SUMMARY
Drosophila hematopoietic progenitor maintenance involves both near neighbor and systemic interactions. This study shows that olfactory receptor neurons (ORNs) function upstream of a small set of neurosecretory cells that express GABA. Upon olfactory stimulation, GABA from these neurosecretory cells is secreted into the circulating hemolymph and binds to metabotropic GABAB receptors expressed on blood progenitors within the hematopoietic organ, the lymph gland. The resulting GABA signal causes high cytosolic Ca2+, necessary and sufficient for progenitor maintenance. Thus the activation of an odorant receptor is essential for blood progenitor maintenance and consequently, larvae raised on minimal odor environments fail to sustain a pool of hematopoietic progenitors. This study links sensory perception and the effects of its deprivation on the integrity of the hematopoietic and innate immune systems in Drosophila.
Keywords: olfaction, GABA, hematopoietic progenitors, calcium homeostasis, neuroimmunology, Drosophila
INTRODUCTION
The maintenance of hematopoietic progenitor cells has been extensively associated with niche interactions (Garrett and Emerson, 2009; Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007; Mercier et al., 2012), however less is known about the systemic mechanisms regulating this process. In vivo, genetic analysis in Drosophila allows mechanistic investigation of hematopoietic progenitor cells in their native microenvironment. Hemocytes, Drosophila blood cells that are akin to vertebrate myeloid cells, develop within a specialized hematopoietic organ called the lymph gland during embryonic and larval stages, and contribute to the blood cells that circulate in pupae and adults (Jung et al., 2005). The differentiated hemocytes, residing in the outermost layer of the lymph gland called the cortical zone (CZ, Figures 1A–B), arise from undifferentiated progenitors located within the inner core region, termed the medullary zone (MZ). MZ progenitor properties include lack of BrdU incorporation as well as differentiation markers and multipotency, as they give rise to all Drosophila blood cell lineages (Jung et al., 2005; Krzemien et al., 2010; Minakhina and Steward, 2010). No direct evidence for asymmetric cell division has yet been demonstrated for Drosophila hematopoiesis (see however (Minakhina and Steward, 2010)). A small group of cells in the lymph gland, termed the Posterior signaling center (PSC), expresses Hedgehog (Hh) and functions as the hematopoietic niche (Mandal et al., 2007). Hh derived from the PSC synergizes with a CZ-derived signal initiated by Adenosine deaminase growth factor-A (Adgf-A) (Mondal et al., 2011) and these signals are together essential for progenitor maintenance in the MZ (Figure 1B). The MZ progenitors also respond to systemic signals that are triggered by amino acid and insulin levels in the animal (Benmimoun et al., 2012; Dragojlovic-Munther and Martinez-Agosto, 2012; Shim et al., 2012; Tokusumi et al., 2012). Circulating larval blood cells also arise from the head mesoderm of the embryo independently of the lymph gland. They reside in segmentally repeated epidermal-muscular pockets where they rely on the peripheral nervous system (PNS), for their localization and survival (Makhijani et al., 2011).
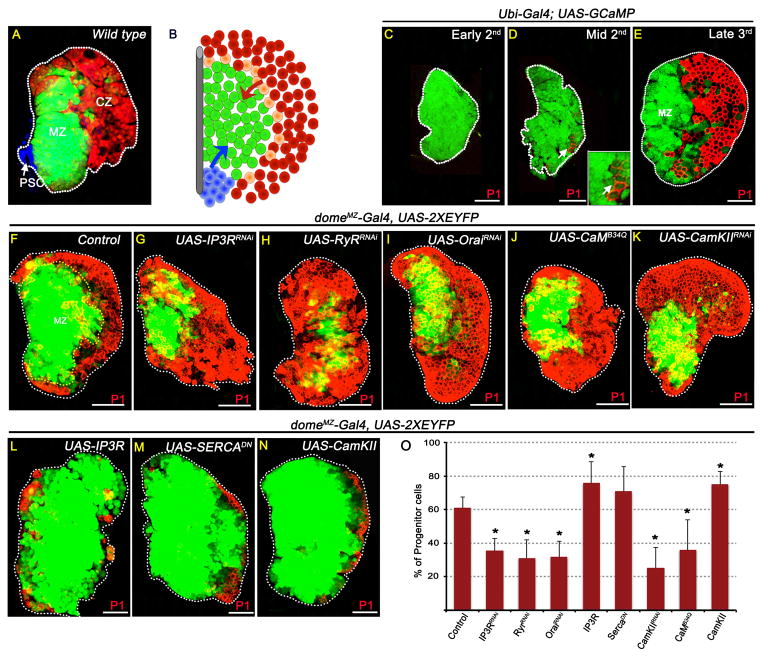
Figure 1. Cytosolic Ca2+ levels regulate blood progenitor maintenance.
Shown are primary lymph gland lobes from wandering 3rd instar larvae except (C, early 2nd) and (D, mid 2nd instar). Error bars in the graphs represent standard deviation. Scale bar: 50μm. See also Figure S1.
(A) Three distinct zones of the lymph gland: PSC functions as hematopoietic niche (blue, Antp staining) and maintains undifferentiated progenitors of the Medullary Zone (MZ, green; dome-Gal4, UAS-2xEYFP). The outermost Cortical Zone (CZ) contains differentiating hemocytes (red; HmlΔ– RFP). (B) Schematic representation of wild-type lymph gland. Blue: PSC; green: MZ; peach: intermediate progenitors expressing mixed markers (Krzemien et al., 2010); red: CZ. Blue arrow: Hh signal; Red arrow: ADGF-A signal.
(C–E) Blood progenitors maintain elevated levels of intracellular Ca2+.
In (C–E), the GCaMP Ca2+ sensor (Ubi-Gal4; UAS-GCaMP) is ubiquitously expressed using the Ubi-Gal4 driver (see Figure S1A–C).
(C) During early 2nd instar, high GCaMP (green) activity is seen in all cells of the lymph gland.
(D) Later, differentiation (P1, red) initiates in a small number of cells (arrow) which attenuate GCaMP (green) activity (inset: high magnification).
(E) By late 3rd instar GCaMP sensor activity is extremely low in mature hemocytes (P1, red) while the MZ continues to display GCaMP sensor activity suggesting that they maintain elevated Ca2+ level in their cytosol.
(F–O) High Ca2+ level is essential for progenitor maintenance. domeMZ-Gal4, UAS-2XEYFP expression (green; see Table S1) marks progenitors and P1 (red) marks differentiated cells. Driver used in (F–O): domeMZ-Gal4. n = # of lymph glands analyzed for statistical analysis (O), percentage of progenitor cells (P1 negative) are counted. p values refer to quantitation shown in (O).
(F) Control (n=5)
(G–K) Lowering Ca2+ signaling in the MZ cells leads to reduced maintenance of progenitors.
(G) IP3RRNAi (p = 2.5E-06; n = 13)
(H) RyRRNAi (p = 4.2E-05; n = 10)
(I) OraiRNAi (p = 1.8E-05; n = 10)
(J) CaMB34Q (Dominant Negative; p = 4.6E-03; n=13)
(K) CaMKIIRNAi (p = 1.3E-05; n=11)
(L–N) Raising Ca2+ signaling in the MZ cells leads to enhanced maintenance of progenitors.
(L) IP3R overexpression (p = 3E-02; n = 4)
(M) SERCADN (p = 9E-02; n=10)
(N) CaMKIIWT overexpression (p = 1.4E-03; n=13)
(O) Quantitation of the genotypes shown in Figures F–N.
In this paper, we describe a link between the activity of olfactory neurons, GABA signaling and the maintenance of blood progenitors. The larval olfactory system consists of a pair of dorsal organs expressing 25 specific odorant receptors (OR) in 21 olfactory receptor neurons (ORNs) (Kreher et al., 2005; Vosshall and Stocker, 2007). An example of such a receptor is Or83b(Orco), an atypical odorant receptor protein, broadly expressed in every ORN, and essential for response to all odors (Larsson et al., 2004). Within the glomeruli of the larval antennal lobe, the ORNs form excitatory synapses with projection neurons (PN) whose axons innervate deeper into regions that represent higher brain centers for information processing (Vosshall and Stocker, 2007). Glomeruli are interconnected either by inhibitory GABAergic (iLN) or excitatory cholinergic (eLN) local interneurons that fine-tune the ORN-PN network (Masse et al., 2009). Here we show that activation of olfactory neurons leads to a secretion of GABA from neurosecretory cells into the circulating blood. Circulating GABA activates metabotropic GABAB receptors (GABABR) on the progenitor cells and mediates changes in the intracellular Ca+2 concentration by activating PLC that regulates Ca2+ stores in the endoplasmic reticulum (ER) (Nilsson et al., 1993; Parramon et al., 1995; Schwirtlich et al., 2010). The resulting high cytosolic Ca2+ is necessary and sufficient for maintenance of the progenitor cells, showing that in Drosophila smell perception is associated with the maintenance of the hematopoetic and immune system.
RESULTS
Intracellular calcium regulates hematopoietic progenitor maintenance
We first investigated whether Ca2+ signaling regulates progenitor cell maintenance. To detect Ca2+ levels within cells of the lymph gland, we expressed the Ca2+ sensor GCaMP (Nakai et al., 2001) under the control of a ubiquitous driver (Ubi-Gal4). GCaMP emits green fluorescence only under conditions of high Ca2+ concentration. In addition to sensing transient Ca2+ pulses in neurons, this sensor has been previously used to monitor developmentally regulated, slow changes in Ca2+ levels (Chen et al., 2012; Muto and Kawakami, 2011) that can be determinants of cell fate and proliferation (Berridge et al., 2003; Schwirtlich et al., 2010). During early stages of lymph gland development, before the onset of any differentiation markers, high sensor activity is detected in all cells of the lymph gland (Figure 1C). At later stages, although the sensor continues to be expressed ubiquitously in cells of the MZ and CZ (Figure S1A–C), high sensor activity is restricted to the MZ progenitors (Figure 1D–E). Fluorescence intensity measurements show a 6.6 fold (660%) reduction in sensor activity in the CZ cells compared with the MZ progenitors (n=10). These results were confirmed with multiple Ca2+ sensing constructs and dyes (Figure S1D–F) and collectively show that during development, hematopoietic progenitors retain stable and high Ca2+ concentration that is depleted as cells initiate differentiation. To determine if the high Ca2+ level is causally related to the maintenance of progenitor fate, Ca2+ concentration was manipulated during hematopoietic development using multiple zone specific drivers (Table S1) and genetic backgrounds (Figure 1 and S1).
In Figure 1F–O, domeMZ-Gal4 was used to express various genetic constructs specifically in the MZ (Figure S1G–H; Table S1). Inhibition of ER-mediated Ca2+ release into the cytosol by knock down of IP3R (Figure 1F,G) or Ryanodine Receptor (RyR) (Figure 1H), reduction of store operated Ca2+ entry by knock down of Orai channel (Figure 1I), or knock down of the downstream components of the pathway, Calmodulin (CaM) (Figure 1J) or Calmodulin dependent kinase II (CaMKII) (Figure 1K) all lead to loss of blood progenitors with a concomitant expansion of differentiated hemocytes (Figure 1F–K, quantitation showed in Figure 1O). Conversely, experiments in which free cytoplasmic Ca2+ concentration is raised within the progenitors such as by over-expression of IP3R (Figure 1L), expression of a dominant negative form of the SERCA pump (Figure 1M) or overexpression of CaMKII (Figure 1N) promote increased progenitor maintenance inhibiting differentiation (Figure 1L–O). The above results have been recapitulated using additional drivers (Figure S1J–W). Altering Ca2+ levels in differentiated cells using CZ-specific drivers does not result in any blood phenotype (Figure S1X–S1AA). Furthermore, an antibody specific to phospho-CaMKII detects the activated kinase specifically in the MZ cells (Figure S1BB). We conclude that high Ca2+ is needed in the MZ cells, but not in the CZ cells for progenitor maintenance.
We used a genetic background (Tep4MZ-Gal4, UAS-mCherry; UAS-GCaMP, UAS-XRNAi/DN) that allows us to restrict expression of the sensor, measure Ca2+ activity, alter Ca2+ activity (controlled by gene X), and also mark MZ and CZ cell fate. At two different stages of development, before and during the onset of normal differentiation, we directly demonstrate that compared to wild type (Figure 2A,B) IP3RRNAi (Figure 2C,D) causes lowering of Ca2+ levels (sensor activity) and SercaDN (Figure 2E,F) causes increase in Ca2+ level (sensor activity) within the cells of the MZ. This experiment also provides a temporal pattern whereby in mutants that block calcium signaling, cells first lose high Ca2+, then lose MZ markers, and finally initiate differentiation.
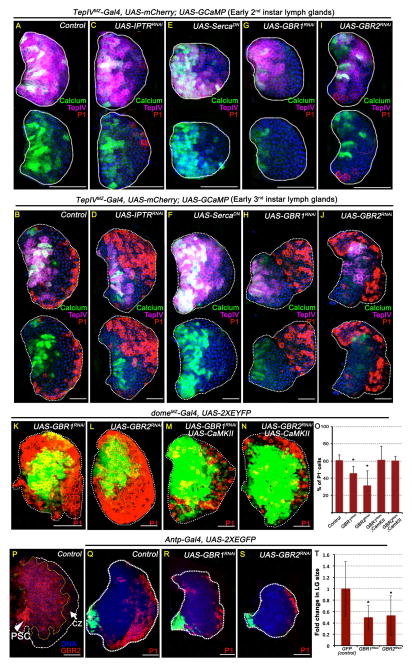
Figure 2. GABABR maintains Ca2+ levels in progenitors.
Scale bar: 50μm. n = # lymph glands analyzed for statistical analyses. Error bars in the graphs represent standard deviation. See also Figure S2.
(A–J) Ca2+ sensor activity in genetic backgrounds that alter Ca2+ signaling. TepIVMZ-Gal4 expression is restricted to a subset of progenitors and was used as a driver in these panels (Table S1). MZ progenitors are marked with mCherry (magenta); cells with high GCaMP activity are green; P1 (red) marks differentiated cells and DNA (DAPI) is blue. Lymph glands shown are dissected either from early 2nd instar (A, C, E, G and I) or from early 3rd instar (B, D, F, H and J) larvae. The same image is shown twice with and without magenta channel to highlight the green fluorescence. See Figures S2F-G for quantitation of GCaMP activity.
(A) Early 2nd instar control (n=8) for comparison with panels C, E, G and I.
(B) Early 3rd instar control (n=8) for comparison with panels D, F, H and J.
(C and D) Lowering Ca2+ levels in the MZ cells due to loss of IP3R reduces GCaMP activity.
(C) IP3RRNAi (p = 1E-03, n =8)
(D) IP3RRNAi (p = 2E-03, n =3)
(E and F) Raising Ca2+ levels in the MZ cells by SercaDN increases GCaMP activity.
(E) SercaDN (p = 0.13, n =10)
(F) SercaDN (p = 9E-04, n =8)
(G–J) Loss of GABABR signaling in the MZ cells reduces GCaMP activity.
(G) GBR1RNAi (p =1.3E-06, n =10)
(H) GBR1RNAi (p =3E-04, n =10)
(I) GBR2RNAi (p = 1.6E-05, n =8)
(J) GBR2RNAi (p = 9E-06, n =6)
(K–O) GABABR regulates hematopoietic progenitor maintenance.
domeMZ-Gal4 expression is restricted to the MZ progenitors (see Table S1 and Figure S1G–H). The percentage of cells counted and the bar graph (in J) refer to progenitor population marked by domeMZ-Gal4, UAS-2xEYFP (green; n=5). In these panels domeMZ-Gal4 is used as a driver.
(K) GABABR1RNAi (p= 1.4E-03; n=10).
(L) GABABR2RNAi (p= 1.5E-03; n=10).
(M) GABABR1RNAi; CaMKII overexpression (p= 0.48 i.e rescued back to control; n=6)
(N) GABABR2RNA; CaMKII overexpression (p= 0.45; i.e., rescued back to control; n=3).
(O) Quantitation of the genotypes represented in Figure panels K–N.
(P) GABABR2 (red) is expressed in the MZ and the PSC (arrowhead) and down-regulated in the CZ (arrow).
(Q–T) GABABR in the PSC controls lymph gland size.
(Q) Control 3rd instar lymph gland. Antp-Gal4, UAS-GFP (n=12) is expressed in the PSC (green) and Antp-Gal4 was used as a driver in these panels. Differentiated cells (red) are marked with P1. Fold change measured in (O) represents comparisons of mutant lymph gland volume compared with control.
(R) GABABR1RNAi (p= 4E-03; n = 10)
(S) GABABR2RNAi (p= 6E-03; n = 11).
(T) Quantification of lymph gland size represented in Figure panels Q–S.
Metabotropic GABABR signaling in Ca2+ homeostasis and progenitor maintenance
Metabotropic receptors, usually associated with neuronal function, are important for controlling intracellular Ca2+ (Bettler and Tiao, 2006). We knocked down several kinds of these receptors in the mid 2nd instar before differentiation program normally sets in and identified the metabotropic GABAB receptor (GABABR) as the signaling entity required for the maintenance of GCaMP sensor activity in the MZ progenitors. RNAi knockdown of either subunit GABABR1 or GABABR2 is sufficient to cause attenuation of GCaMP activity in the progenitors (Figure 2G–J and Figure S2A–G). This is accompanied by loss of progenitor markers and enhanced differentiation (Figure 2K–L, O and Figure S2H–J). Unlike the MZ and CZ, the PSC size and expression of Antenapedia or Hh proteins by PSC cells is not affected (Figure S2K–M). GABABR mutant phenotypes can be over-ridden by over-expression of either the Orai channel (Figure S2N–P and S2T-U) or wild-type CaMKII (Figure 2M–O and Figure S2Q–U). These results place activation of GABABR signaling upstream of the intracellular Ca2+ sensing mechanism in the progenitors.
The above genetic data are further supported by expression analysis that localizes GABABR protein to MZ progenitors. The level of GABABR declines as the cells differentiate and form part of the CZ (Figure 2P). Additionally, GABABR is also detected in the PSC (Figure 2P), and knocking down the gene in the PSC using the driver Antp-Gal4 (Mandal et al., 2007) leads to a small lymph gland size without causing any increase in progenitor differentiation (Figure 2Q–T). This small lymph gland phenotype, superposed on the progenitor maintenance phenotype, is seen in several contexts of Ca2+ loss-of-function genetic backgrounds (indicated in relevant figures by differences in scale bars). When PSC knock down is caused later in development (using gal80ts; see Experimental Procedures), no change in lymph gland size (or any other mutant phenotype) is apparent (Figure S2V–W). This suggests that GABABR function in the PSC controls the size of the lymph gland (see, for example, (Pennetier et al., 2012)). This function is earlier than, and separable from, the progenitor maintenance phenotype of the MZ.
Neuronally derived GABA activates GABABR signaling in the lymph gland
The neurotransmitter, γ-aminobutyric acid (GABA) is the endogenous ligand that activates GABAR and is synthesized by the enzyme Glutamic acid decarboxylase1 (Gad1). GABA immunoreactivity is detected on the cell-surface of MZ progenitors (Figure 3A; Figure S3A), and is extremely detergent sensitive (see Suppl methods; Figure S3B, C). Lymph gland-specific expression of Gad1 RNAi does not cause any differentiation or progenitor loss phenotypes (Figure S3D,E), nor does this eliminate GABA immunoreactivity in the MZ of the lymph gland (Figure S3F,G). Moreover, Gad1 is not expressed in the lymph gland (Figure S3H) as shown by staining with a specific antibody (Jackson et al., 1990). We conclude that the GABA detected on the lymph gland is synthesized elsewhere and binds to the GABAR on the surface of the MZ progenitors. Consistent with this notion, lymph gland-independent GABA is also detected in the lumen of the heart/aorta (dorsal vessel, DV; Figure S3I,J) in direct contact with the hemolymph. In fact, circulating GABA is readily measured by an Elisa assay in the hemolymph (see control in Figure 3D).
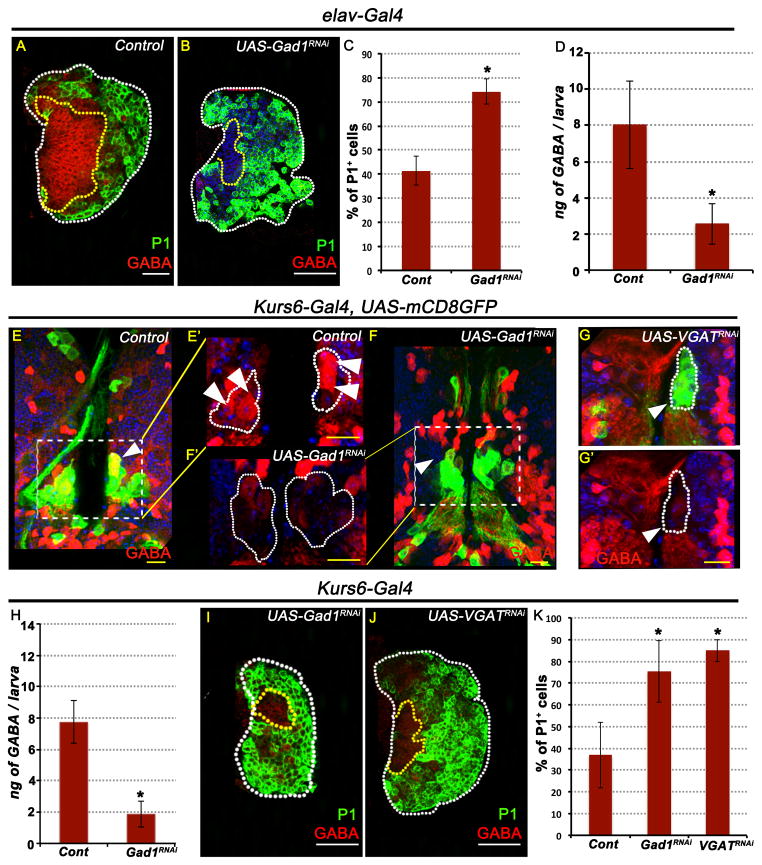
Figure 3. GABA released by a small number of neurosecretory cells promotes blood progenitor maintenance.
In all panels, representative images of lymph glands and brain samples shown are from wandering 3rd instar larvae. TOPRO3 (blue) marks nuclei. White scale bar: 50μm and yellow scale bar: 10μm. n = # lymph glands analyzed for statistical analyses. Error bars in the graphs represent standard deviation. See also Figure S3.
(A) Control: strong GABA (red) staining is seen in the MZ while the CZ cells show much lower levels of GABA staining. Differentiated cells are marked with P1 (green, n= 5)
(B) Pan-neuronal loss of Gad1 function (elav-Gal4; UAS-Gad1RNAi) causes loss of GABA staining (red) in the lymph gland (compare with A). Differentiated cells (green, p= 1.1E-05; n= 9) are expanded compared with control.
(C) Proportion of differentiated cells (green, P1) in (B) compared with (A).
(D) Quantitation of hemolymph GABA levels. 3 ELISA trials per genotype; 10 larvae used per assay.
Control: elav-Gal4, UAS-2xEGFP (8 ± 2.4 ng GABA / larva).
elav-Gal4, UAS-2xEGFP, UAS- Gad1RNAi (2.5 ± 1.1 ng GABA / larva; p = 2.0E-02).
(E–K) GABA positive Kurs6 neurosecretory cells control blood progenitors.
(E) Control: Kurs6-Gal4, UAS-mCD8GFP; 2 Kurs6+ neurosecretory cells (green, GFP) in each lobe of the central brain are also GABA+ (red; yellow due to overlap with green; arrowhead). E′ is a magnified view shown in the red channel to highlight GABA expression (see also Figure S3L).
(F) Gad1RNAi
(G) VGATRNAi
F′ and G′ show a magnified view of of the Kurs6+ cells within the inset shown in the red channel to demonstrate loss of GABA (compare with control, E′).
(H) Loss of Gad1 expression exclusively in the Kurs6+ neurosecretory cells leads to reduction of hemolymph GABA level. n= number of Elisa assays; 10 animals per trial.
Control (Kurs6-Gal4; 7.3 ± 1.3 ng GABA / larva; n=10)
Gad1RNAi (1.8 ± 0.8 ng of GABA / larva; p = 9.86E-05; n=3)
(I–K) Loss of GABA secretion from Kurs6+GABA+ increases blood differentiation.
(I) Gad1RNAi (p= 6.3E-07; n= 10)
(J) VGATRNAi (p= 4.2E-011; n= 13)
(K) Quantitation of percentage of differentiated cells marked by P1 expression (green) in panels I–J. The control shown is: Kurs6-Gal4, UAS-mCD8GFP (n= 15).
In contrast to the above results, when GABA production is specifically blocked in all neurons (elav-Gal4; UAS-Gad1RNAi), GABA immunoreactivity on the lymph gland is lost (Figure 3B), MZ progenitor maintenance is severely affected (Figure 3B,C), and hemolymph GABA is reduced (Figure 3D). Thus, neuronally derived GABA is secreted and detected in circulation and on the lymph gland.
GABA released as a neurotransmitter at synapses within the brain is not expected to cross the blood brain barrier. In Drosophila, several populations of neurosecretory cells have been described, that secrete small molecules and hormones into circulation, and drivers that identify these rare cells within the brain have been developed (Nassel and Winther, 2010; Siegmund and Korge, 2001). Of interest to this study are the Kurs6+ neurons. Unlike in the ventral nerve cord, which has many Kurs6+ neurons, only 6 cluster pairs of 3–4 neurosecretory cells each express Kurs6 within the central brain (Figure S3K). These were of interest as a single pair of a 4-cell Kurs6+ cluster near the sub-esophageal ganglion region, contains 2 cells per cluster that exhibit high GABA immunoreactivity (Figure 3E, Figure S3L). Blocking GABA synthesis with Gad1RNAi or its vesicular packaging using VGATRNAi in the Kurs6+ group of neurons results in down-regulation of GABA levels specifically in these Kurs6+GABA+ cells (Figure 3F–G), while the overall brain GABA expression remains unaffected (Figure 3F). More importantly, lymph glands from Kurs6-GAL4; UAS-Gad1RNAi and Kurs6-GAL4; UAS-VGATRNAi backgrounds have reduced circulating GABA in the hemolymph when compared to sibling controls (Figure 3H) and they show severely reduced GABA immunoreactivity, and reduced maintenance of blood progenitors in the lymph gland (Figure 3I–K).
Olfactory sensing regulates Drosophila larval hematopoietic development
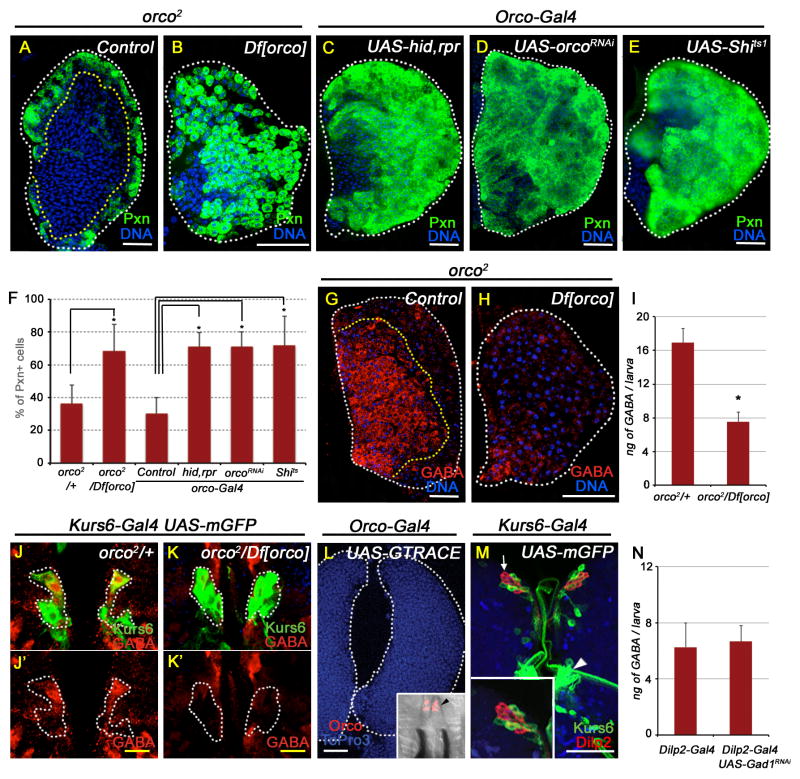
The critical issue of physiological relevance of the above findings requires investigation of upstream mechanisms by which GABA levels are maintained in the neurosecretory cells contained within the central brain. Our past work (Shim et al., 2012) suggests that system-level changes can influence blood progenitor behavior, and we investigated if sensory input, such as that provided by olfaction, may play a role in this process. The common odorant co-receptor 83b (Orco) is broadly expressed in every ORN, essential for all olfaction (Larsson et al., 2004) and the mutant combination orco2/Df[orco] is viable. By investigating the lymph glands of these mutants, we find clear evidence for lymph gland lobes with loss of blood progenitor cells and a concomitant increase in differentiated blood cells (Figure 4A–B, F). A connection between odorant receptor function and blood development is a rather novel and important finding and was therefore confirmed in several different ways. The blood differentiation phenotype of the orco mutant is also seen upon 1) ablating all olfactory receptor neurons (ORNs) using Orco-Gal4 driven expression of pro-apoptotic genes hid-rpr (Figure 4C), 2) upon expression of OrcoRNAi (Figure 4D), and 3) blocking ORN function by expression of Shits1 (Figure 4E). In addition to the blood differentiation phenotypes, blocking olfactory function in these backgrounds results in dramatic reduction of GABA immunoreactivity in the lymph gland (Figure 4G–H). The systemic level of hemolymph GABA extracted from orco mutants (orco2/Df[orco]) is also significantly reduced (Figure 4I). Upstream of the lymph gland, GABA expression in the Kurs6+GABA+ cells of the brain in orco mutant (orco2/Df[orco]) is significantly reduced, demonstrating that olfactory stimulation is essential for maintaining normal GABA levels within the neurosecretory cells (Figures 4J,K). The blood phenotypes seen upon loss of olfaction are not recapitulated upon downregulation of orco function in the lymph gland (Figure S4B–D). This is expected, as expression of odorant receptors is restricted to the olfactory neurons of the dorsal organ, not detected in the lymph gland. This was also confirmed with lineage-traced expression of orco (Figure 4L). PSC markers and GABABR expression remain normal in orco mutant background (Figure S4E–H).
Figure 4. Olfaction mediates maintenance of blood progenitors.
All lymph gland and brain images shown are obtained from wandering 3rd instar larvae. TOPRO3 (blue) marks nuclei. White scale bar: 50 μm; yellow scale bar: 10 μm. n = # of lymph glands statistically analyzed. In (F), percentage of differentiated cells (Pxn positive) are counted. Error bars in the graphs represent standard deviation.
(A–F) Odorant receptor coreceptor (Orco) is essential for blood progenitor maintenance.
(A) Control lymph gland (orco2/+) showing expression of Pxn (green) in differentiated cells (n=8).
(B) orco2/Df[orco] (p=1.3E-04; n=10).
(C–F) Control and driver for these panels is Orco-Gal4
(C) hid, rpr overexpression (p=7.9E-09; n=12).
(D) orcoRNAi (p=3.2E-08; n=11).
(E) Shits1 (p=2.0E-05; n=9).
(F) Quantitation of the genotypes represented in Figure panels A–E.
(G–K) Orco function regulates GABA levels.
(G) Control: orco2/+, GABA (red) staining in lymph gland progenitors.
(H) orco2/Df[orco], causes loss of GABA (red).
(I) Loss of orco function leads to significant reduction in hemolymph GABA levels. Six Elisa assays per genotype, 10 animals per trial.
Df[orco]/+ (control; 16.9 ± 3 ng GABA / larva)
orco2/Df[orco] (7.5 ± 1 ng GABA / larva; p= 3E-04).
(J) Control (orco/+; Kurs6-Gal4, UAS- mCD8GFP) brain showing Kurs6+ neurosecretory cells (green) stained with GABA (red, marked in white dotted line). J′ shows these cells in the red channel to highlight GABA expression.
(K) orco2/Df[orco]; Kurs6-Gal4, UAS-mCD8GFP. K′ shows the Kurs6+ cells in K only in the red channel to demonstrate GABA loss (compare with control, J′).
(L) Lineage tracing of orco-Gal4 (orco-Gal4; GTRACE see Table S1) shows lack of orco expression in the lymph gland. Orco expression is exclusively restricted to ORNs in the dorsal organ (black arrowhead in inset).
(M) Kurs6+ cells (green, Kurs6-Gal4, UAS-mCD8GFP) do not overlap with Dilp2 expressing neurons (red; white arrow and see the high magnification inset). Arrowhead points to the Kurs6 cluster that includes the GABA positive neurons.
(N) Loss of Gad1 expression in the Dilp2+ neurons (Dilp2-Gal4; UAS-Gad1RNAi) does not affect hemolymph GABA levels. Three Elisa trials for each genotype; 10 animals per trial.
Control (Dilp2-Gal4; 6.3 ± 2 ng GABA / larva)
Gad1RNAi (6.7 ± 1.5 ng GABA / larva; p= 0.8; not significantly different).
Olfactory and Nutritional effects are independently controlled
As lack of nutrition can lead to starvation-like phenotypes in the animal and affect blood development (Shim et al., 2012), we addressed whether the blood phenotype caused by losing olfaction is due to starvation. Mutating orco (orco2/Df[orco]) or ablating ORNs using orco-Gal4; UAS hid, rpr genetic backgrounds did not alter FOXO nuclear localization (Figure S4I–J), food intake (Figure S4K), or Dilp2 secretion (Figure S4L,L′), all hallmarks of the starvation phenotype (Shim et al., 2012). This indicates that insulin pathway activity in the olfactory receptor neurons is not involved in causing blood phenotypes in olfactory mutants.
By using appropriate markers, we established that Kurs6+ cells in the brain indeed do not overlap with the Dilp producing neurons (Figure 4M). Furthermore, expression of Gad1RNAi in the Kurs6+ neurons does not affect Dilp expression in the brain insulin producing cells (IPCs) (Figure S4L″). Finally, Gad1RNAi expressed specifically in the Dilp neurons does not cause any progenitor differentiation phenotype or change in GABA immunoreactivity in the lymph gland (Figure S4M) or in the hemolymph (Figure 4N).
Signaling downstream of ORNs
We investigated the role of the olfactory circuit, including the role of projection neurons (PNs) and inhibitory interneurons (iLN) (Figure 5A) in linking ORNs to GABA release by neurosecretory cells. We found that expression of Shits1 using the GH146-Gal4 driver to block the function of the antennal lobe PNs, reduces GABA expression in the Kurs6+GABA+ cells (Figure 5B,C), causes reduction in circulating GABA (Figure 5D), and leads to lymph gland phenotypes that are identical to that seen upon disruption of ORN function (Figure 5E–H, L and Figure S5A,B). The fold change in GABA fluorescence in comparing Figure 5E with Figure 5G is 11.34 (>1000%). This results from the approximately 2–3 fold (200–300%) change detected in circulation in various genetic backgrounds.
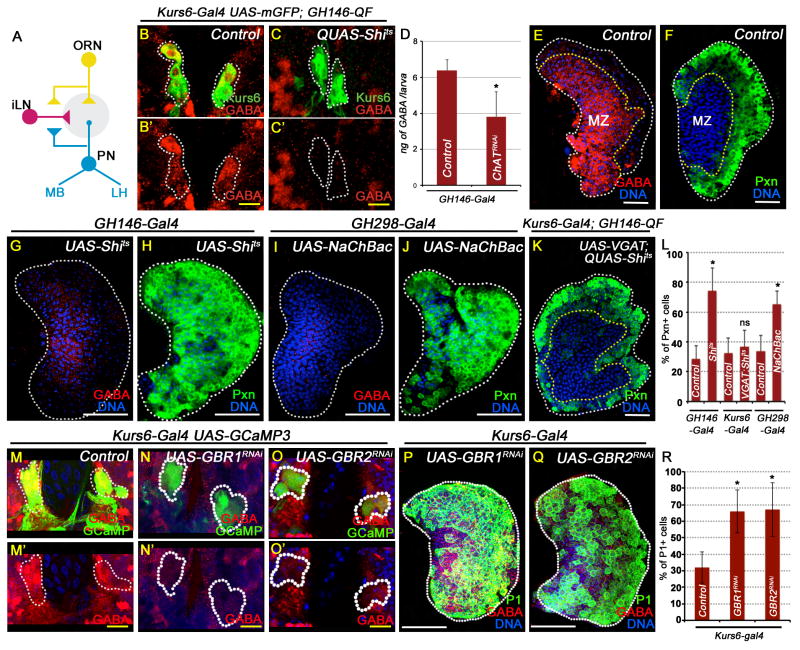
Figure 5. Regulation of GABA and progenitors by ORNs and Kurs6+ cells.
All lymph gland and brain images shown are obtained from wandering 3rd instar larvae. GABA is marked in red. TOPRO3 (blue) marks nuclei. White scale bar: 50 μm; yellow scale bar: 10 μm. n = # lymph glands analyzed for statistical analyses. See also Figure S5.
(A) Schematic diagram of ORN and its downstream neurons. The ORNs (yellow) form excitatory connections with projection neurons (PN, blue), while GABAergic local interneurons (iLN, red) form inhibitory GABAergic synapses between the glomeruli (grey). Axons from the PNs innervate into the lateral horn (LH) and the mushroom body (MB) for higher neuronal processing. Error bars in the graphs represent standard deviation.
(B–L) Projection neuron activity regulates GABA levels in Kurs6+GABA+ cells and in circulation.
(B) Control: Kurs6-Gal4, UAS-mCD8GFP; GH146-QF/+; A small subset of Kurs6+ neurosecretory cells (green, GFP) are also GABA+ (red; yellow due to overlap with green). B′ shows these cells in the red channel to highlight GABA expression.
(C) Kurs6-Gal4, UAS-mCD8GFP; GH146-QF, QUAS-Shits. GABA expression is lost in the Kurs6+ neurons. C′ shows these cells in the red channel (compare with B′).
(D) Quantitation of hemolymph GABA levels. 3 ELISA trials per genotype; 10 larvae per assay.
Control (GH146-Gal4; 6.4 ± 0.6 ng GABA / larva).
ChATRNAi (4 ± 1.2 ng GABA / larva; p= 4E-02; n= 3).
(E) Control lymph glands stained with anti-GABA (red) antibody
(F) Control lymph gland (GH146-Gal4) stained with anti-Pxn (green; n=10).
(G–H) Blocking PN activity causes lymph gland phenotypes.
(G) GH146-Gal4; UAS-Shits causes reduction in lymph gland GABA staining (red, compare with E)
(H) GH146-Gal4; UAS-Shits (Pxn, green, p=5.4E-08; n=11) (compare with F).
(I–J) Activating iLN activity causes lymph gland phenotypes.
(I) GH298-Gal4; UAS-NaChBac leads to reduction in lymph gland GABA expression (red, compare with E)
(J) GH298-Gal4; UAS-NaChBac (Pxn, green; p=3.3E-08; n=12) (compare with F).
(K) Kurs6-Gal4, UAS-VGAT; GH146-QF, QUAS-Shits (Pxn, green; p=0.24, not significant compared with Kurs6-Gal4 control; n=5). Blocking PN activity and activating release of GABA from Kurs6+ cells significantly rescues the phenotype seen in (H).
(L) Quantitation of Pxn positive cells (green) in F, H, J and K.
Controls: Kurs6-Gal4, UAS-mCD8GFP (n=10) and GH298-Gal4 (n=12)
(M–O) GABABR signaling in Kurs6+GABA+ cells regulates Ca2+ and GABA levels.
(M) Control: Kurs6-Gal4; UAS-GCaMP; Ca2+ sensor activity (green, GCaMP) in the Kurs6+GABA+ cells (red; yellow due to overlap with green). M′ shows these cells in the red channel to highlight GABA (red) expression. Driver used in (M–R) is Kurs6-Gal4.
(N) GABABR1RNAi
(O) GABABR2RNAi
N′ and O′ show these Kurs6+ cells in the red channel to demonstrate loss of GABA (compare with control, M′).
(P–R) GABABR signaling in Kurs6+GABA+ cells maintains blood progenitors. Control: Kurs6-Gal4 (n= 15).
(P) GABABR1RNAi (p=3.7E-09; n= 16)
(Q) GABABR2RNAi (p=2.5E-06; n= 7)
(R) Quantitation of Pxn positive cells (green) in P and Q.
Error bars in the graphs represent standard deviation.
Inhibitory tuning of the olfactory signal is mediated by GABAergic local interneurons (iLNs) and increased iLN activity attenuates olfactory behavior (Das et al., 2011). Temporally controlled activation of iLNs by expressing the bacterial channel protein, NaChBac using iLN specific drivers, is sufficient to induce progenitor differentiation similar to that seen by blocking PNs or ORNs (Figure 5I–J).
In an epistasis experiment, the blood differentiation phenotype due to reduced PN function (GH146-QF, QUAS-shits) is suppressed by overexpression of VGAT in the Kurs6+ cells (Kurs6-Gal4; UAS VGAT; GH146-QF, QUAS-shits; Figures 5K). These lymph glands follow a normal developmental profile. Thus, the Kurs6+GABA+ secretory cells function downstream of the olfactory system, including the projection neurons.
A positive feedback loop maintains GABA production in the Kurs6+ cells
The Kurs6+ GABA+ cells express GABABR2 as detected by immunostaining (Figure S5C). These cells are also positive for the Ca2+ sensor GCaMP (Figure 5M). When GABABRRNAi is expressed in the Kurs6+ cells, with the GCaMP sensor in the background, both GABA expression and calcium sensor activity are attenuated (Figure 5N–O). This indicates that the Kurs6+ cells receive a GABA signal and in turn up-regulate their GABA levels. More importantly for this study, lymph glands from Kurs6-Gal4; UAS-GABABR1RNAi or Kurs6-Gal4; UAS-GABABR2RNAi backgrounds show low GABA staining and extensive blood progenitor differentiation phenotype (Figure 5P–R), similar to that observed in Kurs6-Gal4; UAS Gad1RNAi or Kurs6-Gal4; UAS VGATRNAi backgrounds. Based on these results we conclude that Kurs6+ cells are responsive to GABA, which activates GABA/ GABABR dependent Ca2+ signaling to promote synthesis of GABA in these neurons. This functions as a positive feed back loop that maintains GABA levels in these cells as has been seen in similar related systems (Bao et al., 1994; Braun et al., 2010). Presumably, the sensory circuit sets up this feed back loop during development.
Specific ORNs regulate hematopoietic function
In the larval dorsal organ, Orco functions as a dimer with odor restricted conventional ORs to allow specific odor sensation (Kreher et al., 2005). To gauge the involvement of the conventional ORs, we utilized a previously described method for blocking function of one OR at a time by using OrX-Gal4 (X: each different OR) driving the expression of shits1 (Suh et al., 2004). Inhibition of a majority of ORs shows no phenotype in the lymph gland (Figure S6A–D). However, specific loss of Or42a either using shits or the Or42af04305 / Df[Or42a] genetic background causes significant loss of blood progenitors (Figure 6A–D) and reduction of GABA immuno-reactivity in the lymph gland and in circulation (Figure 6E–H). The observed blood phenotypes are as strong as those seen with loss of orco function and therefore establish that olfaction mediated by the dimeric Or42a/Orco complex is essential for the maintenance of GABA level and hematopoietic progenitor maintenance. Or42a has a very broad spectrum of attractive odor response to linear aliphatic compounds including acetate esters and maps to the glomerulus VM7 of the antennal lobe (Kreher et al., 2005). The activation of this glomerulus is presumably critical, via further circuitry involving the PNs and deeper aspects of the brain, for the control of GABA in specific subsets of Kurs6+ neurosecretory cells that influence hematopoiesis. Our results do not, however, rule out involvement of other receptors working redundantly in a combinatorial fashion.
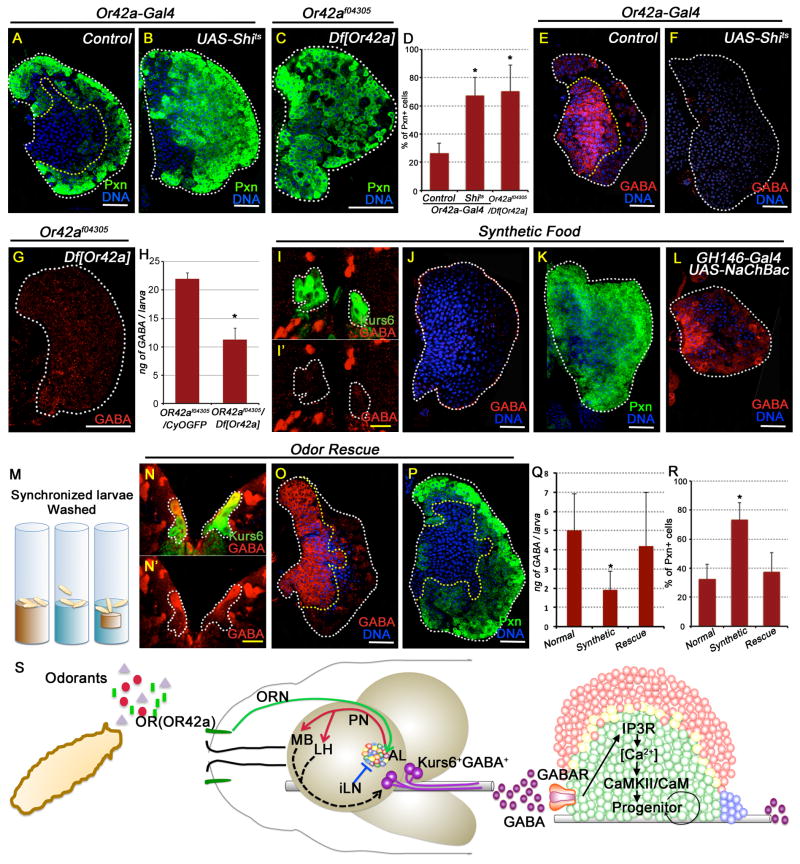
Figure 6. OR42a mediated blood progenitor maintenance and its physiological relevance.
All lymph gland and brain images shown are obtained from wandering 3rd instar larvae. TOPRO3 (blue) marks nuclei. Scale bar: white, 50 μm; yellow, 10 μm. n= # of lymph glands used for statistical analyses. Error bars in the graphs represent standard. deviation. See also Figure S6.
(A–H) Or42a function is essential for progenitor maintenance via regulating GABA.
(A) Control: Or42a-Gal4 (Pxn, green; n=10).
(B) Or42a-Gal4, UAS-Shits (n=10; p=4E-08)
(C) Or42af04305/Df[Or42a] (n=9; p=1.4E-06)
(D) Quantitation of the percentage of Pxn positive cells in A–C,.
(E) Control: Or42a-Gal4 (GABA shown in red).
(F) Or42a-Gal4; UAS-Shits (loss of GABA)
(G) Or42af04305/Df[Or42a] (loss of GABA).
(H) Quantitation of hemolymph GABA levels. n= # of Elisa assays; 10 animals per assay.
Or42af04305/CyO (control; 22 ± 1 ng of GABA/larva; n= 2)
Or42af04305/Df[Or42a] (11.3 ± 2 ng of GABA/larva; p= 1E-02, n= 3).
(I–L) Odor sensing is essential for progenitor maintenance in wild-type flies.
(I) Wild-type larva grown in odorless food shows dramatic loss of GABA (red) in the usually Kurs6+GABA+ neurons (demarcated with white dotted line).
I′ shows these cells in the red channel to highlight loss of GABA (red) staining.
(J) Wild-type larva grown on odorless medium shows severe reduction of GABA (red) staining in the lymph gland.
(K) Growth on odorless medium also induces extensive progenitor differentiation (Pxn, green).
(L) GH146-Gal4; UAS-NaChBac (activated PN) raised on odorless media restores GABA (red) in the MZ progenitors.
(M–R) Odor supplementation rescues lymph gland progenitor differentiation and GABA phenotypes.
(M) Schematic representation of odor deprivation and rescue experiment. The three vials depict the different food conditions: brown represents wild type larvae reared on normal cornmeal/ molasses agar food; blue represents larvae reared in odorless food. The third vial contains a dialysis-tubing pouch sealed in with normal food inserted into the minimal odor medium (referred to below as the “odor rescue condition”).
(N) Kurs6+GABA+ cells are identified (yellow) in larvae grown under “odor rescue condition”. N′ shows these cells in the red channel to highlight GABA expression. Compare with I′ which shows loss of GABA (red) expression on odorless medium.
(O) Lymph gland GABA staining is restored under “odor rescue” growth. Compare with J.
(P) Rescue of lymph gland progenitor differentiation (Pxn, green). Compare with K.
(Q) Quantitation of hemolymph GABA levels. 5 Elisa assays each genotype, 10 animals per assay.
Normal food (control; 5± 1.9 ng GABA/larva); Odorless food: (1.9 ± 1 ng GABA/larva; p=3E-03); Odor rescue condition: (4.2 ± 2.8 ng GABA/larva; p=0.5 i.e., not different from control).
(R) Quantitation of Pxn positive cells. n= # of lymph glands.
wild type (in A; n=10)
Wild type grown on odorless food (K; p=8.9E-08; n=10)
Wild-type grown in odorless food with “odor rescue” (P; p= 0.18 i.e., not significantly different compared with (A) and p=2.9E-06 compared with (K))
(S) Schematic representation of olfaction mediated control of blood progenitor maintenance via regulation of systemic GABA levels (see Discussion section for details).
Smell and hematopoiesis
The effect of odor experience in the environment on hematopoietic development has not been adequately addressed in any animal. Our results highlight the importance of olfactory stimulation on blood progenitor maintenance. As these results were obtained with various mutant strains, we next undertook experiments to address the physiological consequences of odor deprivation on hematopoiesis in wild-type animals. As larvae spend significant part of their early development digging in the food, we reasoned that the odor generating components of the food likely stimulate ORN activity. We therefore prepared a synthetic food medium containing nutrients such as carbohydrates, fat, monophosphates, amino acids, and nitrogen base (Table S2 and Experimental Procedures). This synthetic medium is devoid of any small volatile molecules that may be abundant in the cornmeal/molasses based standard fly media that could lead to an odor response. Synchronized late 2nd instar larvae reared on this synthetic food until 3rd instar larval life for 24 hours did not cause a starvation phenotype (Figure S6E–F). Remarkably, this odor deprivation during larval growth significantly reduces GABA expression in the brain Kurs6+GABA+ cells (Figure 6I–I′), GABA immunoreactivity in the lymph gland (Figure 6J), and maintenance of blood progenitors (Figure 6K). Constitutive activation of PN neurons that function downstream of ORNs in animals reared on minimal odor synthetic media rescues localization of GABA in the lymph gland (Figure 6L) showing that GABA secretion is downstream of odor sensation. Furthermore, the small increase in GABA in the Kurs6-Gal4, UAS-VGAT background, which does not have a phenotype on its own, is sufficient to rescue the differentiation phenotype seen when larvae are raised on the odor-free medium (Figure S6G).
The identity of the specific odorous molecules sensed by Or42a important in the context of blood progenitor maintenance is not clear. We therefore devised a rescue experiment in which a sealed dialysis tubing with low molecular weight cutoff (2kDa; see Experimental Procedures for details), containing normal food, is inserted into the minimal odor synthetic medium (Figure 6M). Larvae released into this supplemented medium will sense small molecular weight compounds and odors that diffuse into the odorless food without access to high molecular weight moieties confined within the tubing. Diffusion of low molecular weight compounds through the membrane into the odor-free synthetic medium is sufficient to recover GABA in Kurs6+ cells, in blood circulation and in the progenitors, in addition to reverting the blood phenotype (Figure 6N–R). These results strongly support our model that olfaction is critical for maintaining GABA that maintains the blood progenitors (Figure 6S).
DISCUSSION
Olfactory sensory stimulation in the control of GABA and maintenance of blood progenitors
Niche-dependent mechanisms of hematopoietic progenitor development and maintenance have been extensively described in both vertebrate and invertebrate literature (Garrett and Emerson, 2009; Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007; Mercier et al., 2012). Mechanisms independent of the niche that operate at a more systemic level but affect progenitor development have recently started to emerge (reviewed by (Gancz and Gilboa, 2013)).
Here, we describe a signal that originates from the brain and regulates blood progenitor maintenance (Figure 6S). This pathway is independent of the nutritional signal that involves Drosophila Insulin and TOR (Shim et al., 2012). We find that olfaction-dependent sensory stimulation relays systemic cues from the central nervous system to the undifferentiated blood progenitors by regulating physiological levels of GABA secreted into the blood stream. GABA is expressed in a small number of neurosecretory cells of the brain and the release of GABA from this class of neurosecretory cells is critically dependent on olfactory stimulation. Olfactory dysfunction decreases GABA expression in neurosecretory cells and also reduces systemic GABA levels in the circulating blood. Blood progenitors express the metabotropic GABAB receptor, which enables them to respond to GABA, raising the concentration of their cytosolic calcium, essential for inhibition of premature differentiation and maintenance of the progenitors. This control is lost when either the olfactory neurons or their network partners in the olfactory glomeruli are disrupted. A consequence of the above mechanism is that wild-type Drosophila larvae reared on odor-limited media have dramatically reduced systemic GABA levels, and consequently, their blood progenitors precociously differentiate. Upon blocking olfaction, GABA levels in the entire central brain region is reduced (Figure S4A), but it is the two GABA expressing neurosecretory cells in each lobe of the central brain that are important in controlling GABA secreted into the aorta that controls hematopoiesis.
Within the lymph gland, GABAB receptor is expressed in the blood progenitors and downregulated as the cells differentiate. Binding of GABA to GABABR maintains high cytosolic Ca2+ in the progenitors, a prerequisite for their remaining undifferentiated. The differentiated blood cells have very low or undetectable levels of Ca2+ and are also unresponsive to its alterations. Downstream of elevated Ca2+, the functions of Calmodulin and CaMKII are essential for progenitor maintenance. Events further downstream currently remain unclear. In principle, Ca2+ could directly or indirectly interact with either ROS or Wg related pathways shown to be important for progenitor maintenance (Kohn and Moon, 2005; Owusu-Ansah and Banerjee, 2009; Sinenko et al., 2009; Yan et al., 2006).
Brain and the innate immune/hematopoietic system
Accumulating evidence has shown that the mammalian nervous system also regulates innate immune responses through hormonal and neuronal routes. Sympathetic and parasympathetic nervous systems directly innervate into immune organs, while neuroendocrine factors control inflammation at a systemic level. Furthermore, immune cells express receptors for various neuronal factors, supporting the idea that there are contributions of the nervous system to immunity (Bhat et al., 2010; Kawashima and Fujii, 2003; Sternberg, 2006). Brain dysfunction, including certain neurodegenerative diseases generate heightened immune reaction, as central nervous system is generally thought to inhibit immune responses (Sternberg, 2006). The mammalian hematopoietic niche is innervated (Mendez-Ferrer et al., 2009) and cells within the niche express a Ca2+ sensing receptor on their surface that they utilize to home towards the peri-endosteal compartment (Adams et al., 2006). However, to our knowledge, a direct involvement of secreted GABA or olfaction in the maintenance of hematopoietic progenitors has not been demonstrated in any other system.
GABA is conserved from bacteria to plants and animals. In plants, GABA functions as a metabolite, a signaling molecule, and in stress response (Roberts, 2007). In vertebrates, GABA function has been primarily studied in neurotransmission (Li and Xu, 2008), but it also functions as a metabolite (Erecinska et al., 1996) and in developmental signaling in both embryonic tissue and in adult regeneration (Nilsson et al., 1993; Ohmasa and Saito, 2004; Soltani et al., 2011). We can readily detect GABA secreted into the Drosophila hemolymph. This is not unprecedented as GABA can be measured in the blood stream of many mammals, including humans (Ferkany et al., 1978). Interestingly, GABABR is expressed in primary human HSCs, and its expression is higher in immature stem cells than in more mature progenitors (Steidl et al., 2004). GABA function in human HSCs remains unclear, and it is not known if its function is controlled through a sensory signal as we have described here in Drosophila.
Dual use of signals for Development and Stress response
In addition to the universally used developmental pathways such as Hh, Dpp and Wg, Drosophila blood precursors utilize several unusual pathways for their development. For example, physiologically generated ROS functions as a signaling molecule that allows the blood progenitors to differentiate (Owusu-Ansah and Banerjee, 2009), while increased ROS, resulting from infection, is a stress signal that causes rapid expansion of this differentiation process (Sinenko et al., 2012). More recently, it was shown that insulin maintains the progenitor population during normal development, and starvation is a stress condition that causes drop in insulin levels and allows premature differentiation (Benmimoun et al., 2012; Dragojlovic-Munther and Martinez-Agosto, 2012; Shim et al., 2012; Tokusumi et al., 2012). Similarly, Hifα, stabilized under normoxic conditions by physiologically generated NO binds Notch and maintains a class of blood cells, while hypoxic conditions sensed as a stress, stabilize additional amounts of Hifα and increase the number of these blood cells (Mukherjee et al., 2011).
To summarize, in all the above instances, we see examples of signals that are used by the myeloid precursors for their normal development in a programmed manner, and the same signals cause rapid expansion of these blood cells upon conditions of stress. In the fly, the conditions that favor blood differentiation, including reduced olfaction, are normally initiated during pupariation when the need for increased numbers of macrophages is critical. As a bonus, these same pathways can cause increased differentiation earlier in larval life when activation of these pathways is perceived as a stress response. This response is reflected in our mutational studies.
Overall, this study describes the mechanism for coordinating inputs from olfactory stimulation to maintain blood progenitors via regulation of systemic GABA levels. As olfaction is an important sensory input for the larva, inability to sense odor could be interpreted as an important stress response. Anosmic larvae cannot survive in a competitive environment due to lack of food-searching behavior (Asahina et al., 2008). Furthermore, a recent study has shown that OR56a senses a microbial odorant to avoid unsuitable breeding and feeding sites (Stensmyr et al., 2012). Thus proper olfaction promotes survival, both by allowing improved competition within a brood and through avoidance of infectious organisms. Increased hematopoietic differentiation in the absence of odor input could also be beneficial to the larva in mounting an immune response, although this remains to be proven in future studies. In humans, loss of olfaction has been associated with abnormalities in many parts of the brain and impaired olfaction leads to amplified inflammation in mammals (Strous and Shoenfeld, 2006). Our data in Drosophila highlight that sensory stress response can directly influence developmental and cell fate decisions of blood progenitors. Whether this is also relevant to higher organisms with more complicated blood lineages remains to be explored.
EXPERIMENTAL PROCEDURES
For details see Supplementary info. Stocks and mutant crosses were maintained at 25°C. Crosses used in RNAi or Gal4/UAS genetic experiments were maintained at 29°C. Lymph gland staining was quantified based on the middle 1/3 confocal sections as previously described (Shim et al., 2012). Amount of GABA in circulation was determined by the GABA research ELISA kit (Labor Diagnostika Nord GmbH & Co. KG, Germany). For minimal odor experiments, a synthetic medium was made with constituents indicated in Table S2. For odor rescue, dialysis tubing with low molecular weight cutoff (Spectra/Por Dialysis tubing, MWCO 2000 D) was used for holding the normal food, and inserted into the odorless media.
Supplementary Material
Olfactory sensory perception controls hematopoietic progenitor maintenance
Olfactory receptor neurons instruct neurosecretory cells to secrete GABA
Secreted systemic GABA is sensed directly by the blood progenitors via GABABR
GABABR signal elevates cytosolic Ca2+ that maintains blood progenitors
Acknowledgments
We thank members of the Banerjee lab and J. Wang for helpful discussions. We acknowledge the Bloomington, VDRC, DGRC and NIG Drosophila stock centers and the DSHB hybridoma bank. We thank the following individuals for stocks and reagents: I. Ando, K. Brueckner, T. Clandinin, S. Cohen, C. Evans, J. Fessler, G. Hasan, A. Hofmann, F. R. Jackson, Y-N Jan, T. Kitamoto, G. Korge, P. Leopold, D. Naessel, K. Ngo, S. Noselli, E. Olson, E. Rulifson, S. Sinenko, and J. Wang. Supported by NHLBI grant, R01 HL067395 to UB; The Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research awards to UB and JS; and a California Institute for Regenerative Medicine postdoctoral fellowship TG2-01169 to JS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Asahina K, Pavlenkovich V, Vosshall LB. The survival advantage of olfaction in a competitive environment. Curr Biol. 2008;18:1153–1155. doi: 10.1016/j.cub.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Nathan B, Hsu CC, Zhang Y, Wu R, Wu JY. Role of Protein Phosphorylation in Regulation of Brain L-Glutamate Decarboxylase Activity. J Biomed Sci. 1994;1:237–244. doi: 10.1007/BF02253308. [DOI] [PubMed] [Google Scholar]
- Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, Rorsman P. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Cichon J, Wang W, Qiu L, Lee SJ, Campbell NR, Destefino N, Goard MJ, Fu Z, Yasuda R, et al. Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron. 2012;76:297–308. doi: 10.1016/j.neuron.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sadanandappa MK, Dervan A, Larkin A, Lee JA, Sudhakaran IP, Priya R, Heidari R, Holohan EE, Pimentel A, et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E646–654. doi: 10.1073/pnas.1106411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Smith LA, Seifert WE, Caprioli RM, Enna SJ. Measurement of gamma-aminobutyric acid (GABA) in blood. Life Sci. 1978;22:2121–2128. doi: 10.1016/0024-3205(78)90456-3. [DOI] [PubMed] [Google Scholar]
- Gancz D, Gilboa L. Hormonal Control of Stem Cell Systems. Annu Rev Cell Dev Biol. 2013 doi: 10.1146/annurev-cellbio-101512-122331. [DOI] [PubMed] [Google Scholar]
- Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–506. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Jackson FR, Newby LM, Kulkarni SJ. Drosophila GABAergic systems: sequence and expression of glutamic acid decarboxylase. J Neurochem. 1990;54:1068–1078. doi: 10.1111/j.1471-4159.1990.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenini-ndependent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Li K, Xu E. The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci Bull. 2008;24:195–200. doi: 10.1007/s12264-008-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and nichederived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332:1210–1213. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Kawakami K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun Integr Biol. 2011;4:566–568. doi: 10.4161/cib.4.5.15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Eriksson PS, Ronnback L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54:605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- Ohmasa M, Saito T. GABAA-receptor-mediated increase in intracellular Ca2+ concentration in the regenerating retina of adult newt. Neurosci Res. 2004;49:219–227. doi: 10.1016/j.neures.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parramon M, Gonzalez MP, Herrero MT, Oset-Gasque MJ. GABAB receptors increase intracellular calcium concentrations in chromaffin cells through two different pathways: their role in catecholamine secretion. J Neurosci Res. 1995;41:65–72. doi: 10.1002/jnr.490410108. [DOI] [PubMed] [Google Scholar]
- Pennetier D, Oyallon J, Morin-Poulard I, Dejean S, Vincent A, Crozatier M. Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc Natl Acad Sci U S A. 2012;109:3389–3394. doi: 10.1073/pnas.1109407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR. Does GABA Act as a Signal in Plants?: Hints from Molecular Studies. Plant Signal Behav. 2007;2:408–409. doi: 10.4161/psb.2.5.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwirtlich M, Emri Z, Antal K, Mate Z, Katarova Z, Szabo G. GABA(A) and GABA(B) receptors of distinct properties affect oppositely the proliferation of mouse embryonic stem cells through synergistic elevation of intracellular Ca(2+) FASEB J. 2010;24:1218–1228. doi: 10.1096/fj.09-143586. [DOI] [PubMed] [Google Scholar]
- Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund T, Korge G. Innervation of the ring gland of Drosophila melanogaster. J Comp Neurol. 2001;431:481–491. doi: 10.1002/1096-9861(20010319)431:4<481::aid-cne1084>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Shim J, Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO reports. 2012;13:83–89. doi: 10.1038/embor.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A. 2011;108:11692–11697. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl U, Bork S, Schaub S, Selbach O, Seres J, Aivado M, Schroeder T, Rohr UP, Fenk R, Kliszewski S, et al. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 2004;104:81–88. doi: 10.1182/blood-2004-01-0373. [DOI] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Shoenfeld Y. To smell the immune system: olfaction, autoimmunity and brain involvement. Autoimmun Rev. 2006;6:54–60. doi: 10.1016/j.autrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Tokusumi Y, Tokusumi T, Shoue DA, Schulz RA. Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland. PLoS One. 2012;7:e41604. doi: 10.1371/journal.pone.0041604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annual review of neuroscience. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.