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J Gen Virol. 2003;84:1687–1699. doi: 10.1099/vir.0.19074-0. [DOI] [PubMed] [Google Scholar]
- Boudreau JE, Bridle BW, Stephenson KB, Jenkins KM, Brunelliere J, Bramson JL, Lichty BD, Wan Y. Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Mol Ther. 2009;17:1465–1472. doi: 10.1038/mt.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Stephenson KB, Hanson S, Kucharczyk M, Duncan R, Bell JC, Lichty BD. The p14 FAST protein of reptilian reovirus increases vesicular stomatitis virus neuropathogenesis. J Virol. 2009;83:552–561. doi: 10.1128/JVI.01921-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning MJ, Huneycutt BS, Huang AS, Reiss CS. Replication-defective viruses modulate immune responses. J Immunol. 1991;147:2685–2691. [PubMed] [Google Scholar]
- Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. Nonsegmented negative-strand viruses as vaccine vectors. J Virol. 2006;80:10293–10306. doi: 10.1128/JVI.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro FA, Bianconi ML, Weissmuller G, Stauffer F, Da Poian AT. Membrane recognition by vesicular stomatitis virus involves enthalpy-driven protein-lipid interactions. J Virol. 2002;76:3756–3764. doi: 10.1128/JVI.76.8.3756-3764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro FA, Lapido-Loureiro PA, Cordo SM, Stauffer F, Weissmuller G, Bian-coni ML, Juliano MA, Juliano L, Bisch PM, Da Poian AT. Probing the interaction between vesicular stomatitis virus and phosphatidylserine. Eur Biophys J. 2006;35:145–154. doi: 10.1007/s00249-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Cary ZD, Willingham MC, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis in U87 Glioblastoma Cells by a Type II Death receptor mechanism and induces cell death and tumor clearance in vivo. J Virol. 2011;85:5708–5717. doi: 10.1128/JVI.02393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave DR, Hendrickson FM, Huang AS. Defective interfering virus particles modulate virulence. J Virol. 1985;55:366–373. doi: 10.1128/jvi.55.2.366-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G, Xu S, Watanabe M, Jayakar HR, Whitt MA, Gingrich JR. Enhanced oncolytic activity of vesicular stomatitis virus encoding SV5-F protein against prostate cancer. J Urol. 2010;183:1611–1618. doi: 10.1016/j.juro.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Sterka DG, Jr, Furr SR, Marriott I, Grdzelishvili VZ. Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology. 2010 doi: 10.1016/j.virol.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang S, Banerjee AK, Chen M. N-terminal phosphorylation of phosphoprotein of vesicular stomatitis virus (VSV) is required for preventing nucleoprotein from binding to cellular RNAs and for functional template formation. J Virol. 2013 doi: 10.1128/JVI.02761-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Restivo A, Reiss CS. Leukotrienes play protective roles early during experimental VSV encephalitis. J Neuroimmunol. 2001;120:94–102. doi: 10.1016/s0165-5728(01)00415-5. [DOI] [PubMed] [Google Scholar]
- Chen N, Warner JL, Reiss CS. NSAID treatment suppresses VSV propagation in mouse CNS. Virology. 2000;276:44–51. doi: 10.1006/viro.2000.0562. [DOI] [PubMed] [Google Scholar]
- Christian AY, Barna M, Bi Z, Reiss CS. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9:195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- Clemens MJ, Vaquero CM. Inhibition of protein synthesis by double-stranded RNA in reticulocyte lysates: evidence for activation of an endoribonuclease. Biochem Biophys Res Commun. 1978;83:59–68. doi: 10.1016/0006-291x(78)90397-2. [DOI] [PubMed] [Google Scholar]
- Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JM. Heptad-repeat sequences in the glycoprotein of rhabdoviruses. Virus Genes. 1995;10:107–114. doi: 10.1007/BF01702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll JM. Synthetic peptides from the heptad repeats of the glycoproteins of rabies, vesicular stomatitis and fish rhabdoviruses bind phosphatidylserine. Arch Virol. 1997;142:2089–2097. doi: 10.1007/s007050050227. [DOI] [PubMed] [Google Scholar]
- Colonno RJ, Lazzarini RA, Keene JD, Banerjee AK. In vitro synthesis of messenger RNA by a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977;74:1884–1888. doi: 10.1073/pnas.74.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Ivanov IE, Gottlieb T, Rindler M, Adesnik M, Sabatini DD. A sorting signal for the basolateral delivery of the vesicular stomatitis virus (VSV) G protein lies in its luminal domain: analysis of the targeting of VSV G-influenza hemagglutinin chimeras. Proc Natl Acad Sci U S A. 1989;86:4112–4116. doi: 10.1073/pnas.86.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JH, Lyles DS. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish TE, Stallknecht DE, Brown CC, Seal BS, Howerth EW. Pathogenesis of experimental vesicular stomatitis virus (New Jersey serotype) infection in the deer mouse (Peromyscus maniculatus) Vet Pathol. 2001;38:396–406. doi: 10.1354/vp.38-4-396. [DOI] [PubMed] [Google Scholar]
- Coulon P, Deutsch V, Lafay F, Martinet-Edelist C, Wyers F, Herman RC, Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J Gen Virol. 1990;71(Pt 4):991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, Brunner LJ, Kobinger GP. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78:912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp EW, Mare CJ, Cupp MS, Ramberg FB. Biological transmission of vesicular stomatitis virus (New Jersey) by Simulium vittatum (Diptera: Simuliidae) J Med Entomol. 1992;29:137–140. doi: 10.1093/jmedent/29.2.137. [DOI] [PubMed] [Google Scholar]
- Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 2009;5:e1000394. doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton DK, Massol RH, Whelan SP, Kirchhausen T. The length of vesicular stomatitis virus particles dictates a need for actin assembly during clathrin-dependent endocytosis. PLoS Pathog. 2010;6:e1001127. doi: 10.1371/journal.ppat.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S, Samuel MA, Suthar MS, Gale M, Diamond MS. Toll-like receptor 3 has a protective role against west nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Nayak D, Zhou Y, Pattnaik AK. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Mathur M, Gupta AK, Janssen GM, Banerjee AK. RNA polymerase of vesicular stomatitis virus specifically associates with translation elongation factor-1 alphabetagamma for its activity. Proc Natl Acad Sci U S A. 1998;95:1449–1454. doi: 10.1073/pnas.95.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC, Davila M, Sobrino F, Ortin J, Domingo E. Establishment of cell lines persistently infected with foot-and-mouth disease virus. Virology. 1985;145:24–35. doi: 10.1016/0042-6822(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dinh PX, Beura LK, Panda D, Das A, Pattnaik AK. Antagonistic effects of cellular poly(c) binding proteins on vesicular stomatitis virus gene expression. J Virol. 2011;85:9459–9471. doi: 10.1128/JVI.05179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Doyle M, Holland JJ. Virus-induced interference in heterologously infected HeLa cells. J Virol. 1972;9:22–28. doi: 10.1128/jvi.9.1.22-28.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreja H, Piechaczyk M. The effects of N-terminal insertion into VSV-G of an scFv peptide. Virol J. 2006;3:69. doi: 10.1186/1743-422X-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet BS, Campbell CL, Stuart MA, Wilson WC. Vector competence of Culicoides sonorensis (Diptera: Ceratopogonidae) for vesicular stomatitis virus. J Med Entomol. 2005;42:409–418. doi: 10.1603/0022-2585(2005)042[0409:vcocsd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Drolet BS, Stuart MA, Derner JD. Infection of Melanoplus sanguinipes grasshoppers following ingestion of rangeland plant species harboring vesicular stomatitis virus. Appl Environ Microbiol. 2009;75:3029–3033. doi: 10.1128/AEM.02368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Old-stone MBA. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC. A let- 7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- Eidelman O, Schlegel R, Tralka TS, Blumenthal R. pH-dependent fusion induced by vesicular stomatitis virus glycoprotein reconstituted into phospho-lipid vesicles. J Biol Chem. 1984;259:4622–4628. [PubMed] [Google Scholar]
- Emerson SU, Wagner RR. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973;12:1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria PA, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, Arana C, van Deursen J, Fontoura BM. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012;8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Flanagan EB, Zamparo JM, Ball LA, Rodriguez LL, Wertz GW. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J Virol. 2001;75:6107–6114. doi: 10.1128/JVI.75.13.6107-6114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger JM, Bronson RT, Huang AS, Reiss CS. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J Virol. 1991;65:4950–4958. doi: 10.1128/jvi.65.9.4950-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr SR, Chauhan VS, Moerdyk-Schauwecker MJ, Marriott I. A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells. J Neuroinflamm. 2011;8:99. doi: 10.1186/1742-2094-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr SR, Chauhan VS, Sterka D, Jr, Grdzelishvili VZ, Marriott I. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol. 2008:1–11. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr SR, Marriott I. Viral CNS infections: role of glial pattern recognition receptors in neuroinflammation. Front Microbiol. 2012;3:201. doi: 10.3389/fmicb.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr SR, Moerdyk-Schauwecker M, Grdzelishvili VZ, Marriott I. RIG-I mediates nonsegmented negative-sense RNA virus-induced inflammatory immune responses of primary human astrocytes. Glia. 2010;58:1620–1629. doi: 10.1002/glia.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy DF, Lyles DS. Vesicular stomatitis viruses expressing wild-type or mutant M proteins activate apoptosis through distinct pathways. J Virol. 2005;79:4170–4179. doi: 10.1128/JVI.79.7.4170-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy DF, Lyles DS. Oncolytic vesicular stomatitis virus induces apoptosis via signaling through PKR, Fas, and Daxx. J Virol. 2007;81:2792–2804. doi: 10.1128/JVI.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Lenard J. Cooperative binding of multimeric phosphoprotein (P) of vesicular stomatitis virus to polymerase (L) and template: pathways of assembly. J Virol. 1995;69:7718–7723. doi: 10.1128/jvi.69.12.7718-7723.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Whitaker-Dowling P, Watkins SC, Griffin JA, Bergman I. Rapid adaptation of a recombinant vesicular stomatitis virus to a targeted cell line. J Virol. 2006;80:8603–8612. doi: 10.1128/JVI.00142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Tsao J, Schein S, Green TJ, Luo M, Zhou ZH. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science. 2010;327:689–693. doi: 10.1126/science.1181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Old-stone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Gillies S, Stollar V. Generation of defective interfering particles of vesicular stomatitis virus in Aedes albopictus cells. Virology. 1980;107:497–508. doi: 10.1016/0042-6822(80)90316-5. [DOI] [PubMed] [Google Scholar]
- Grdzelishvili VZ, Smallwood S, Tower D, Hall RL, Hunt DM, Moyer SA. A single amino acid change in the L-polymerase protein of vesicular stomatitis virus completely abolishes viral mRNA cap methylation. J Virol. 2005;79:7327–7337. doi: 10.1128/JVI.79.12.7327-7337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grdzelishvili VZ, Smallwood S, Tower D, Hall RL, Hunt DM, Moyer SA. Identification of a new region in the vesicular stomatitis virus L polymerase protein which is essential for mRNA cap methylation. Virology. 2006;350:394–405. doi: 10.1016/j.virol.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Green TJ, Luo M. Structure of the vesicular stomatitis virus nucleocapsid in complex with the nucleocapsid-binding domain of the small polymerase cofactor, P. Proc Natl Acad Sci U S A. 2009;106:11713–11718. doi: 10.1073/pnas.0903228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Das T, Banerjee AK. Casein kinase II is the P protein phosphorylating cellular kinase associated with the ribonucleoprotein complex of purified vesicular stomatitis virus. J Gen Virol. 1995;76(Pt 2):365–372. doi: 10.1099/0022-1317-76-2-365. [DOI] [PubMed] [Google Scholar]
- Hall MP, Burson KK, Huestis WH. Interactions of a vesicular stomatitis virus G protein fragment with phosphatidylserine: NMR and fluorescence studies. Biochim Biophys Acta. 1998;1415:101–113. doi: 10.1016/s0005-2736(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- Hansen DE, Thurmond MC, Thorburn M. Factors associated with the spread of clinical vesicular stomatitis in California dairy cattle. Am J Vet Res. 1985;46:789–795. [PubMed] [Google Scholar]
- Hardy MP, Owczarek CM, Jermiin LS, Ejdeback M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84:331–345. doi: 10.1016/j.ygeno.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Harty RN, Brown ME, McGettigan JP, Wang G, Jayakar HR, Huibregtse JM, Whitt MA, Schnell MJ. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J Virol. 2001;75:10623–10629. doi: 10.1128/JVI.75.22.10623-10629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty RN, Paragas J, Sudol M, Palese P. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J Virol. 1999;73:2921–2929. doi: 10.1128/jvi.73.4.2921-2929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie E, Grdzelishvili V. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93:2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiber JF, Barber GN. Vesicular stomatitis virus expressing tumor suppressor p53 is a highly attenuated, potent oncolytic agent. J Virol. 2011;85:10440–10450. doi: 10.1128/JVI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Heinrich BS, Cureton DK, Rahmeh AA, Whelan SP. Protein expression redirects vesicular stomatitis virus RNA synthesis to cytoplasmic inclusions. PLoS Pathog. 2010;6(6):e1000958. doi: 10.1371/journal.ppat.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercyk N, Horikami SM, Moyer SA. The vesicular stomatitis virus L protein possesses the mRNA methyltransferase activities. Virology. 1988;163:222–225. doi: 10.1016/0042-6822(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Clague MJ, Puri A, Morris SJ, Blumenthal R, Grimaldi S. Effect of erythrocyte transbilayer phospholipid distribution on fusion with vesicular stomatitis virus. Biochemistry. 1990;29:4054–4058. doi: 10.1021/bi00469a005. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Wu YJ, Gerber M, Berger-Rentsch M, Heimrich B, Schwemmle M, Zimmer G. Fusion-active glycoprotein G mediates the cytotoxicity of vesicular stomatitis virus M mutants lacking host shut-off activity. J Gen Virol. 2010;91:2782–2793. doi: 10.1099/vir.0.023978-0. [DOI] [PubMed] [Google Scholar]
- Holland JJ, Domingo E, de la Torre JC, Steinhauer DA. Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol. 1990;64:3960–3962. doi: 10.1128/jvi.64.8.3960-3962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JJ, Villarreal LP, Breindl M. Factors involved in the generation and replication of rhabdovirus defective T particles. J Virol. 1976;17:805–815. doi: 10.1128/jvi.17.3.805-815.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Huang AS, Baltimore D. Defective viral particles and viral disease processes. Nature. 1970;226:325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huneycutt BS, Bi Z, Aoki CJ, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DM. Vesicular stomatitis virus mutant with altered polyadenylic acid polymerase activity in vitro. J Virol. 1983;46:788–799. doi: 10.1128/jvi.46.3.788-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt DM. Effect of analogues of S-adenosylmethionine on in vitro polyadenylation by vesicular stomatitis virus. J Gen Virol. 1989;70:535–542. doi: 10.1099/0022-1317-70-3-535. [DOI] [PubMed] [Google Scholar]
- Hwang BY, Schaffer DV. Engineering a serum-resistant and thermostable vesicular stomatitis virus G glycoprotein for pseudotyping retroviral and lentiviral vectors. Gene Ther. 2013 doi: 10.1038/gt.2013.1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland DD, Bang T, Komatsu T, Reiss CS. Delayed administration of interleukin-12 is efficacious in promoting recovery from lethal viral encephalitis. Viral Immunol. 1999;12:35–40. doi: 10.1089/vim.1999.12.35. [DOI] [PubMed] [Google Scholar]
- Ireland DD, Reiss CS. Gene expression contributing to recruitment of circulating cells in response to vesicular stomatitis virus infection of the CNS. Viral Immunol. 2006;19:536–545. doi: 10.1089/vim.2006.19.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Liu Y, Drolet BS, Carnero E, Garcia-Sastre A, Harty RN. Cytopathogenesis of vesicular stomatitis virus is regulated by the PSAP motif of M protein in a species-dependent manner. Viruses. 2012;4:1605–1618. doi: 10.3390/v4091605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks N, Myers R, Greiner SM, Thompson J, Mader EK, Greenslade A, Griesmann GE, Federspiel MJ, Rakela J, Borad MJ, Vile RG, Barber GN, Meier TR, Blanco MC, Carlson SK, Russell SJ, Peng KW. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum Gene Ther. 2010;21:451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Nace R, Barber GN, Russell SJ. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol. 2010;84:1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Lindholm D, Kreutzberg GW. Interleukin-6 and transforming growth factor-beta 1 mRNAs are induced in rat facial nucleus following motoneuron axotomy. Eur J Neurosci. 1993;5:775–781. doi: 10.1111/j.1460-9568.1993.tb00929.x. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Barna M, Reiss CS. Interleukin-12 promotes recovery from viral encephalitis. Viral Immunol. 1997;10:35–47. doi: 10.1089/vim.1997.10.35. [DOI] [PubMed] [Google Scholar]
- Kopecky SA, Lyles DS. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol. 2003;77:4658–4669. doi: 10.1128/JVI.77.8.4658-4669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth RS, Munis JR, Oh PS, Meylan PR, Richman DD. Characterization of a macrophage-tropic HIV strain that does not alter macrophage cytokine production yet protects macrophages from superinfection by vesicular stomatitis virus. AIDS Res Hum Retroviruses. 1990;6:1023–1026. doi: 10.1089/aid.1990.6.1023. [DOI] [PubMed] [Google Scholar]
- Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83:10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib M, Zamay AS, Muharemagic D, Chechik A, Bell JC, Berezovski MV. Electrochemical sensing of aptamer-facilitated virus immunoshielding. Anal Chem. 2012;84:1677–1686. doi: 10.1021/ac202978r. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc I, Luyet PP, Pons V, Ferguson C, Emans N, Petiot A, Mayran N, Demaurex N, Faure J, Sadoul R, Parton RG, Gruenberg J. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol. 2005;7:653–664. doi: 10.1038/ncb1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, Kanji F, Auer R, Brown CW, Lichty BD, Parato K, Atkins H, Kirn D, Bell JC. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F, Niknejad N, Wang J, Auer R, Weberpals JI, Bell JC, Dimitroulakos J. Sensitivity of cervical carcinoma cells to vesicular stomatitis virus-induced oncolysis: Potential role of human papilloma virus infection. Int J Cancer. 2012;131:E204–E215. doi: 10.1002/ijc.27404. [DOI] [PubMed] [Google Scholar]
- Lee AS, Burdeinick-Kerr R, Whelan SP. A ribosome-specialized translation initiation pathway is required for cap-dependent translation of vesicular stomatitis virus mRNAs. Proc Natl Acad Sci U S A. 2013;110:324–329. doi: 10.1073/pnas.1216454109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth GJ, Rodriguez LL, Del cbarrera J. Vesicular stomatitis. Vet J. 1999;157:239–260. doi: 10.1053/tvjl.1998.0303. [DOI] [PubMed] [Google Scholar]
- Levy DE, Marie IJ, Durbin JE. Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol. 2011;1:476–486. doi: 10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Fontaine-Rodriguez EC, Whelan SP. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J Virol. 2005;79:13373–13384. doi: 10.1128/JVI.79.21.13373-13384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rahmeh A, Morelli M, Whelan SP. A conserved motif in region v of the large polymerase proteins of nonsegmented negative-sense RNA viruses that is essential for mRNA capping. J Virol. 2008;82:775–784. doi: 10.1128/JVI.02107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Tainsky MA. Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One. 2011;6:e28683. doi: 10.1371/journal.pone.0028683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan X, He X, Sun H, Zou Z, Yuan H, Xu H, Wang C, Shi X. MicroRNA-466l inhibits antiviral innate immune response by targeting interferon-alpha. Cell Mol Immunol. 2012;9:497–502. doi: 10.1038/cmi.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Yan M, Lu Y, Chen IS. Retargeting VSV-G pseudotyped lentiviral vectors with enhanced stability by in situ synthesized polymer shell. Hum Gene Ther Methods. 2013 doi: 10.1089/hgtb.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: reinventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lichty BD, McBride H, Hanson S, Bell JC. Matrix protein of Vesicular stomatitis virus harbours a cryptic mitochondrial-targeting motif. J Gen Virol. 2006;87:3379–3384. doi: 10.1099/vir.0.81762-0. [DOI] [PubMed] [Google Scholar]
- Linge C, Gewert D, Rossmann C, Bishop JA, Crowe JS. Interferon system defects in human malignant melanoma. Cancer Res. 1995;55:4099–4104. [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles DS, Rupprecht CE. Rhabdoviridae. In: DM, Knipe PM, Howley e, editors. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1363–1408. [Google Scholar]
- Marozin S, Altomonte J, Stadler F, Thasler WE, Schmid RM, Ebert O. Inhibition of the IFN-beta response in hepatocellular carcinoma by alternative spliced isoform of IFN regulatory factor-3. Mol Ther. 2008;16:1789–1797. doi: 10.1038/mt.2008.201. [DOI] [PubMed] [Google Scholar]
- Marozin S, De Toni EN, Rizzani A, Altomonte J, Junger A, Schneider G, Thasler WE, Kato N, Schmid RM, Ebert O. Cell cycle progression or translation control is not essential for vesicular stomatitis virus oncolysis of hepatocellular carcinoma. PLoS One. 2010;5:e10988. doi: 10.1371/journal.pone.0010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FC, Anton PA, Gornbein JA, Shanahan F, Merrill JE. Production of interleukin-1 by microglia in response to substance P: role for a non-classical NK-1 receptor. J Neuroimmunol. 1993;42:53–60. doi: 10.1016/0165-5728(93)90212-h. [DOI] [PubMed] [Google Scholar]
- Masters PS, Banerjee AK. Complex formation with vesicular stomatitis virus phosphoprotein NS prevents binding of nucleocapsid protein N to nonspecific RNA. J Virol. 1988;62:2658–2664. doi: 10.1128/jvi.62.8.2658-2664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin SF, Rackley RR, Sadhukhan PC, Kim MS, Novick AC, Bandyopadhyay SK. Impaired alpha-interferon signaling in transitional cell carcinoma: lack of p48 expression in 5637 cells. Cancer Res. 2001;61:2261–2266. [PubMed] [Google Scholar]
- Matlin KS, Reggio H, Helenius A, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol. 2005;169:116–125. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mead DG, Gray EW, Noblet R, Murphy MD, Howerth EW, Stallknecht DE. Biological transmission of vesicular stomatitis virus (New Jersey serotype) by Simulium vittatum (Diptera: Simuliidae) to domestic swine (Sus scrofa) J Med Entomol. 2004;41:78–82. doi: 10.1603/0022-2585-41.1.78. [DOI] [PubMed] [Google Scholar]
- Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, Feldmann H, Hensley LE, Geisbert TW. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012:6. doi: 10.1371/journal.pntd.0001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire CE, Whitt MA. The protease-sensitive loop of the vesicular stomatitis virus matrix protein is involved in virus assembly and protein translation. Virology. 2011;416:16–25. doi: 10.1016/j.virol.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerdyk-Schauwecker M, Hwang SI, Grdzelishvili VZ. Analysis of virion associated host proteins in vesicular stomatitis virus using a proteomics approach. Virol J. 2009;6:166. doi: 10.1186/1743-422X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: Role of type I interferon signaling. Virology. 2013;436:221L, 34. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsurro V, Beghelli S, Wang R, Barbi S, Coin S, Di Pasquale G, Bersani S, Castellucci M, Sorio C, Eleuteri S, Worschech A, Chiorini JA, Pederzoli P, Alter H, Marincola FM, Scarpa A. Anti-viral state segregates two molecular phenotypes of pancreatic adenocarcinoma: potential relevance for adenoviral gene therapy. J Transl Med. 2010;8:10. doi: 10.1186/1479-5876-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi M, Fazli L, Tearle H, Guo Y, Cox M, Bell J, Ong C, Jia W, Rennie PS. Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res. 2010;70:1367–1376. doi: 10.1158/0008-5472.CAN-09-2377. [DOI] [PubMed] [Google Scholar]
- Moyer SA, Baker SC, Lessard JL. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986;83:5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd JA, Leavitt RW, Kingsbury DT, Holland JJ. Natural selection of mutants of vesicular stomatitis virus by cultured cells of Drosophila melanogaster. J Gen Virol. 1973;20:341–351. doi: 10.1099/0022-1317-20-3-341. [DOI] [PubMed] [Google Scholar]
- Muharemagic D, Labib M, Ghobadloo SM, Zamay AS, Bell JC, Berezovski MV. Anti-Fab aptamers for shielding virus from neutralizing antibodies. J ACS. 2012;134:17168–17177. doi: 10.1021/ja306856y. [DOI] [PubMed] [Google Scholar]
- Muik A, Dold C, Geiß Y, Volk A, Werbizki M, Dietrich U, von Laer D. Semireplication-competent vesicular stomatitis virus as a novel platform for oncolytic virotherapy. J Mol Med (Berl) 2012;90:959L, 70. doi: 10.1007/s00109-012-0863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A, Kneiske I, Werbizki M, Wilflingseder D, Giroglou T, Ebert O, Kraft A, Dietrich U, Zimmer G, Momma S, von Laer D. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011;85:5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Besmer DM, Moerdyk-Schauwecker M, Moestl N, Ornelles DA, Mukherjee P, Grdzelishvili VZ. Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma. J Virol. 2012;86:3073–3087. doi: 10.1128/JVI.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Dutta K, Basu A. RIG-I mediates innate immune response in mouse neurons following Japanese encephalitis virus infection. PloS One. 2011;6:e21761. doi: 10.1371/journal.pone.0021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Bauer SP, Harrison P. Interference induced in GL-V3 monkey kidney cells by rabies virus strains. J Gen Virol. 1981;53:347–351. doi: 10.1099/0022-1317-53-2-347. [DOI] [PubMed] [Google Scholar]
- Noser JA, Mael AA, Sakuma R, Ohmine S, Marcato P, Lee PW, Ikeda Y. The RAS/Raf1/MEK/ERK signaling pathway facilitates VSV-mediated oncolysis: implication for the defective interferon response in cancer cells. Mol Ther. 2007;15:1531–1536. doi: 10.1038/sj.mt.6300193. [DOI] [PubMed] [Google Scholar]
- Novella IS, Ball LA, Wertz GW. Fitness analyses of vesicular stomatitis strains with rearranged genomes reveal replicative disadvantages. J Virol. 2004;78:9837–9841. doi: 10.1128/JVI.78.18.9837-9841.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novella IS, Clarke DK, Quer J, Duarte EA, Lee CH, Weaver SC, Elena SF, Moya A, Domingo E, Holland JJ. Extreme fitness differences in mammalian and insect hosts after continuous replication of vesicular stomatitis virus in sandfly cells. J Virol. 1995;69:6805–6809. doi: 10.1128/jvi.69.11.6805-6809.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novella IS, Hershey CL, Escarmis C, Domingo E, Holland JJ. Lack of evolutionary stasis during alternating replication of an arbovirus in insect and mammalian cells. J Mol Biol. 1999;287:459–465. doi: 10.1006/jmbi.1999.2635. [DOI] [PubMed] [Google Scholar]
- Novella IS, Presloid JB, Zhou T, Smith-Tsurkan SD, Ebendick-Corpus BE, Dutta RN, Lust KL, Wilke CO. Genomic evolution of vesicular stomatitis virus strains with differences in adaptability. J Virol. 2010;84:4960–4968. doi: 10.1128/JVI.00710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T, Banerjee AK. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol Cell. 2007;25:85–97. doi: 10.1016/j.molcel.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Oritani K, Kincade PW, Zhang C, Tomiyama Y, Matsuzawa Y. Type I interferons and limitin: a comparison of structures, receptors, and functions. Cytokine Growth F R. 2001;12:337–348. doi: 10.1016/s1359-6101(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Osiak A, Utermohlen O, Niendorf S, Horak I, Knobeloch KP. ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus. Mol Cell Biol. 2005;25:6338–6345. doi: 10.1128/MCB.25.15.6338-6345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag D, Hoblitzell-Ostertag TM, Perrault J. Overproduction of double-stranded RNA in vesicular stomatitis virus-infected cells activates a constitutive cell-type-specific antiviral response. J Virol. 2007;81:503–513. doi: 10.1128/JVI.01218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MJ, Lucas-Lenard J. The influence of the host cell on the inhibition of virus protein synthesis in cells double infected with vesicular stomatitis virus and mengovirus. J Gen Virol. 1980;50:293–307. doi: 10.1099/0022-1317-50-2-293. [DOI] [PubMed] [Google Scholar]
- Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28:1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozduman K, Wollmann G, Ahmadi SA, van den Pol AN. Peripheral immunization blocks lethal actions of vesicular stomatitis virus within the brain. J Virol. 2009;83:11540–11549. doi: 10.1128/JVI.02558-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglino JC, van den Pol AN. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J Virol. 2011;85:9346–9358. doi: 10.1128/JVI.00723-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik AK, Hwang L, Li T, Englund N, Mathur M, Das T, Banerjee AK. Phosphorylation within the amino-terminal acidic domain I of the phospho-protein of vesicular stomatitis virus is required for transcription but not for replication. J Virol. 1997;71:8167–8175. doi: 10.1128/jvi.71.11.8167-8175.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Peltier DC, Simms A, Farmer JR, Miller DJ. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol. 2010;184:7010–7021. doi: 10.4049/jimmunol.0904133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso RW, Moyer SA. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988;162:369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- Perrault J, Leavitt RW. Inverted complementary terminal sequences in single-stranded RNAs and snap-back RNAs from vesicular stomatitis defective interfering particles. J Gen Virol. 1978;38:35–50. doi: 10.1099/0022-1317-38-1-35. [DOI] [PubMed] [Google Scholar]
- Pfeffer LM, Wang C, Constantinescu SN, Croze E, Blatt LM, Albino AP, Nanus DM. Human renal cancers resistant to IFN’s antiproliferative action exhibit sensitivity to IFN’s gene-inducing and antiviral actions. J Urol. 1996;156:1867–1871. [PubMed] [Google Scholar]
- Plakhov IV, Aoki C, Reiss CS, Huang AS. Pathogenesis of murine encephalitis limited by defective interfering particles. An immunohistochemical study. J Neurovirol. 1995a;1:207–218. doi: 10.3109/13550289509113967. [DOI] [PubMed] [Google Scholar]
- Plakhov IV, Arlund EE, Aoki C, Reiss CS. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995b;209:257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- Preble OT, Costello LE, Huang DD, Barmada MA. Neurovirulence mutant of vesicular stomatitis virus with an altered target cell tropism in vivo. Infect Immun. 1980;29:744–757. doi: 10.1128/iai.29.2.744-757.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qanungo KR, Shaji D, Mathur M, Banerjee AK. Two RNA polymerase complexes from vesicular stomatitis virus-infected cells that carry out transcription and replication of genome RNA. Proc Natl Acad Sci U S A. 2004;101:5952–5957. doi: 10.1073/pnas.0401449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz E, Moreno N, Peralta PH, Tesh RB. A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am J Trop Med Hyg. 1988;39:312–314. doi: 10.4269/ajtmh.1988.39.312. [DOI] [PubMed] [Google Scholar]
- Rajani KR, Pettit Kneller EL, McKenzie MO, Horita DA, Chou JW, Lyles DS. Complexes of vesicular stomatitis virus matrix protein with host Rae1 and Nup98 involved in inhibition of host transcription. PLoS Pathog. 2012;8(9):e1002929. doi: 10.1371/journal.ppat.1002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raux H, Obiang L, Richard N, Harper F, Blondel D, Gaudin Y. The matrix protein of vesicular stomatitis virus binds dynamin for efficient viral assembly. J Virol. 2010;84:12609–12618. doi: 10.1128/JVI.01400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, Reis e Sousa C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Reif JS, Webb PA, Monath TP, Emerson JK, Poland JD, Kemp GE, Cholas G. Epizootic vesicular stomatitis in Colorado, 1982: infection in occupational risk groups. Am J Trop Med Hyg. 1987;36:177–182. doi: 10.4269/ajtmh.1987.36.177. [DOI] [PubMed] [Google Scholar]
- Reiss CS, Plakhov IV, Komatsu T. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci. 1998;855:751–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- Roche S, Albertini AA, Lepault J, Bressanelli S, Gaudin Y. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell Mol Life Sci. 2008;65:1716–1728. doi: 10.1007/s00018-008-7534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth SL, Whittaker GR. Promotion of vesicular stomatitis virus fusion by the endosome-specific phospholipid bis(monoacylglycero)phosphate (BMP) FEBS Lett. 2011;585:865–869. doi: 10.1016/j.febslet.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau CK, Thompson CB, Cherry S. Ars2 regulates both miRNA- and siRNA-dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138:340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V, Sterman DH, Harty RN, Okumura A, Barber GN, Vile RG, Federspiel MJ, Russell SJ, Litzky L, Albelda SM. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S, Theofanis T, Reiss CS. Interferon-induced tetherin restricts vesicular stomatitis virus release in neurons. DNA Cell Biol. 2011;30:965–974. doi: 10.1089/dna.2011.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R, Tralka TS, Willingham MC, Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell. 1983;32:639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Schlehuber LD, Rose JK. Prediction and identification of a permissive epitope insertion site in the vesicular stomatitis virus glycoprotein. J Virol. 2004;78:5079–5087. doi: 10.1128/JVI.78.10.5079-5087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Buonocore L, Whitt MA, Rose JK. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle M, Weining KC, Richter MF, Schumacher B, Staeheli P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Seganti L, Superti F, Girmenia C, Melucci L, Orsi N. Study of receptors for vesicular stomatitis virus in vertebrate and invertebrate cells. Microbiologica. 1986;9:259–267. [PubMed] [Google Scholar]
- Sharif-Askari E, Nakhaei P, Oliere S, Tumilasci V, Hernandez E, Wilkinson P, Lin R, Bell J, Hiscott J. Bax-dependent mitochondrial membrane permeabilization enhances IRF3-mediated innate immune response during VSV infection. Virology. 2007;365:20–33. doi: 10.1016/j.virol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, Yang J, Fu S, Zhang D. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem. 2011;286:4517–4524. doi: 10.1074/jbc.M110.159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ebert O, Suriawinata A, Thung SN, Woo SL. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J Virol. 2005;79:13705–13713. doi: 10.1128/JVI.79.21.13705-13713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Banerjee AK. Transcriptional activity and mutational analysis of recombinant vesicular stomatitis virus RNA polymerase. J Virol. 1993;67:1334–1339. doi: 10.1128/jvi.67.3.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PloS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P, Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991;65:4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners CP, Goldberg VJ. On the mechanism of neurotropism of vesicular stomatitis virus in newborn hamsters. Studies with temperature-sensitive mutants. J Gen Virol. 1975;29:281–296. doi: 10.1099/0022-1317-29-3-281. [DOI] [PubMed] [Google Scholar]
- Steinhauer DA, de la Torre JC, Holland JJ. High nucleotide substitution error frequencies in clonal pools of vesicular stomatitis virus. J Virol. 1989;63:2063–2071. doi: 10.1128/jvi.63.5.2063-2071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA, Holland JJ. Direct method for quantitation of extreme polymerase error frequencies at selected single base sites in viral RNA. J Virol. 1986;57:219–228. doi: 10.1128/jvi.57.1.219-228.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol. 2000a;74:9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000b;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Sun WH, Pabon C, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC, Rosen ST. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood. 1998;91:570–576. [PubMed] [Google Scholar]
- Sun X, Yau VK, Briggs BJ, Whittaker GR. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology. 2005;338:53–60. doi: 10.1016/j.virol.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Superti F, Seganti L, Ruggeri FM, Tinari A, Donelli G, Orsi N. Entry pathway of vesicular stomatitis virus into different host cells. J Gen Virol. 1987;68:387–399. doi: 10.1099/0022-1317-68-2-387. [DOI] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, Diamond MS, Gale M. IPS-1 is essential for the control of West Nile Virus infection and immunity. PloS Pathog. 2010;6(2):e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinteck BD, Lyles DS. Plasma membrane microdomains containing vesicular stomatitis virus M protein are separate from microdomains containing G protein and nucleocapsids. J Virol. 2008;82:5536–5547. doi: 10.1128/JVI.02407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- Tani H, Morikawa S, Matsuura Y. Development and applications of VSV vectors based on cell tropism. Front Microbiol. 2011;2:272. doi: 10.3389/fmicb.2011.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Chaniotis BN, Johnson KM. Vesicular stomatitis virus, Indiana serotype: multiplication in and transmission by experimentally infected phle-botomine sandflies (Lutzomyia trapidoi) Am J Epidemiol. 1971;93:491–495. doi: 10.1093/oxfordjournals.aje.a121284. [DOI] [PubMed] [Google Scholar]
- Thomsen AR, Nansen A, Andersen C, Johansen J, Marker O, Christensen JP. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int Immunol. 1997;9:1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Jr, Palian BM, Reiss CS. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Lyles DS, Reiss CS. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J Neurovirol. 2007;13:433–445. doi: 10.1080/13550280701460565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PE, Elena SF. Cost of host radiation in an RNA virus. Genetics. 2000;156:1465–1470. doi: 10.1093/genetics/156.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Dalton KP, Rose JK. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol. 2002;76:1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Davis JN. Highly attenuated recombinant vesicular stomatitis virus VSV-12′GFP displays immunogenic and oncolytic activity. J Virol. 2013;87:1019–1034. doi: 10.1128/JVI.01106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen JC, van der Marel GA, van Boom JH, Bayly SF, Player MR, Torrence PF. 2,5-oligoadenylate-peptide nucleic acids (2-5A-PNAs) activate RNase L. Bioorg Med Chem. 1999;7:449–455. doi: 10.1016/s0968-0896(98)00258-2. [DOI] [PubMed] [Google Scholar]
- von Kobbe C, van Deursen JM, Rodrigues JP, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo-Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Waibler Z, Detje CN, Bell JC, Kalinke U. Matrix protein mediated shutdown of host cell metabolism limits vesicular stomatitis virus-induced interferon-alpha responses to plasmacytoid dendritic cells. Immunobiology. 2007;212:887–894. doi: 10.1016/j.imbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang BX, Rahbar R, Fish EN. Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res. 2011;31:545–552. doi: 10.1089/jir.2010.0158. [DOI] [PubMed] [Google Scholar]
- Wang PH, Arjona A, Zhang Y, Sultana H, Dai JF, Yang L, LeBlanc PM, Doiron K, Saleh M, Fikrig E. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol. 2010;11:U912–U961. doi: 10.1038/ni.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner JM, Jiang D, Pan XB, Chang J, Block TM, Guo JT. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz GW, Moudy R, Ball LA. Adding genes to the RNA genome of vesicular stomatitis virus: positional effects on stability of expression. J Virol. 2002;76:7642–7650. doi: 10.1128/JVI.76.15.7642-7650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SP, Ball LA, Barr JN, Wertz GT. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P, Youngner JS, Widnell CC, Wilcox DK. Superinfection exclusion by vesicular stomatitis virus. Virology. 1983;131:137–143. doi: 10.1016/0042-6822(83)90540-8. [DOI] [PubMed] [Google Scholar]
- Whitlow ZW, Connor JH, Lyles DS. Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome. J Virol. 2006;80:11733–11742. doi: 10.1128/JVI.00971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow ZW, Connor JH, Lyles DS. New mRNAs are preferentially translated during vesicular stomatitis virus infection. J Virol. 2008;82:2286–2294. doi: 10.1128/JVI.01761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J, Kurilla MG, Keene JD. A host protein (La) binds to a unique species of minus-sense leader RNA during replication of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1983;80:5827–5831. doi: 10.1073/pnas.80.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Tattersall P, van den Pol AN. Targeting human glioblas-toma cells: comparison of nine viruses with oncolytic potential. J Virol. 2005;79:6005–6022. doi: 10.1128/JVI.79.10.6005-6022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–1573. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J Biol Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- Wu L, Huang TG, Meseck M, Altomonte J, Ebert O, Shinozaki K, García-Sastre A, Fallon J, Mandeli J, Woo SL. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy. Hum Gene Ther. 2008;19:635–647. doi: 10.1089/hum.2007.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyers F, Blondel D, Petitjean AM, Dezelee S. Restricted expression of viral glycoprotein in vesicular stomatitis virus-infected Drosophila melanogaster cells. J Gen Virol. 1989;70:213–218. doi: 10.1099/0022-1317-70-1-213. [DOI] [PubMed] [Google Scholar]
- Wyers F, Richard-Molard C, Blondel D, Dezelee S. Vesicular stomatitis virus growth in Drosophila melanogaster cells: G protein deficiency. J Virol. 1980;33:411–422. doi: 10.1128/jvi.33.1.411-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Ohnishi S. Vesicular stomatitis virus binds and fuses with phospholipid domain in target cell membranes. Biochemistry. 1986;25:3703–3708. doi: 10.1021/bi00360a034. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Imaizumi T, Lee SJ, Tanji K, Sakaki H, Matsumiya T, Ishikawa A, Taima K, Yuzawa E, Mori F, Wakabayashi K, Kimura H, Satoh K. Retinoic acid-inducible gene-I mediates RANTES/CCL5 expression in U373MG human astrocytoma cells stimulated with double-stranded RNA. Neurosci Res. 2007;58:199–206. doi: 10.1016/j.neures.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Youngner JS, Jones EV, Kelly M, Frielle DW. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981;108:87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Yuan H, Yoza BK, Lyles DS. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251:383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]
- Zhang KX, Matsui Y, Hadaschik BA, Lee C, Jia W, Bell JC, Fazli L, So AI, Rennie PS. Down-regulation of type I interferon receptor sensitizes bladder cancer cells to vesicular stomatitis virus-induced cell death. Int J Cancer. 2010;127:830–838. doi: 10.1002/ijc.25088. [DOI] [PubMed] [Google Scholar]
- Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci U S A. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]