Abstract
Synthesis of acetylcholine (ACh) by non-neuronal cells is now well established and plays diverse physiologic roles. In neurons, the Na+-dependent, high affinity choline transporter (CHT1) is absolutely required for ACh synthesis. By contrast, some non-neuronal cells synthesize ACh in the absence of CHT1 indicating a fundamental difference in ACh synthesis compared to neurons. The aim of this study was to identify choline transporters, other than CHT1, that play a role in non-neuronal ACh synthesis. ACh synthesis was studied in lung and colon cancer cell lines focusing on the choline transporter-like proteins, a five gene family (CTL1-5). Supporting a role for CTLs in choline transport in lung cancer cells, choline transport was Na+-independent and CTL1-5 were expressed in all cells examined. CTL1,2,&5 were expressed at highest levels and knockdown of CTL1,2&5 decreased choline transport in H82 lung cancer cells. Knockdowns of CTL1,2,3&5 had no effect on ACh synthesis in H82 cells. By contrast, knockdown of CTL4 significantly decreased ACh secretion by both lung and colon cancer cells. Conversely, increasing expression of CTL4 increased ACh secretion. These results indicate that CTL4 mediates ACh synthesis in non-neuronal cell lines and presents a mechanism to target non-neuronal ACh synthesis without affecting neuronal ACh synthesis.
Keywords: choline, acetylcholine, lung cancer, choline transporter, CTL, choline transporter-like protein
Introduction
Synthesis and secretion of acetylcholine (ACh) to act as an autocrine or paracrine hormone by non-neuronal tissues is now well established (Wessler and Kirkpatrick 2008). Choline acetyltransferase (ChAT) and ACh synthesis has been demonstrated in airway epithelial cells (Klapproth et al. 1997; Proskocil et al. 2004; Wessler and Kirkpatrick 2008), colon epithelial cells (Porter et al. 1996; Cheng et al. 2008; Yajima et al. 2011), keratinocytes (Kurzen et al. 2007), glia (Wessler et al. 1997), lymphocytes (Kawashima and Fujii 2000), and ovarian follicular cells (Mayerhofer and Kunz 2005) among other cell types. Non-neuronal ACh plays multiple physiologic and pathologic roles, affecting growth, development, secretion and ciliary movement among many actions (Lips et al. 2007; Wessler and Kirkpatrick 2008; En-Nosse et al. 2009; Novotny et al. 2011; Hollenhorst et al. 2012). In both lung cancer and colon cancer, ACh acts as an autocrine growth factor for cancer growth and development (Song et al. 2003; Song et al. 2007; Song et al. 2008). The wide-spread expression of ACh in non-neuronal tissues makes it important to understand how non-neuronal ACh synthesis differs from neuronal ACh synthesis.
There are clear similarities and differences between neuronal and non-neuronal cholinergic signaling. Both neuronal and non-neuronal cell types express choline acetyltransferase, the vesicular acetylcholine transporter, cholinesterases, nicotinic and muscarinic receptors (Proskocil et al. 2004; Wessler and Kirkpatrick 2008). Regulation of ACh secretion is obviously different as non-neuronal cells generally are not excitable and secretion is not triggered by action potentials. Another clear difference is transport of choline that is used for ACh synthesis.
In neurons choline used for ACh synthesis is transported from extracellular spaces via a Na+-dependent, high-affinity choline transporter (CHT1) (Apparsundaram et al. 2000; Okuda et al. 2000) and in the absence of CHT1, neurons cannot synthesize ACh (Ferguson et al. 2004). By contrast, colon epithelial cells (Yajima et al. 2011) and lung cancer cells can synthesize ACh in the absence of CHT1 (Song et al. 2003; Song and Spindel 2008). Thus, choline transport in non-neuronal cells cannot be solely dependent on CHT1 and must by necessity utilize other choline transporters such as the recently described family of five choline transporters designated as the choline transporter-like proteins 1–5 (CTL1–5) (O’Regan et al. 2000; Traiffort et al. 2005). The CTLs are Na+-independent and have an intermediate-affinity for choline and hemicholinium-3 (HC-3) as compared to CHT1 (O’Regan et al. 2000; Inazu et al. 2005; Traiffort et al. 2005). CTL1 in particular has been shown to transport choline in renal tubule epithelia (Yabuki et al. 2009), keratinocytes (Uchida et al. 2009), neuroblastoma (Machova et al. 2009; Yamada et al. 2010) and lung adenocarcinoma cells (Nakamura et al. 2010). CTL2 has also been shown to transport choline in lung adenocarcinoma cells (Nakamura et al. 2010). CTL3 has recently been demonstrated to be expressed in neutrophils and has been identified as the human neutrophil alloantigen-3a (Greinacher et al. 2010). While it has been suggested that CTL1 may be linked to non-neuronal ACh synthesis, this has only been proposed on the frequent co-expression of CTL1 with non-neuronal ACh (Yamada et al. 2010).
We thus undertook to determine which of the CTL’s is positively linked to ACh synthesis in two different non-neuronal systems, examining both small cell lung carcinoma (SCLC) cells using the H82 cell line that synthesizes ACh but does not express CHT1 and the H508 colon cancer cell line that similarly synthesizes ACh but does not express detectable levels of CHT1. This was done by a combination of pharmacologic characterization of choline transport and characterizing the effect of knockdown of the different CTL’s and expression of CTL4 on ACh synthesis. In this paper, we show that of the CTLs, only CTL4 appears positively linked to ACh synthesis in two different non-neuronal cell types.
Materials and Methods
Cell lines and tumors
Small cell lung carcinoma cell lines H417, H592, H69, H82, H146, H345, and H1694 cell lines were obtained from ATCC. HA-E, SV-E, MO-A, RG-1 and YR-A cell lines were established as previously described (Campling et al. 1992). H82 cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 1% fetal bovine serum, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite. The medium for maintaining other SCLC cell lines was RPMI1640 containing 10% fetal calf serum. Archival samples of SCLC were obtained from the Department of Pathology in Oregon Health & Sciences University and from the Department of Neuroscience in University of Pennsylvania. Colon cancer cell lines HT-29, H747, H508, SNU-C1, WiDr, Caco-2 and HCT-15 were obtained from ATCC and maintained as suggested by ATCC.
PCR
RT-PCR and realtime PCR were used to detect mRNAs for CHT1 and the CTLs in SCLC cell lines and tumors. RNA was prepared and PCR performed as previously described (Song et al. 2003). Realtime assays were run in duplex using 18s RNA as an internal standard. Primers used for PCR and realtime-PCR are listed in supplement table 1.
CTL knockdown with siRNAs
On-Target Plus smart pool siRNAs against CTL1, 2, 3, 5 and CHT1 were purchased from Dharmacon (Chicago, IL, USA). SiRNA against CTL4 (sRNA ID# s37329) was from Ambion (Austin, TX, USA). SiRNAs were transfected according to the manufacturer’s protocol, with DharmaFECT 1 (Dharmacon), at a concentration of 100 nM and treated cells were used at approximately 96 hours post transfection.
CTL4 lentiviral transduction
The CTL4 cDNA was obtained from the image consortium and subcloned into the lentiviral shuttle vector pLVI-IRES which contains a CMV promoter to drive cDNA expression and a bicistronic-expressed GFP marker. Lentivirus was prepared and titrated as previously described (Dissen et al. 2009). H82 cells were infected at a ratio of 5 transforming units per cell in the presence of polybrene 0.16 μg/ml. Colon cancer cells were similarly infected but without polybrene. One week post infection, cells were sorted by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA) and used for studies described hereafter.
ACh and ChAT analysis
To investigate effects of choline concentration on ACh synthesis and secretion, 24-well plates were seeded with H82 cells at a concentration of 5 × 105 cells per well in 1 ml and different concentrations of choline chloride (Sigma, St. Louis, MO, USA) added. After plating, in order to inhibit cholinesterase activity, 50 μM neostigmine (Sigma) was immediately added to each well. The concentration of choline in unsupplemented RPMI-1640 medium is 20 μM. Additional choline at concentrations of 30, 100, 300 and 1000 μM respectively were added into the wells of cell culture plates and incubated for 24 h. After incubation, cells were centrifuged and supernatants quick frozen and stored at −80 °C. ACh was extracted from cell pellets with 90% methanol in 0.1 M formic acid.
To investigate effects of individual CTL knockdown or CTL4 transduction, the lung or colon cancer cells were harvested and incubated with baseline choline and 50 μM neostigmine after knockdown of each CTL or after CTL4 transduction as described as above. To measure Na+ dependence of ACh synthesis, 3 × 106 H82 cells per well were incubated in Na+ buffer or Na+-free buffer. For Na+-free buffer, NaCl was replaced with an equimolar concentration of N-methyl-D-glucamine chloride as described by Inazu et al (Inazu et al. 2005). Samples for ACh assay were harvested after 3 h incubation.
Acetylcholine was measured by HPLC with enzyme-coupled electrochemical assay as previously described (Song et al. 2003) or by mass spectrometry (4000 Q TRAP™ LC/MS/MS System, Applied Biosystems, Foster City, CA, USA) using D4-ACh as an internal standard, as described by Philips et al (2010). Results by both methods were similar.
Choline uptake assay
Choline uptake was measured with [3H]choline (86Ci/mM, PerkinElmer, Boston, USA) as described by (Inazu et al. 2005). To measure choline uptake, H82 cells were harvested and washed twice with choline-free uptake buffer, consisting of 125 mM NaCl, 4.8 mM KCl, 1.2 mM CaCl2 and 1.2 mM KH2PO4, 5.6 mM glucose, 1.2 mM MgSO4 and 25 mM HEPES (pH to 7.4). 5 × 105 H82 cells were incubated with 20 nM [3H]choline at room temperature for different time periods (5, 10, 20, 40, and 80 min). Uptake was terminated by adding ice-cold buffer and then cells were centrifuged and washed five times with ice-cold buffer. Cells were lysed in 0.4 ml of 1% SDS/0.2 M NaOH, and cellular incorporation of [3H]choline was determined by scintillation counting. Nonspecific choline uptake was determined by adding 1 mM hemicholinium-3 (HC-3). For choline uptake saturation analysis in the Na+ buffer or Na+-free buffer, 20 nM [3H]choline plus different concentrations (0.78, 1.56, 3.125, 6.25, 12.5, 50 and 100 μM) of choline were added. To measure the effect of specific CTL1, 2, 4 and 5 knockdown on choline transport, H82 cells were harvested 4 days after transfection and resuspended in Na+-free buffer for choline uptake analysis. [3H]-choline uptake data was normalized by protein measurement with BCA assay (Thermo Scientific, Rockford, USA).
Cell proliferation
H82 cells were used for evaluation of SCLC cell growth. H82 cells were plated at 5,000 cells/well in 96-well culture plates and increasing concentrations of choline were added to the wells. For characterization of the role of cholinergic receptors, either the nAChR antagonist mecamylamine at 10−6 and 10−5 M or the mAChR antagonist atropine at 10−6 and 10−5 M were added to wells containing 10−3.5 M choline. Cell number after 9 days was then measured using the MTS assay as previously described (Song et al. 2003). For characterization of the effect of CTL knockdown, cells were plated in 96-well plates and siRNAs (100 nM final concentration) and DharmaFECT 1 were added and 4 days after transfection, cell proliferation was assayed with CellTiter-Blue™ reagent (Promega, Madison, WI, USA).
Statistical analysis
All data are presented as Mean ± SE. Data for cell growth and choline uptake, ACh secretion were analyzed by ANOVA with Newman-Keuls multiple-comparison tests. The effects of CTL4 transduction on choline transport and ACh secretion were analyzed by two sample t-test. Kinetic parameters were calculated by non-linear regression methods using Prism (GraphPad Software, Inc., San Diego, CA, USA).
Results
ACh secretion by H82 SCLC cells is dependent on media choline concentration
As shown in figure 1, ACh secretion by lung cancer cells is directly affected by media choline levels. ACh secretion was increased in a dose-dependent manner by increased concentration of choline in the media (R2 =0.945, p <0.05) (Fig. 1A, B). Addition of neostigmine significantly increased ACh levels, showing the presence of cholinesterase in the media, and further confirming the specificity of the ACh assay. Realtime PCR assay of ChAT mRNA levels showed no changes in ChAT mRNA levels with increased choline levels, and assay of ChAT enzymatic activity also showed no changes with increased choline levels (data not shown). Ninety percent of ACh synthesized by H82 cells was constitutively released as determined by measuring levels of ACh in cell pellets (Fig. 1C). Thus, ACh levels in the medium accurately reflect ACh synthesis and are dependent on choline transport into the cell.
Figure 1.
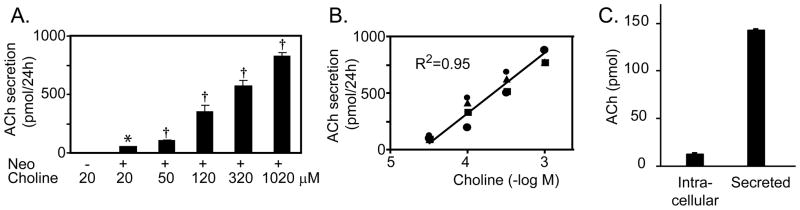
Choline increases ACh secretion by H82 SCLC cells. A. Choline significantly increased ACh secretion by H82 cells in a concentration-dependent manner. Addition of the cholinesterase inhibitor neostigmine (50 μM) resulted in increased levels of ACh in the medium. Secretion represent total amount of ACh secreted by 500,000 cells over 24 hours. B. There is a linear relationship between choline concentration and ACh secretion by H82 cells. Data are mean ± SE of 4 separate experiments. * p<0.05 versus no neostigmine; † p<0.05 versus 20 μM choline + neostigmine. C. Relative levels of ACh in cells versus medium. 500,000 H82 cells were cultured in standard medium (20 μM choline) in the presence of neostigmine for 24 hours and levels of ACh in media and cell pellet measured.
Choline transport and ACh synthesis by H82 cells
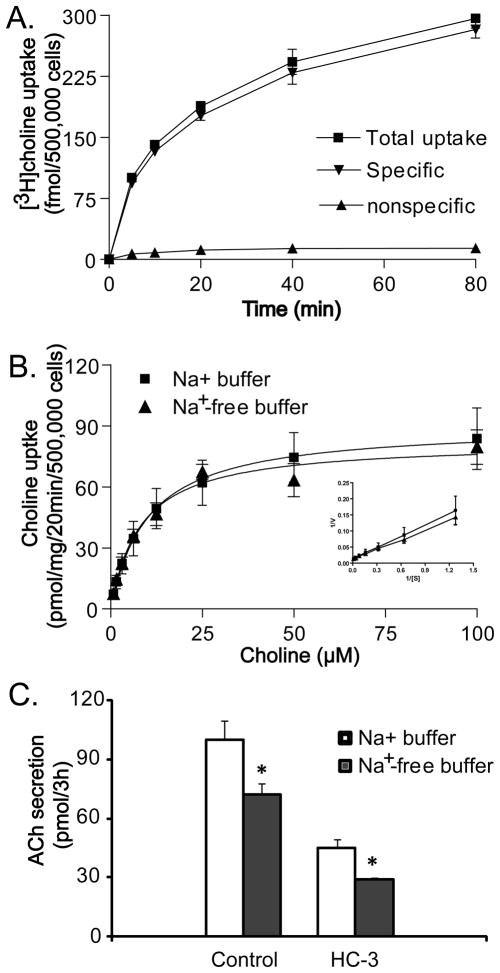
As shown in Table 1, H82 cells as well as the majority of SCLC cell lines tested do not express CHT1; therefore SCLC must utilize other choline transporters for ACh synthesis. To begin to characterize the transporters involved in choline transport into lung cancer cells, kinetics of [3H]choline uptake was examined. As shown in Fig. 2A, [3H]choline uptake in H82 cells was increased in a time-dependent manner in 125 mM Na+ buffer. Non-specific [3H]choline uptake was less than 10% of total [3H]choline uptake in the presence of 1 mM HC-3 at all choline concentrations tested. Based on these findings, subsequent saturation curve experiments were performed using an uptake period of 20 min in both the presence and absence of Na+. Kinetic analysis of specific choline uptake data in 125 mM Na+ buffer and Na+-free buffer yielded Michaelis-Menton constant (Km) of 10.2 ± 3.2 μM and 8.2 ± 1.6 μM and Maximal velocity (Vmax) 90.3 ± 8.6 and 82.2 ± 4.8 pmol/20 min, respectively. The close kinetic parameters in both 125 mM Na+ buffer and Na+-free buffer show that Na+ does not significantly affect choline transport and that choline uptake in H82 cells is mediated by a Na+-independent mechanism. This further rules out a role high affinity choline transporter CHT1 which is Na+ dependent.
Table 1.
Expression of CHT1 and CTLs in SCLC cell lines and tumors.
| Cell line | CHT1 | CTL1 | CTL2 | CTL3 | CTL4 | CTL5 |
|---|---|---|---|---|---|---|
| H69 | − | + | + | + | + | + |
| H82 | − | + | + | + | + | + |
| H146 | − | + | + | + | − | + |
| H345 | + | + | + | + | + | + |
| H417 | − | + | + | + | + | + |
| H592 | − | + | + | + | − | + |
| H1694 | + | + | + | + | + | + |
| HA-E | − | + | + | + | + | + |
| SV-E | − | + | + | + | + | + |
| MO-A | + | + | + | + | + | + |
| RG-1 | − | + | + | + | + | + |
| YR-A | + | + | + | + | + | + |
| Tumor1 | − | + | + | + | + | + |
| Tumor2 | + | + | + | + | + | + |
|
| ||||||
| Avg Ct | Not Done | 31.4 ± 0.4 | 29.5 ± 0.5 | > 38 | 36.2 ± 0.5 | 31.0 ± 1.0 |
Conventional RT-PCR was done for cell lines and tumors to show the presence (+) or absence (−) of the specified choline transporters. In addition, realtime-PCR was performed to quantitate levels of the transporters in the cell lines. While CT levels between RNAs cannot be precisely compared, the difference of ~5 CT suggests approximately a 30-fold difference. Ct values > 38 represent concentrations below the level of reliable detection.
Fig. 2.
Na+-independent choline uptake and ACh synthesis by H82 cells. A. Time course of [3H]choline (20 nM) uptake. Each point represents the mean ± SE of four experiments in 125 mM Na+ buffer. Non-specific [3H]choline uptake was determined in the presence of 1 mM hemicholinium-3 (HC-3). B. Choline uptake is Na+-independent in H82 cells. Substrate saturation curves of choline uptake in 125 mM NaCl and Na+-free buffer were established as described in the material and methods. Uptake of choline by H82 cells was measured for 20 min at increasing concentrations of choline. Non-specific choline uptake was also determined by adding 1 mM HC-3. Inset: Eadie-Hofstee plots of the data. V, uptake rates in pmol/mg/20min; S, choline concentration. Data are mean ± SE of six replicates in three experiments. C. Effect of HC-3 and Na+-free buffer on ACh synthesis in H82 cells standard medium (20 μM choline) in the presence of neostigmine. Each point represents the mean ± SE of four replicates in two experiments, * p<0.05 versus 125 mM Na+ buffer.
The Eadie-Hofstee plot (Fig. 2B, inset) shows a single line in both 125 mM Na+ buffer (r2 =0.99, p<0.001) and Na+-free buffer (r2=0.96, p<0.001), suggesting that the majority of choline uptake into H82 cells is primarily mediated by a single class of transporters. Linkage to ACh synthesis appears more complicated since as shown in Fig. 2C, ACh secretion was decreased by 30% in Na+-free buffer compared to buffer that included Na+ and by 50% in the presence of HC-3 compared to incubation without HC-3. This suggests that the primary choline transporter specifically linked to ACh is Na+-independent and HC-3 sensitive, but that other transporters may also link to ACh synthesis.
The CTLs transport choline in SCLC
The data above showing that choline transport in SCLC was Na+-independent and sensitive to HC-3 is consistent with the known pharmacology of the CTLs (O’Regan et al. 2000; Inazu et al. 2005; Traiffort et al. 2005) therefore suggesting a role for the CTL’s in choline transport in SCLC. On this basis, RT-PCR was performed to characterize the expression of the CTLs in a panel of SCLC cell lines and tumors. As shown in Table 1, all five subtypes of CTL could be detected by conventional RT-PCR in all SCLC cell lines tested and tumors, except for H146 and H592 which lacked CTL4. By contrast, CHT1 could not be detected in more than half of SCLC tumors and cell lines tested. Realtime PCR showed levels of CTL4 was substantially lower than levels for CTL1, 2 and 5 and CTL3 was essentially undetectable (Table 1). Increasing levels of choline in the cell culture medium did not increase levels of the CTL mRNAs.
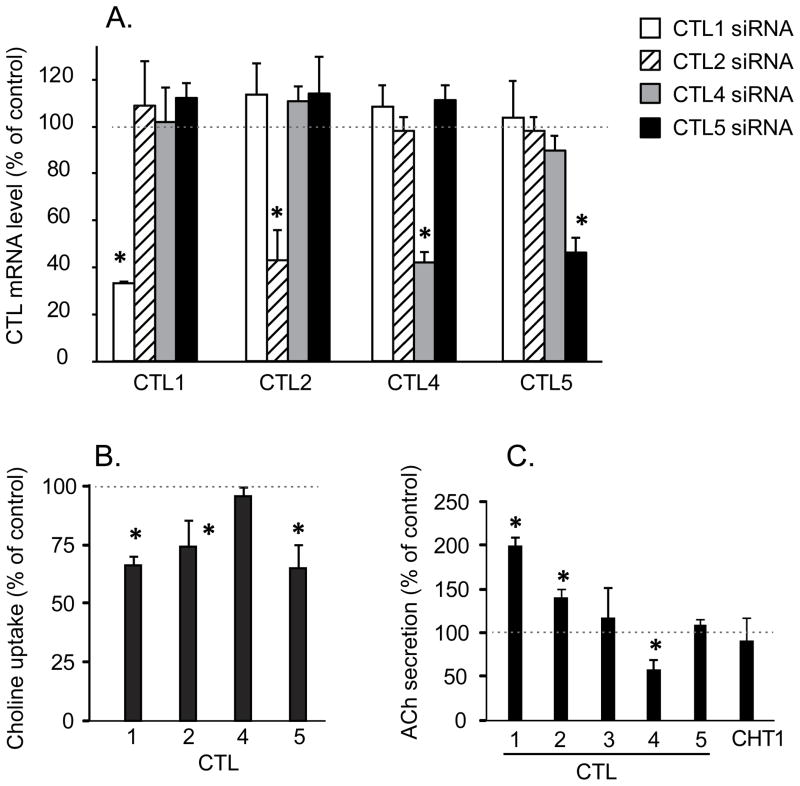
Knockdown of CTL1, 2 and 5 significantly decreased choline transport in H82 cells, demonstrating the role of CTL1, 2 and 5 in choline transport in SCLC (Fig. 3A,B). No effect on choline transport was seen by knockdown of CTL4 which is consistent with its lower levels compared to CTL1, 2 and 5. The effect of CTL3 knockdown on choline transport was not measured because of its very low levels of expression.
Fig. 3.
Effect of CTL knockdown on choline transport and ACh secretion. CTL subtypes 1–5 and CHT1 were knocked-down by RNAi and cells processed as described in the methods. A. Isoform specific siRNAs decreased levels of CTL1, 2, 4 and 5 mRNA respectively by 50–70% compared to control siRNA-treated cells without affecting levels of other CTL’s. Knockdown for CHT1 and CTL3 are not shown since they were below the level of detection in H82 cells and were included as negative controls for effects on ACh secretion. Data are mean ± SE of at least four replicates in two separate experiments. *P < 0.05 compared to RNA levels in cells treated with control (nonsense) siRNA. B. Choline uptake in Na+-free buffer was significantly decreased compared to control siRNA in H82 cells by knockdown of CTL 1, 2 or 5, but not by knockdown of CTL4 (control choline uptake equaled 10.04 ± 1.66 pmol/mg/15min, choline concentration = 20 nM). Data are mean ± SE of 4 replicates in two separate experiments. *P < 0.05 compared to choline uptake in cells treated with control siRNA. C. Effect of knockdown of CTLs 1–5 and CHT1 on ACh secretion by H82 cells. Only knockdown of CTL4 significantly reduced ACh secretion CTL4 (control ACh secretion equaled 80.2 ± 29.9 pmol/0.5 million cells/24h). Knockdown of CTL3, 5 and CHT1 had no effect, while knockdown of CTL1 and 2 significantly increased levels of ACh secretion. 50 μM neostigmine added to all cultures to prevent ACh degradation. Data are mean ± SE of 6 replicates in three separate experiments. *P <0.05 compared to the cells treated with control siRNA.
Choline transporter-like protein 4 is linked to ACh synthesis and secretion in lung cancer cells
To determine if a specific CTL subtype was linked to ACh synthesis in SCLC, the effect of knockdown of each CTL subtype on ACh secretion was tested in H82 cells. As shown in Fig. 3C only the knockdown of CTL4 significantly reduced ACh secretion. The CTL4 knockdown also significantly reduced the increase in ACh secretion caused by increased media choline (data not shown). Surprisingly, knockdown of CTL 1 and 2 actually increased ACh secretion by H82 cells (Fig. 3C). CHT1 which is not expressed in H82 cells served as a negative control, and as expected, had no effects on ACh secretion (Fig. 3C). Specificity of isoform knockdowns was confirmed by measuring levels of the other transporters and detecting no changes in the non-targeted forms (Fig. 3A).
The linkage between CTL4, choline transport and ACh synthesis was confirmed by transduction of H82 cells with a lentivirus expressing CTL4 (Fig. 4). CTL4 transduction significantly increased choline uptake in the presence of 20 nM and 10 μM choline (Fig. 4A). Importantly, as shown in figure 4B, CTL4 transduction significantly increased ACh secretion in H82 cells and this effect was enhanced by higher concentrations of choline. Levels of CTL4 mRNA in the transduced cells were increased more than 200-fold and levels of the other CTL’s were not affected (Fig. 4C).
Fig. 4.
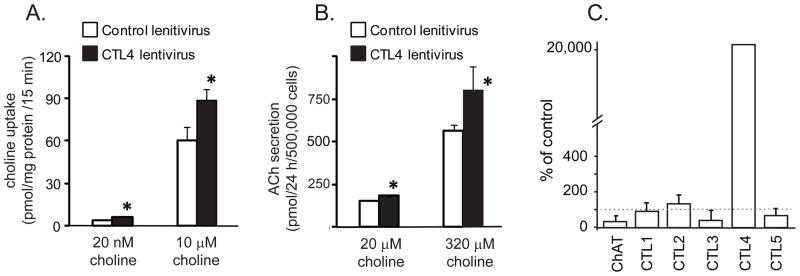
CTL4 expression in H82 cells increased choline transport and ACh synthesis. Lentivirus CTL4 vector construction and transduction as described in the material and methods. A. CTL4 transduction increased choline uptake by H82 cells. Data are mean ± SE of 6 replicates in three separate experiments. *P< 0.05 compared to control vector. B. CTL4 transduction significantly increased ACh synthesis in H82 cells. Data are mean ± SE of 4 replicates in two separate experiments. 50 μM neostigmine added to all cultures to prevent ACh degradation. Cell number was also measured at 24 hours and was not affected by lentivirus transduction (data not shown). * P<0.05 compared to control vector. C. Transduction of H82 cells with CTL4 lentivirus increased levels of CTL4 more than 200-fold compared to cells transduced with a control lentivirus. Other CTL’s and ChAT were not significantly changed.
Choline transporter-like protein 4 is also linked to ACh synthesis and secretion in colon cancer cell lines
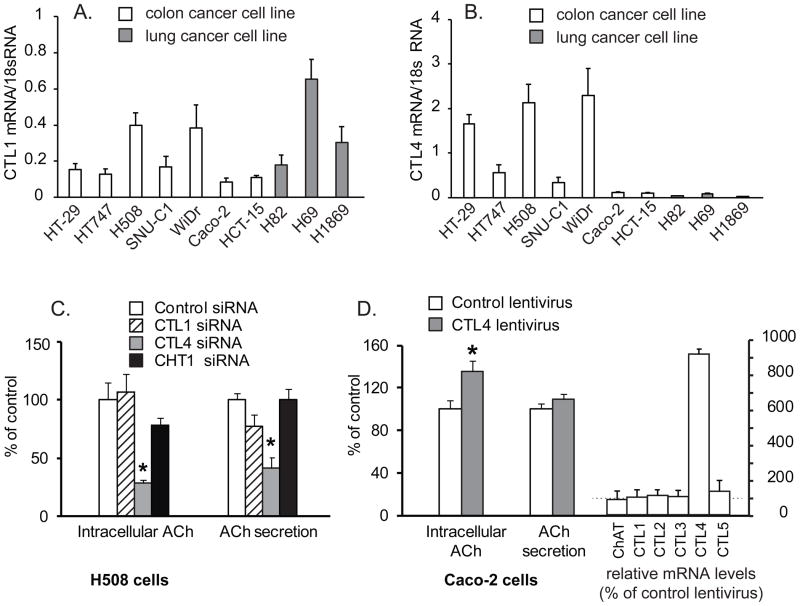
To generalize the role of CTL4 in non-neuronal ACh synthesis we studied a second cell type which is well established to secrete ACh, notably colonic epithelial cells as exemplified by colon carcinoma cell lines. As shown in figure 5, colon cancer cell lines express both CTL1 and CTL4, though colon cancer cells express significantly higher levels of CTL4 than do lung cancer cells (Fig 5A,B). Levels of CHT1 in colon cancer cells were essentially undetectable (data not shown). As shown in figure 5C, CTL4 knockdown in H508 colon cancer cells which have high levels of CTL4 significantly decreased both intracellular and secreted ACh, while knockdown of CTL1 and CHT1 had no significant effect on ACh synthesis and secretion. As shown in figure 5D, expression of CTL4 in Caco-2 colon cancer cells which have low levels of CTL4 significantly increased intracellular ACh levels, though secretion of ACh into the media was not significantly changed.
Fig. 5.
CTL4 modulates ACh synthesis in colon cancer cells. A. Expression of CTL1 in colon and lung cancer cell lines. B. Expression of CTL4 in colon and lung cancer cell lines. C.Effect of knockdown of CTLs 1, 4 and CHT1 on ACh synthesis of H508 cells. Specific siRNAs decreased levels of CTL1 and CTL4 mRNA respectively by 50–60% compared to control siRNA-treated cells (data not shown, but similar knockdown and specificity as shown in figure 3). Data are mean ± SE of 7 replicates in two separate experiments and show percent of control intracellular ACh (0.07 pmol by 100,000 H508 cells treated with control siRNA) or percent of control media ACh concentration (0.14 pmol per 100,000 H508 cells treated with control siRNA in 48 h). *p < 0.01versus control siRNA. 50 μM neostigmine added to all cultures to prevent ACh degradation. D. CTL4 transduction increased ACh synthesis by Caco-2 cells. Transduction of CTL4 significantly elevated intracellular ACh of Caco-2 cells by 35%, but did not significantly impact ACh secretion. Data are mean ± SE of 10 replicates in two separate experiments and presented as percentage of Caco-2 cells ACh secretion by cells transduced with control lentivirus or ACh secretion (1.76 pmol by 100,000 Caco-2 cells within 48 h) or percent of intracellular ACh in cell cells transduced with control lentivirus (3.91 pmol in 100,000 Caco-2 cells). 50 μM neostigmine added to all cultures to prevent ACh degradation. Right hand bars show that CTL4 transduction increased CTL4 mRNA levels 9.1-fold compared to cells transduced with control lentivirus, but did not significantly affect levels of the other CTL or ChAT mRNA. *p < 0.01 versus control lentivirus.
Targeting CTL4 can modulate cell growth
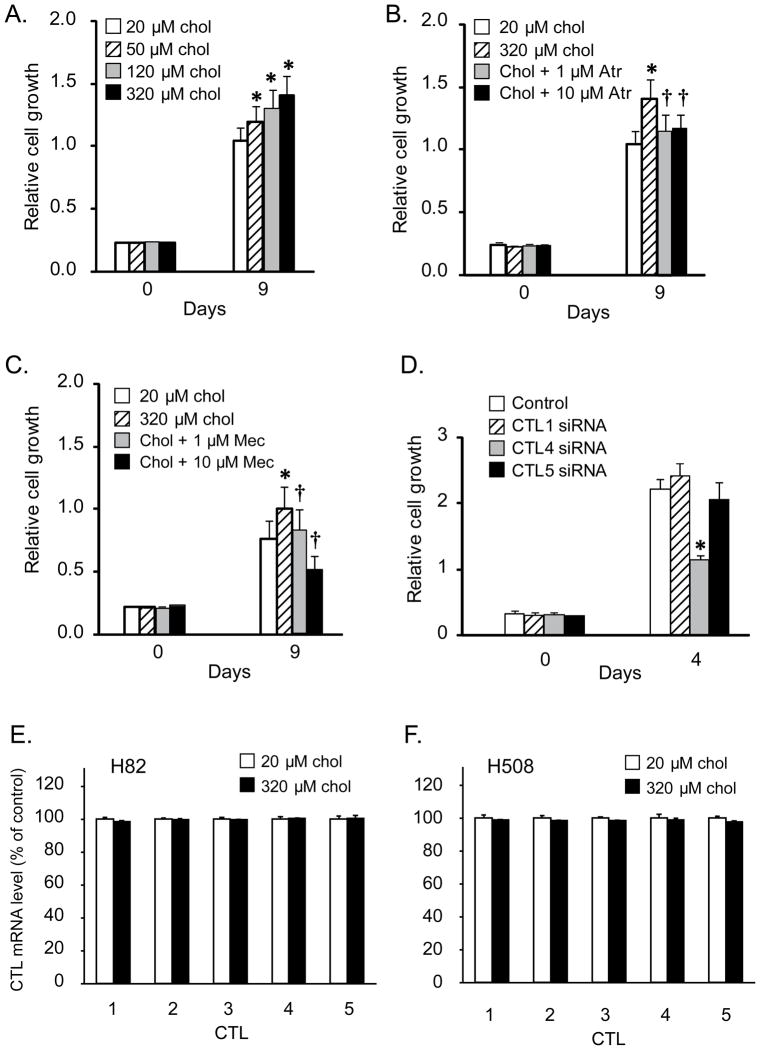
As an illustration of the potential importance of CTL4 as a way to target non-neuronal ACh synthesis, we investigated the ability of CTL4 knockdown as a way to modulate growth of lung cancer cells. First as shown in figure 1C, ACh secretion by H82 lung cancer cells is increased in a concentration-dependent manner by increased extracellular choline. In turn, as shown in Fig. 6A, increased choline significantly increased H82 cell proliferation in a concentration-dependent manner. This increase in proliferation appears to be mediated by secreted ACh as it was reduced by both the muscarinic antagonist atropine (Fig. 6B) and the nicotinic antagonist mecamylamine (Fig 6C). Consistent with the potential importance of CTL4 as a way to specifically target effects of non-neuronal ACh, CTL4 knockdown significantly decreased the growth of H82 cells (Fig. 6D). By contrast, knockdown of CTL1 and CTL5 did not significantly affect cell growth (Fig. 6D). Increased choline by itself did not affect CTL levels in either lung or colon carcinoma cells (Fig 6E,F).
Figure 6.
Modulation of SCLC cell growth by choline and cholinergic receptor antagonists. H82 cells were plated as described in the material and methods. Drugs were added immediately after plating cells and incubated for 9 days as described in methods. A. Choline caused a concentration-dependent increase of H82 cell proliferation at 9 days (* P < 0.05). B. The mAChR antagonist atropine at 1 and 10 μM inhibited the increase of H82 cell proliferation induced by increased choline (320 μM). C. The nAChR antagonist mecamylamine at 1 and 10 μM inhibited the increase of H82 cell proliferation induced by increased choline (320 μM). Data were mean ± SE of 12 replicates in two separate experiments. * p< 0.05 versus baseline choline. † p<0.05 versus 320 μM choline alone. chol, choline; Atr, atropine; Mec, mecamylamine. D. Effect of CTL knockdown on H82 cell growth (choline concentration = 20 μM). Consistent with the effects of CTL knockdowns on ACh secretion shown in panel C, knockdown of CTL4 significantly decreased H82 cell growth while knockdown of CTL5 had no effect and knockdown of CTL1 had a small stimulatory effect. Data were mean ± SE of 20 replicates in two separate experiments. *P<0.05 compared to the cells treated with control siRNA. E., F. Increased levels of choline did not affect levels of CTL expression in either H82 or H508 cells.
Discussion
Acetylcholine is synthesized from choline and acetyl-CoA by the action of ChAT. For ACh synthesis to proceed, choline must be transported into the cell by a choline transporter that is linked in some manner to ChAT. Choline is an organic cation and does not freely cross cell membranes. Its uptake from extracellular spaces requires specific choline transporters present on cell membranes. In neurons, the high affinity choline transporter CHT1 is absolutely required for ACh synthesis (Apparsundaram et al. 2000; Okuda et al. 2000; Ferguson et al. 2004), but as shown in table 1, H82 cells and the majority of SCLC cell lines examined do not express CHT1 yet synthesize ACh (Song et al. 2003); therefore, lung cancers must utilize a different choline transporter than CHT1 for ACh synthesis. This is not unique to lung cells as colon crypt cells also lack CHT1 but synthesize ACh (Yajima et al. 2011). Thus two quite different types of epithelial cells do not utilize CHT1 for choline transport for ACh synthesis and therefore rely on other choline transporters for this purpose. Multiple choline transporters have been identified in humans and other species (Kleinzeller et al. 1994; Pfeil et al. 2003), therefore the purpose of this study was to begin to identify the choline transporters that are linked to non-neuronal ACh synthesis.
As shown in figure 1, ACh secretion by H82 SCLC cells is directly related to media choline concentration (Fig. 1A, 1B and 1C) and there is a clear correlation between choline concentration and ACh secretion (Fig. 1D). The increased ACh secretion caused by increased choline levels was not caused by increased ChAT activity, nor was the increase caused by changes in cholinesterase levels since the changes were seen in the presence of neostigmine, a cholinesterase inhibitor. In this regard, H82 cells are similar to neurons where choline also increases ACh synthesis (Ulus et al. 1989) although obviously other significant differences such as the mechanisms underlying ACh secretion are present (Song and Spindel 2008). This is reflected by the relatively low ACh levels in H82 cell pellets in which ACh in cell pellets is only 1/10 as much as is in the media, suggesting a degree of constitutive secretion.
Recently, a new family of intermediate affinity choline transporters, the choline transporter-like proteins, (CTLs) has been shown to be widely expressed in mammalian tissues. Five CTL genes have been described (CTL1-5) and complex alternative spicing also occurs (O’Regan et al. 2000; Traiffort et al. 2005). In human and rat, CTL1-4 mRNA are mainly detected in peripheral tissues, while CTL1 is also widely expressed throughout the nervous system (Traiffort et al. 2005). The CTLs are Na-independent and inhibited by HC-3 at millimolar concentrations as opposed to the nanomolar inhibition of CHT1 by HC-3. This pharmacology of the CTLs is similar to what we observed in H82 cells in which choline transport was also primarily Na+-independent with a Km of 8.2 μM and was inhibited by μM concentrations of HC-3 (Fig. 2). Consistent with a role for the CTL’s in choline transport in lung cells, RT-PCR showed that CTL1-5 are expressed in essentially all lung cancer cell lines tested (Table 1). Quantitation by real-time PCR showed abundant levels of CTL1, 2 and 5 in all cell lines and tumors while levels of CTL3 and 4 were significantly lower. This distribution is similar to that reported by Tomi et al who found levels of CTL3 and CTL4 were 100-fold lower than levels of CTL1 in rat retinal capillary endothelial cells (Tomi et al. 2007).
The roles of CTL 1, 2 and 5 in transporting choline in H82 cells was confirmed by sRNA knockdown, in which knockdown of each of those transporter subtypes decreased choline transport by ~30% (Fig. 3B). This confirms that the multiple CTLs expressed by SCLC can indeed transport choline. Knockdown of CTL4 did not affect choline uptake, suggesting CTL4 does not significantly contribute to total choline uptake consistent with its lower levels of expression. Our findings are consistent with those of Nakamura al (Nakamura et al. 2010) who also saw a decrease in choline transport by knockdown of CTL1 and CTL2 in A549 lung adenocarcinoma cells and Machova et al who showed CTL1 knockdown decreased choline transport in neuroblastoma cells (Machova et al. 2009). Thus our and others’ findings clearly demonstrate a role for the CTL’s in choline transport.
To determine if one of the CTL’s was specifically linked to ACh synthesis in lung cells, CTL1-5 were individually knocked-down with siRNAs. As seen in Figure 3C, knockdown of CTL 1, 2, 3, 5 and CHT1 did not decrease ACh synthesis. Only the knockdown of CTL4 significantly reduced ACh synthesis and secretion. This suggests that in SCLC, of the 5 CTL’s, only CTL4 is specifically coupled to ACh synthesis; similar to CHT1 which in neurons is specifically coupled to ACh synthesis. Confirming the linkage of CTL4-mediated choline transport to ACh synthesis in lung cell lines, expression of CTL4 in H82 cells increased both choline transport and ACh synthesis (Fig. 4A and 4B). An unexpected finding was that ACh synthesis after knockdown of CTL1 and CTL2 actually increased. One possible explanation is that in the absence of CTL1 and CTL2, more choline is transported by CTL4 thus leading to increased ACh synthesis. Another possibility is that CTL1 and 2 play a role in ACh reuptake as has been proposed for the organic cation transporter N1 (OCTN1) (Pochini et al. 2011) and therefore the CTL1 and 2 knockdowns increased media ACh levels by inhibiting reuptake.
It is interesting to note that stimulation of ACh secretion and cell growth occurs at choline levels higher than the saturating levels for choline transport shown in figure 2. This suggests that that CTL4 may have lower affinity for choline than some of the other CTL’s or reflect the mechanism by which choline for ACh synthesis is segregated from the general pool of intracellular choline.
To rule out the possibility that lung cancer cells are unique among non-neuronal cell types in their use of CTL4 for ACh synthesis we examined a second epithelial cell type known to synthesize ACh, namely colonic epithelial cells as exemplified by colon cancer cells which have been clearly shown to synthesize ACh (Porter et al. 1996; Yajima et al. 2011). As shown in figure 5B, 7 of 7 colon cancer cell lines expressed CTL4. CHT1 was generally undetectable in these lines (data not shown). Colon cancer cells consistently expressed higher levels of CTL4 than did lung cancer cells (Fig. 5B). Confirming the role of CTL4 in ACh synthesis in colon cancer cells knockdown of CTL4 significantly decreased levels of ACh synthesis and secretion in H508 colon cancer cells which express relatively high levels of CTL-4 and CTL4 transduction increased levels of intracellular ACh in Caco-2 colon cancer cells which express relatively low levels of CTL4. Increased levels of secretion of ACh into the media in Caco-2 cells transduced with CTL4 was not seen which likely represents the relatively low levels of ChAT that are expressed by those cells and thereby likely limiting ACh synthesis. This finding confirms the role of CTL4 in two diverse types of epithelial cells in non-neuronal ACh synthesis and suggests that CTL4 is likely involved in ACh synthesis by cell types that do not depend on CHT1.
It is important to note that CTL-4 may not be the only choline transporter linked to non-neuronal ACh synthesis. For example the organic cation transporter 1 (OCT1) has also been suggested to play such a role as well as being involved in ACh secretory processes (Lips et al. 2007; Yajima et al. 2011). Other choline or cation transporters may also turn out to be linked to non-neuronal ACh synthesis role as well and there may be tissue specificity in the choline transporters used for ACh synthesis in different tissues. The finding of the linkage of CTL-4 to non-neuronal ACh synthesis suggests apparent compartmentalization of choline transport and ACh synthesis. In neurons, compartmentalization appears to derive from localization of CHT1 to synaptic vesicles, endosomes, or the cell membrane (Ribeiro et al. 2006; Cuddy et al. 2012). Whether similar localization of CTL4 in analogous compartments (eg, secretory vesicles, endosomes or cell membrane) plays a role in non-neuronal ACh synthesis remains to be determined. Regardless of the mechanism of linkage, it is clear that not just any choline transporter will support non-neuronal ACh synthesis as knockdowns of CTL1, 2, 5 and CHT1 did not affect ACh secretion in the cell lines tested.
Identification of the choline transporters linked to non-neuronal ACh synthesis has clear importance both for a fundamental understanding of the mechanisms of non-neuronal ACh synthesis but also to provide a way to target non-neuronal ACh synthesis without targeting neuronal ACh synthesis. An example is shown in figure 6 in which the growth of a lung cancer cell line can be inhibited by knocking down levels of CTL4 while knockdown of CTL1 or 5 had no effect on cell growth. This is consistent with the multiple reports that, choline uptake is increased in lung cancers compared to normal cells (Ackerstaff et al. 2003; Glunde et al. 2006) which may partially underlie the increased levels of ACh in lung cancer compared to normal lung (Song et al. 2008; Song and Spindel 2008). The data shown in figure 6 suggests that increased choline can stimulate cell proliferation through both nicotinic and muscarinic mechanisms, and it is likely that increased choline also stimulates cell growth secondary to increased phospholipid synthesis. These data also suggest that other diseases found to be characterized by increased non-neuronal ACh synthesis could also potentially be treated by CTL4 knockdown.
In summary our data shows that the choline transporter CTL4 appears to be specifically linked to non-neuronal acetylcholine synthesis and secretion as exemplified by lung and colon cancer cells. By contrast the choline transporters CHT1, CTL1, CTL2, and CTL5 do not appear necessary for non-neuronal ACh synthesis and/or secretion. These findings suggest some degree of functional linkage of CTL4 to ACh synthesis and secretion in non-neuronal cells and that therefore, CTL4 can be targeted as a way to change non-neuronal ACh secretion without affecting the choline transported in neurons by CHT1 that is needed for neuronal ACh synthesis.
Supplementary Material
Acknowledgments
We thank Tanaya Neff for assistance with lentivirus preparation, Stanley Shiigi for assistance with cell sorting, Jan Krzysztof Blusztajn for assistance with ChAT assays and Dennis Koop and Jenny Luo of the OHSU pharmacokinetics core for ACh assays.
Grant Support
NIH Gants OD011092, HL087710, CA151601 (ERS) and American Thoracic Society/Boehringer Ingelheim Research Grant ACR07-002 (PS).
Abbreviations used
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- CHT1
choline high affinity transporter
- CTL
choline-transporter like protein
- mAChR
muscarinic acetylcholine receptor
- nAChR
nicotinic acetylcholine receptor
- SCLC
small cell lung carcinoma
- HC-3
hemicholinium-3
- MAPK
mitogen-activated protein kinase
Footnotes
The authors have no conflict of interests to report.
References
- Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem. 2003;90:525–533. doi: 10.1002/jcb.10659. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun. 2000;276:862–867. doi: 10.1006/bbrc.2000.3561. [DOI] [PubMed] [Google Scholar]
- Campling BG, Haworth AC, Baker HM, Greer DL, Holden JJ, Bradley EC, Pym J, Dexter DF. Establishment and characterization of a panel of human lung cancer cell lines. Can. 1992;69:2064–2074. doi: 10.1002/1097-0142(19920415)69:8<2064::aid-cncr2820690811>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine Release by Human Colon Cancer Cells Mediates Autocrine Stimulation of Cell Proliferation. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy LK, Gordon AC, Black SA, Jaworski E, Ferguson SS, Rylett RJ. Peroxynitrite donor SIN-1 alters high-affinity choline transporter activity by modifying its intracellular trafficking. J Neurosci. 2012;32:5573–5584. doi: 10.1523/JNEUROSCI.5235-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Lomniczi A, Neff TL, Hobbs TR, Kohama SG, Kroenke CD, Galimi F, Ojeda SR. In vivo manipulation of gene expression in non-human primates using lentiviral vectors as delivery vehicles. Methods. 2009;49:70–77. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- En-Nosse M, Hartmann S, Trinkaus K, Alt V, Stigler B, Heiss C, Kilian O, Schnettler R, Lips KS. Expression of non-neuronal cholinergic system in osteoblast-like cells and its involvement in osteogenesis. Cell Tissue Res. 2009 doi: 10.1007/s00441-009-0871-1. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci U S A. 2004;101:8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla ZM. Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharm. 2006;3:496–506. doi: 10.1021/mp060067e. [DOI] [PubMed] [Google Scholar]
- Greinacher A, Wesche J, Hammer E, Furll B, Volker U, Reil A, Bux J. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–48. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- Hollenhorst MI, Lips KS, Wolff M, Wess J, Gerbig S, Takats Z, Kummer W, Fronius M. Luminal cholinergic signalling in airway lining fluid: a novel mechanism for activating chloride secretion via Ca(2+) dependent Cl(-) and K(+) channels. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inazu M, Takeda H, Matsumiya T. Molecular and functional characterization of an Na+-independent choline transporter in rat astrocytes. J Neurochem. 2005;94:1427–1437. doi: 10.1111/j.1471-4159.2005.03299.x. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K, Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A, Dodia C, Chander A, Fisher AB. Na(+)-dependent and Na(+)-independent systems of choline transport by plasma membrane vesicles of A549 cell line. Am J Physiol. 1994;267:C1279–C1287. doi: 10.1152/ajpcell.1994.267.5.C1279. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The Non-neuronal Cholinergic System of Human Skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007;51:1042–1053. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Machova E, O’Regan S, Newcombe J, Meunier FM, Prentice J, Dove R, Lisa V, Dolezal V. Detection of choline transporter-like 1 protein CTL1 in neuroblastoma x glioma cells and in the CNS, and its role in choline uptake. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06218.x. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Kunz L. A non-neuronal cholinergic system of the ovarian follicle. Ann Anat. 2005;187:521–528. doi: 10.1016/j.aanat.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Fujiwara R, Ishiguro N, Oyabu M, Nakanishi T, Shirasaka Y, Maeda T, Tamai I. Involvement of choline transporter-like proteins, CTL1 and CTL2, in glucocorticoid-induced acceleration of phosphatidylcholine synthesis via increased choline uptake. Biol Pharm Bull. 2010;33:691–696. doi: 10.1248/bpb.33.691. [DOI] [PubMed] [Google Scholar]
- Novotny A, Ryberg K, Heiman UJ, Nilsson L, Khorram-Manesh A, Nordgren S, Delbro DS, Nylund G. Is acetylcholine a signaling molecule for human colon cancer progression? Scand J Gastroenterol. 2011;46:446–455. doi: 10.3109/00365521.2010.539252. [DOI] [PubMed] [Google Scholar]
- O’Regan S, Traiffort E, Ruat M, Cha N, Compaore D, Meunier FM. An electric lobe suppressor for a yeast choline transport mutation belongs to a new family of transporter-like proteins. Proc Natl Acad Sci U S A. 2000;97:1835–1840. doi: 10.1073/pnas.030339697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Pfeil U, Lips KS, Eberling L, Grau V, Haberberger RV, Kummer W. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am J Respir Cell Mol Biol. 2003;28:473–477. doi: 10.1165/rcmb.2002-0190OC. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Yang L, Shulkes A, Vonlaufen A, Poljak A, Bustamante S, Warren A, Xu Z, Guilhaus M, Pirola R, Apte MV, Wilson JS. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochini L, Scalise M, Galluccio M, Pani G, Siminovitch KA, Indiveri C. The human OCTN1 (SLC22A4) reconstituted in liposomes catalyzes acetylcholine transport which is defective in the mutant L503F associated to the Crohn’s disease. Biochim Biophys Acta. 2011;1818:559–565. doi: 10.1016/j.bbamem.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Schemann M, Costa M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gas. 1996;111:401–408. doi: 10.1053/gast.1996.v111.pm8690205. [DOI] [PubMed] [Google Scholar]
- Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, Spindel ER. Acetylcholine Is an Autocrine or Paracrine Hormone Synthesized and Secreted by Airway Bronchial Epithelial Cells. Endocrinology. 2004;145:2498–2506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Black SA, Prado VF, Rylett RJ, Ferguson SS, Prado MA. The “ins” and “outs” of the high-affinity choline transporter CHT1. J Neurochem. 2006;97:1–12. doi: 10.1111/j.1471-4159.2006.03695.x. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, Saha S, Spindel ER. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–4700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, Mark GP, Grando SA, Spindel ER. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- Song P, Spindel ER. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J Pharmacol Sci. 2008;106:180–185. doi: 10.1254/jphs.fm0070091. [DOI] [PubMed] [Google Scholar]
- Tomi M, Arai K, Tachikawa M, Hosoya KI. Na(+)-Independent Choline Transportin Rat Retinal Capillary Endothelial Cells. Neurochem Res. 2007 doi: 10.1007/s11064-007-9367-0. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Ruat M, O’Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J Neurochem. 2005;92:1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Inazu M, Takeda H, Yamada T, Tajima H, Matsumiya T. Expression and functional characterization of choline transporter in human keratinocytes. J Pharmacol Sci. 2009;109:102–109. doi: 10.1254/jphs.08291fp. [DOI] [PubMed] [Google Scholar]
- Ulus IH, Wurtman RJ, Mauron C, Blusztajn JK. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989;484:217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Reinheimer T, Klapproth H, Schneider FJ, Racke K, Hammer R. Mammalian glial cells in culture synthesize acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:694–697. doi: 10.1007/pl00005107. [DOI] [PubMed] [Google Scholar]
- Yabuki M, Inazu M, Yamada T, Tajima H, Matsumiya T. Molecular and functional characterization of choline transporter in rat renal tubule epithelial NRK-52E cells. Arch Biochem Biophys. 2009;485:88–96. doi: 10.1016/j.abb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Yajima T, Inoue R, Matsumoto M, Yajima M. Non-neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol. 2011;589:953–962. doi: 10.1113/jphysiol.2010.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Inazu M, Tajima H, Matsumiya T. Functional expression of choline transporter-like protein 1 (CTL1) in human neuroblastoma cells and its link to acetylcholine synthesis. Neurochem Int. 2010 doi: 10.1016/j.neuint.2010.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.