Ventralight ST with SorbaFix fixation exhibited more favorable strength of tissue ingrowth and histologic response with similar mesh contracture and adhesion characteristics compared with Physiomesh fixed with Securestrap.
Keywords: Absorbable fixation, Tensile strength, Adhesions, Ventral hernia, Tissue ingrowth, Absorbable barrier mesh
Abstract
Background and Objectives:
The objective of this study was to compare mesh contracture, adhesion characteristics, tissue ingrowth, and histologic response of Ventralight ST/SorbaFix (C.R. Bard/Davol, Warwick, RI, USA) with Physiomesh/Securestrap (Ethicon, Somerville, NJ, USA) in a porcine model of laparoscopic ventral hernia repair.
Methods:
Standard laparoscopic technique was used to bilaterally implant meshes in 10 female Yorkshire swine. Each animal received either two Ventralight ST meshes (oval shaped, 10.2 × 15.2 cm) or two Physiomesh meshes (oval shaped 10 × 15 cm), one on either side of the midline. The meshes were fixated to the intact peritoneum with either SorbaFix (for animals receiving Ventralight ST) or Securestrap (for animals receiving Physiomesh). There were 5 animals in each group, yielding 10 of each mesh-fixation combination. Mesh contracture, adhesion characteristics, tissue ingrowth, and histologic response were evaluated after 14 days by image analysis, mechanical testing, and histologic staining (hematoxylin-eosin, Masson trichrome, picrosirius red, and von Willebrand factor).
Results:
Ventralight ST/SorbaFix and Physiomesh/Securestrap exhibited a similar percentage of mesh contracture, percentage of adhesion coverage, adhesion tenacity, collagen deposition, and levels of necrosis (P > .05 in all cases). However, Ventralight ST/SorbaFix exhibited significantly less inflammation (P = .0001), fibrosis (P = .0017), hemorrhage (P = .0001), and angiogenesis (P = .0032) and significantly greater strength of tissue ingrowth (P = .0003) than Physiomesh/Securestrap after the 14-day implantation period.
Conclusions:
Ventralight ST/SorbaFix exhibited more favorable strength of tissue ingrowth and histologic response and similar mesh contracture and adhesion characteristics compared with Physiomesh/Securestrap over a short-term 14-day implantation period in a preclinical porcine model.
INTRODUCTION
As laparoscopic ventral hernia repair (LVHR) has gained popularity, there has been a substantial increase in the number and variety of mesh materials and fixation devices marketed specifically for this application. Mesh materials have evolved to include permanent or absorbable barrier layers to minimize adhesion of the abdominal viscera to the mesh when placed intraperitoneally. These barrier layers are composed of a variety of materials ranging from expanded polytetrafluoroethylene (as a permanent barrier) to materials such as omega-3 fatty acids, oxidized regenerated cellulose, type I collagen, poliglecaprone-25, or hydrogels (as absorbable barriers).1,2
Absorbable barriers represent an attractive option for LVHR. The absorbable barrier layer is typically resorbed over a period of 30 to 240 days (1–8 months),3–6 minimizing adhesions during the critical early postoperative period7 and allowing adequate time for the formation of a neoperitoneum. Because the barrier is temporary, the amount of residual permanent foreign material at the repair site is minimized. Examples of meshes with absorbable barriers include C-QUR (Atrium Medical, Hudson, New Hampshire, USA), Parietex Composite (Covidien, Mansfield, Massachusetts, USA), Physiomesh (Ethicon, Somerville, New Jersey, USA), Proceed (Ethicon), Sepramesh IP Composite (C.R. Bard/Davol, Warwick, Rhode Island, USA), Ventralight ST (C.R. Bard/Davol), and Ventrio ST (C.R. Bard/Davol).
Numerous fixation devices are also available for LVHR applications, and again, there are permanent (ie, transfascial sutures and metallic tacks/clips) and absorbable (ie, polymer constructs and fibrin sealants) fixation options.8,9 The greatest advantage of an absorbable fixation device is that most of these devices are resorbed over the first postoperative year, minimizing the amount of foreign material at the repair site over the long-term. This may help to prevent adhesions and chronic pain, which can be associated with the fixation device rather than the mesh material used for the repair.10 However, absorbable fixation devices rely on strong tissue ingrowth through the mesh and/or supplemental transfascial sutures to provide long-term fixation of the mesh and prevent migration. Examples of absorbable fixation devices include AbsorbaTack (Covidien), Permasorb (C.R. Bard/Davol), Securestrap (Ethicon), and SorbaFix (C.R. Bard/Davol).
The composition, expected resorption times, and effectiveness of many of these meshes and fixation devices have been reviewed in the literature,1,2,5,8,9,11–15 and an overview is presented in Table 1. However, the available materials are constantly changing. For instance, meshes such as Physiomesh and Ventralight ST and fixation devices such as Securestrap and SorbaFix have recently become commercially available and do not appear in many of the studies comparing the other meshes/fixation devices. Consequently, this study was designed to explore the properties of these newest absorbable barrier mesh designs and absorbable fixation devices. The objective of this study was to compare mesh contracture, adhesion characteristics (area covered by adhesions and tenacity of adhesions), strength of tissue ingrowth, and host tissue response associated with Ventralight ST/SorbaFix compared with Physiomesh/Securestrap after a 14-day implantation period in a porcine model of LVHR.
Table 1.
Mesh Materials and Fixation Devices
| Product | Components | Resorption Time |
|---|---|---|
| Mesh material | ||
| C-QUR (Atrium Medical) | Polypropylene | Not biodegradable |
| Omega-3 fatty acid gel coating | 90–120 d | |
| Parietex Composite (Covidien) | Polyethylene terephthalate | Not biodegradable |
| Type I collagen layer | 30 d | |
| Physiomesh (Ethicon) | Polypropylene | Not biodegradable |
| Polydioxanone | 180 d | |
| Poliglecaprone-25 (Monocryl) | 240 d | |
| Proceed (Ethicon) | Polypropylene | Not biodegradable |
| Polydioxanone | 180 d | |
| Oxidized regenerated cellulose | 28 d | |
| Sepramesh IP Composite (C.R. Bard/Davol) | Polypropylene | Not biodegradable |
| Polyglycolic acid | 50–80 d | |
| Sodium hyaluronate | 30 d | |
| Carboxymethylcellulose | 30 d | |
| Polyethylene glycol | 30 d | |
| Ventralight ST (C.R. Bard/Davol) | Polypropylene | Not biodegradable |
| Polyglycolic acid | 50–80 d | |
| Sodium hyaluronate | 30 d | |
| Carboxymethylcellulose | 30 d | |
| Polyethylene glycol | 30 d | |
| Ventrio ST (C.R. Bard/Davol) | Polypropylene | Not biodegradable |
| Polyglycolic acid | 50–80 d | |
| Sodium hyaluronate | 30 d | |
| Carboxymethylcellulose | 30 d | |
| Polyethylene glycol | 30 d | |
| Polydioxanone | 170–220 d | |
| Fixation device | ||
| AbsorbaTack (Covidien) | Co-polymer poly-(glycolide-co-L-lactide) | 365 d |
| Permasorb (C.R. Bard/Davol) | Poly-d,l-lactide | 480 d |
| Securestrap (Ethicon) | Polydioxanone and a co-polymer of lactide/glycolide | 365 d |
| SorbaFix (C.R. Bard/Davol) | Poly-d,l-lactide | 365 d |
MATERIALS AND METHODS
Materials
Two mesh–fixation device combinations were evaluated in this porcine model of LVHR. The first combination consisted of Ventralight ST fixated with SorbaFix. The second combination consisted of Physiomesh fixated with Securestrap.
Ventralight ST is composed of a permanent polypropylene (PP) fiber co-knitted with an absorbable polyglycolic acid (PGA) fiber, producing a mesh with a PP side and a PGA side.2,16 The PGA side of the mesh is subsequently coated with an absorbable hydrogel layer composed of sodium hyaluronate (HA), carboxymethylcellulose (CMC), and polyethylene glycol (PEG). The purpose of this absorbable HA/CMC/PEG layer is to minimize adhesion formation between the mesh and the viscera when the mesh is placed inside the abdomen during LVHR.5 This HA/CMC/PEG layer is expected to be fully resorbed within approximately 1 month in vivo. For the purposes of this study, Ventralight ST was used in conjunction with SorbaFix.
SorbaFix is composed of poly-d,l-lactide and is expected to be fully resorbed within approximately 12 months in vivo.17 SorbaFix devices are approximately 3.4 mm in diameter and 6.7 mm in length with a tissue penetration depth of approximately 5 mm.17
Physiomesh is composed of a permanent PP mesh layer encapsulated with polydioxanone (PDO) on both sides to facilitate the bonding of two poliglecaprone-25 (Monocryl; Ethicon) absorbable barrier layers, one to each side of the PP mesh.2,6 The purpose of these poliglecaprone-25 layers is to minimize adhesion formation between the mesh and the viscera when the mesh is placed inside the abdomen during LVHR. These poliglecaprone-25 layers are expected to be fully resorbed within approximately 8 months in vivo. A purple stripe (D&C Violet No. 2) of PDO material is also applied to one side of the mesh on top of the poliglecaprone-25 layer for orientation purposes during laparoscopic implantation of the Physiomesh material. For the purposes of this study, Physiomesh was used in conjunction with Securestrap.
Securestrap is composed of a combination of PDO and a copolymer of lactide/glycolide and is expected to be fully resorbed within approximately 12 months in vivo.18 Securestrap devices are shaped like staples and span across mesh fibers with two prongs (6.7 mm in length) that provide two sites of fixation per device.18
Methods
Study compliance.
The Institutional Animal Care and Use Committee of the DaVinci Biomedical Research Products facility (South Lancaster, Massachusetts, USA), where the study was conducted, approved the experimental protocol before the start of the study, and standard operating procedures were followed at all times.
Animal care and operative technique.
Ten female Yorkshire swine weighing approximately 50 to 55 kg were acquired for the study and acclimated to the facility for 6 to 7 days. Animals were fasted for 8 hours before surgery and were sedated with 20 mg/kg ketamine, 2 mg/kg xylazine, and 0.04 mg/kg atropine administered by intramuscular injection. After sedation, the animals were intubated and maintained under anesthesia with isoflurane (0.5%–4% to effect throughout the procedure). Animals were positioned in dorsal recumbency, and the operative area was shaved, cleaned with 3 alternating scrubs of povidone-iodine and 70% isopropyl alcohol solutions, and draped in the standard fashion for aseptic surgery. Buprenorphine hydrochloride (0.01 mg/kg, intramuscularly), Ceftiofur (5 mg/kg, intramuscularly), and Carprofen (2 mg/kg, subcutaneously) were administered preoperatively for antibiotic prophylaxsis and pain relief, respectively.
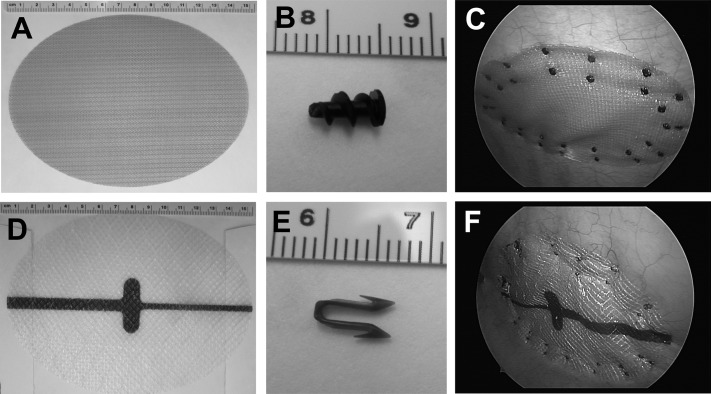
Standard laparoscopic technique was used to bilaterally implant the meshes. Each animal received either two Ventralight ST meshes (oval shaped, 10.2 × 15.2 cm) (Figure 1A) or two Physiomesh meshes (oval shaped, 10 × 15 cm) (Figure 1D), one on either side of the midline. The meshes were fixated to the intact peritoneum with either SorbaFix (for animals receiving Ventralight ST) (Figure 1B) or Securestrap (for animals receiving Physiomesh) (Figure 1E). There were 5 animals in each group, yielding 10 of each mesh-fixation combination. Each mesh was fixated with 32 of the appropriate fixation constructs (16 forming an inner ring and 16 forming an outer ring), as shown in Figures 1C and 1F.
Figure 1.
A, Ventralight ST before implantation. B, SorbaFix before implantation. C, Ventralight ST after laparoscopic implantation and placement of SorbaFix fixation devices. D, Physiomesh before implantation. E, Securestrap before implantation. F, Physiomesh after laparoscopic implantation and placement of Securestrap fixation devices.
Once the animals recovered from anesthesia, they were returned to their pens with free access to food and water. Buprenorphine was administered (0.01 mg/kg, intramuscularly) twice a day for the first postoperative day for pain relief and then discontinued because none of the animals exhibited signs of pain or discomfort beyond this point. Animals were observed daily during the 14-day implantation period for abnormalities of appearance or behavior that might indicate adverse health effects. The abdominal region of each animal was examined to assess the condition of the wound and the subcutaneous tissues for evidence of seromas and/or hematomas.
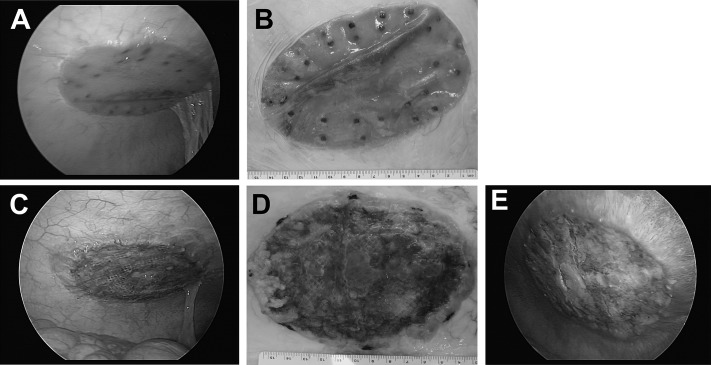
After the 14-day implantation period, each animal was sedated and anesthetized as previously described. Laparoscopy was performed to visually assess each mesh, and photographs were taken to document findings (Figures 2A and 2C). Humane euthanasia was achieved by administering an overdose of pentobarbital sodium (60–150 mg/kg, intravenously to effect) according to the American Veterinary Medical Association guidelines.19 After euthanasia, the skin and adipose tissue were removed from the abdominal wall and the entire abdomen was dissected to expose the meshes. The entire abdominal wall was then explanted en bloc, photographed (Figures 2B, 2D, and 2E), and visually inspected to assess the integrity of the repair site, peritoneal tissue attachments (adhesions), and mesh contracture. Each mesh was placed in saline solution (0.9% sodium chloride) and refrigerated overnight before mechanical testing the following day.
Figure 2.
Ventralight ST/SorbaFix after 14-day implantation period: laparoscopic view (A) and macroscopic view (B). Physiomesh/Securestrap after 14-day implantation period: laparoscopic view (C), macroscopic view (D), and inhibition of tissue integration at PDO marker and “reactive peritoneum” on and surrounding mesh (E).
Mesh Contracture and Adhesion Area
Each mesh was photographed with a ruler included for scale. Mesh dimensions and percent area covered by adhesions were assessed with ImageJ software (http://rsbweb.nih.gov/ij/) as described previously.12
Adhesion Tenacity
Adhesion tenacity was evaluated with the following scoring as described previously12: 0, no adhesions observed; 1, loose adhesion requiring blunt dissection only; 2, firm adhesion requiring sharp dissection without extensive vascularity; 3, firm adhesion requiring sharp dissection with extensive vascularity; 4, firm adhesion requiring sharp dissection with extensive fibrotic ingrowth and extensive vascularity; and 5, grade 4 with firm attachment to visceral organs (bowel, liver, spleen).
Mechanical Testing
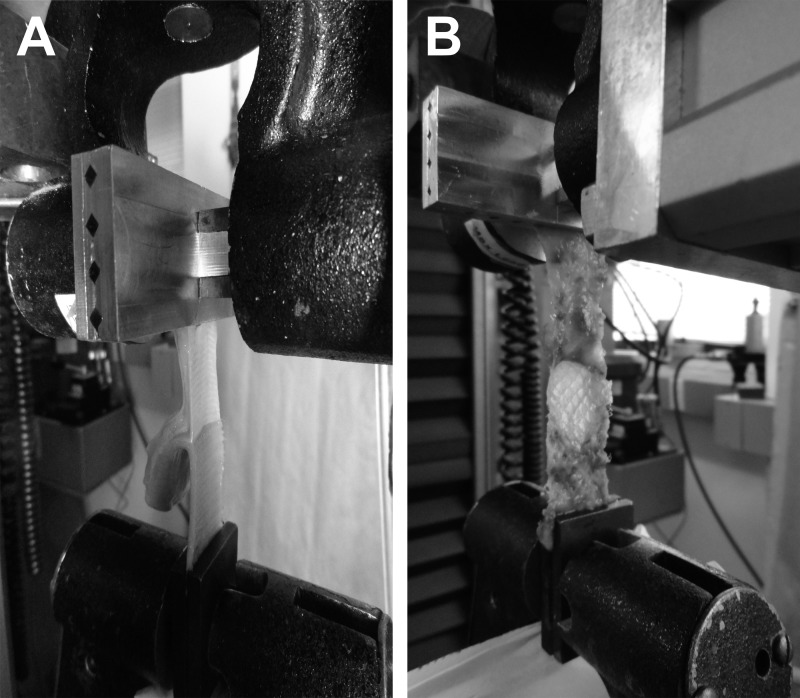
Mechanical testing (T-peel test) was conducted as follows. Specimens were brought to room temperature and prepared into uniform specimens measuring 2 × 6 cm (avoiding areas containing fixation devices) by use of an arbor press and a rectangular die. Three mesh specimens were prepared for each Physiomesh/Securestrap to evaluate the strength of tissue ingrowth through an area containing the PDO marker (n = 1), as well as the strength of tissue ingrowth through areas without the PDO marker (n = 2). Only two mesh specimens were prepared for each Ventralight ST/SorbaFix because this mesh is uniform across the entire surface and does not contain a marker region. The mesh was separated from the abdominal wall tissue at one end of each rectangular specimen to create a separated area measuring 1.5 × 2 cm. The mesh layer was placed in one of the pneumatic grips of the Instron servohydraulic test frame (Instron, Norwood, Massachusetts, USA) and secured with a pressure of 45 psi, and the abdominal wall tissue was similarly secured in the other pneumatic grip (Figures 3A and 3B). The mesh was then peeled from the underlying abdominal wall tissue at a rate of 25 mm/min until the mesh was disrupted from the abdominal wall tissue. The force-versus-displacement data were used to calculate the T-peel force by averaging the consistent separation data. The T-peel force was then normalized to the width of the specimen by dividing by 20 mm, yielding T-peel strength in units of newtons per centimeter.
Figure 3.
A, Ventralight ST/SorbaFix specimen during T-peel testing (abdominal wall tissue in upper grip and mesh in lower grip of machine). B, Physiomesh/Securestrap specimen during T-peel testing (the central region shows the PDO marker with minimal tissue ingrowth visible).
Histology
One specimen (2 × 2 cm) was also prepared from each mesh for histologic assessment. Each specimen was stored in 10% neutral buffered formalin, embedded, sectioned, and stained with hematoxylin-eosin, Masson trichrome, picrosirius red, and von Willebrand factor. All slides were evaluated by a board-certified veterinary pathologist, and representative photographs were taken of each slide. Slides were scored for inflammatory cell infiltrates (mast cells, neutrophils, eosinophils, lymphocytes, plasma cells, macrophages, and giant cells), collagen deposition (type I and type III collagen), fibroplasia/fibrosis (granulation tissue), hemorrhage, necrosis, and angiogenesis by use of the following scoring as described previously12: 0, no response; 1, minimal/barely detectable; 2, mild/slightly detectable; 3, moderate/easily detectable; and 4, marked/very evident.
Statistical Analysis
Systat software (version 12.0; Systat Software, Chicago, IL, USA) was used to perform all statistical analyses. For continuous data in which two groups of data were compared (ie, percent mesh contracture and percent area covered by adhesions), an unpaired, 2-tailed t test was performed. For continuous data in which 3 groups of data were compared (ie, T-peel strength), a 1-way analysis of variance was performed followed by a Fisher Least Significant Difference post-test as appropriate. For scores such as adhesion tenacity and histologic parameters in which two groups of data were compared, a nonparametric test (Mann-Whitney) was performed. Statistical significance was set at the P < .05 level. All data are reported as mean ± standard error of the mean.
RESULTS
Macroscopic Appearance at Explantation
Localized red discoloration or redness was observed for many of the Ventralight ST/SorbaFix specimens (Figures 2A and 2B) in the regions where the mesh was slightly wrinkled/folded with no appreciable or pathologically meaningful microscopic correlate. However, frequent and widespread to diffuse red discoloration was observed for all of the Physiomesh/Securestrap specimens (Figures 2C, 2D, and 2E). These macroscopic observations correlated microscopically with varying degrees of hemorrhage that was associated with active and ongoing granulation tissue ingrowth, incomplete integration of the mesh, and incomplete tissue coverage. In addition, omental adhesions were observed for 3 of 10 Ventralight ST/SorbaFix specimens (30%) and 5 of 10 Physiomesh/Securestrap specimens (50%) at the time of explantation. Furthermore, serous fluid accumulation was observed between the mesh and abdominal wall (particularly under the PDO marker region) in 5 of 10 Physiomesh/Securestrap meshes (50%) and in 1 of 10 Ventralight ST/SorbaFix meshes (10%). Inflammation of the spiral colon was observed in 1 of 10 Physiomesh/Securestrap specimens (10%).
Mesh Contracture
Image analysis showed that Ventralight ST/SorbaFix contracted 16.9% ± 2.7% compared with 17.0% ± 2.6% for Physiomesh/Securestrap (Table 2). Statistically, the difference was not significant (P = .9746).
Table 2.
Summary of Results
| Ventralight ST/SorbaFix (Mean ± SEM) | Physiomesh/Securestrap (Mean ± SEM) |
||
|---|---|---|---|
| Mesh | PDO Marker | ||
| Mesh contracture (%) | 16.9 ± 2.7 | 17.0 ± 2.6 | not applicable |
| Adhesion area (%) | 1.2 ± 0.7 | 6.0 ± 3.5 | not applicable |
| Adhesion tenacity | 0.4 ± 0.2 | 1.1 ± 0.4 | not applicable |
| T-peel strength (N/cm) | 1.4 ± 0.1 | 0.9 ± 0.1a | 0.2 ± 0.0b |
| Inflammation | 2.1 ± 0.1 | 3.3 ± 0.2c | not applicable |
| Type I collagen | 3.3 ± 0.2 | 3.5 ± 0.2 | not applicable |
| Type III collagen | 0.7 ± 0.2 | 0.5 ± 0.2 | not applicable |
| Fibrosis | 0.0 ± 0.0 | 1.2 ± 0.3d | not applicable |
| Hemorrhage | 0.0 ± 0.0 | 2.0 ± 0.3c | not applicable |
| Necrosis | 0.0 ± 0.0 | 0.0 ± 0.0 | not applicable |
| Angiogenesis | 2.8 ± 0.1 | 3.6 ± 0.2e | not applicable |
P = .0003 compared with Ventralight ST/SorbaFix.
P < .0001 compared with Ventralight ST/SorbaFix.
P = .0001 compared with Ventralight ST/SorbaFix.
P = .0017 compared with Ventralight ST/SorbaFix.
P = .0032 compared with Ventralight ST/SorbaFix.
Adhesion Area
Adhesions covered 1.2% ± 0.7% of Ventralight ST/SorbaFix compared with 6.0% ± 3.5% of Physiomesh/Securestrap (Table 2), indicating a very small area of adhesion on each mesh. Most adhesions were observed near the periphery of the mesh or at one of the fixation points. Statistically, the difference was not significant (P = .1896).
Adhesion Tenacity
The tenacity of adhesions covering Ventralight ST/SorbaFix scored 0.4 ± 0.2 compared with 1.1 ± 0.4 for Physiomesh/Securestrap (Table 2). Statistically, the difference was not significant (P = .2140). Adhesion tenacity in this range of 0.4 to 1.1 (on a scale of 0–5) indicates that the observed adhesions were loose and filmy, requiring only blunt dissection to free them from the surface of the mesh. This compares well with the low percentage of mesh covered by adhesions, as well as the narrative descriptions at the time of explantation, which indicated that adhesions between the omentum and the mesh were observed for 30% of Ventralight ST/SorbaFix specimens and 50% of Physiomesh/Securestrap specimens.
Mechanical Testing
At the time of explantation, a visual difference was observed between the poliglecaprone-25 regions of the Physiomesh/Securestrap and those regions containing the purple PDO orientation marker. It appeared that very little tissue ingrowth occurred in the region of the PDO marker compared with the rest of the mesh (as shown in Figures 2D, 2E, and 3B). Thus, two sets of specimens were prepared for mechanical testing of the Physiomesh/Securestrap group. One set of specimens was prepared in such a way as to avoid the PDO region completely, whereas another set was prepared that contained only this PDO region. In this way the strength of tissue ingrowth associated with each region of the mesh could be evaluated mechanically by the T-peel test. Because Ventralight ST/SorbaFix is homogeneous in composition and does not contain a PDO marker region, only one set of specimens was prepared for this material. The results of the T-peel test showed significantly reduced tissue ingrowth strength in the PDO region of Physiomesh/Securestrap (0.2 ± 0.0 N/cm, P = .0001) (Table 2) compared with the mesh region of Physiomesh/Securestrap (0.9 ± 0.1 N/cm). In addition, tissue ingrowth into Ventralight ST/SorbaFix (1.4 ± 0.1 N/cm) was significantly greater than the Physiomesh/Securestrap mesh region (P = .0003) and PDO marker region (P < .0001). This is not surprising given that Physiomesh/Securestrap contains an adhesion barrier layer on both sides of the mesh material. These poliglecaprone-25 (ie, Monocryl) absorbable barrier layers are intended to minimize adhesions of the abdominal viscera to the underlying mesh. However, the results of this study suggest that the presence of this barrier layer on both sides of the mesh may inhibit tissue ingrowth on the abdominal wall side of the mesh as shown by the low-–tissue ingrowth strength achieved with Physiomesh/Securestrap compared with Ventralight ST/SorbaFix.
Histology
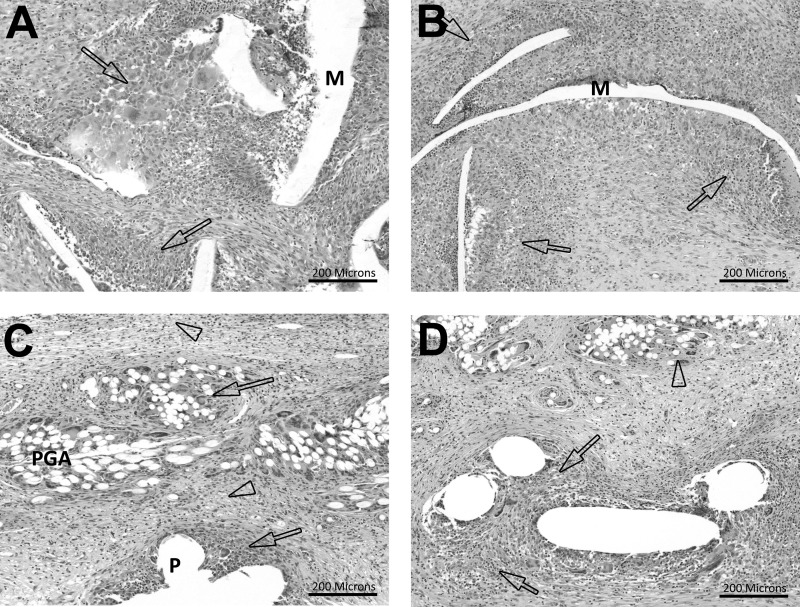
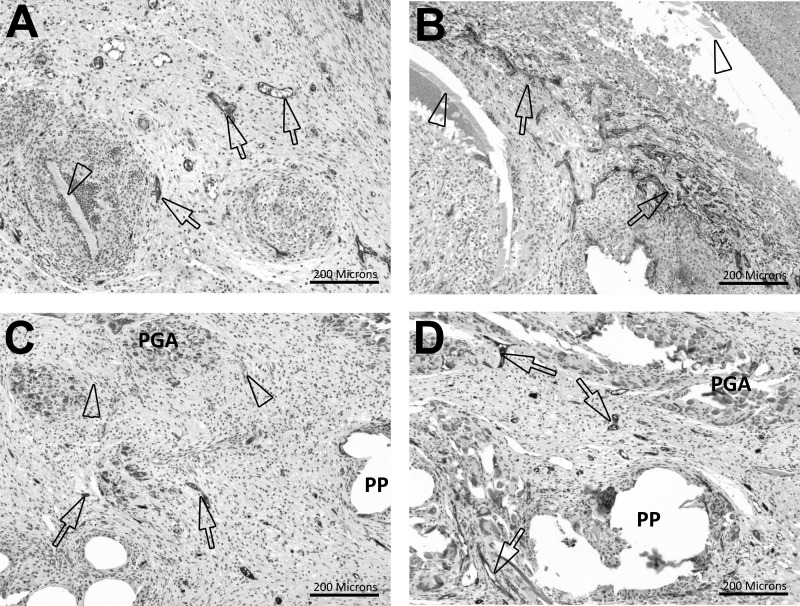
Ventralight ST/SorbaFix produced an inflammatory response with a score of 2.1 ± 0.1 compared with 3.3 ± 0.2 for Physiomesh/Securestrap (Figure 4, Table 2). Inflammation scores in this range indicated mild/slight inflammation in Ventralight ST/SorbaFix specimens and moderate inflammation in Physiomesh/Securestrap specimens. Statistically, Physiomesh/Securestrap produced a significantly greater inflammatory response than Ventralight ST/SorbaFix (P = .0001). This inflammatory response was primarily associated with the Monocryl layer of the Physiomesh materials and, to a lesser degree, with the PP fibers (Figures 4A and 4B). In Ventralight ST/SorbaFix specimens, inflammation was associated with the PP fibers but was also observed around and between the PGA fiber bundles (Figures 4C and 4D).
Figure 4.
Hematoxylin-eosin stain, original magnification ×10. A, Inflammation score 3 in Physiomesh/Securestrap specimen. The arrows indicate moderate inflammation. B, Inflammation score 4 in Physiomesh/Securestrap specimen. The arrows indicate marked inflammation. C, Inflammation score 2 in Ventralight ST/SorbaFix specimen. The arrows indicate a mild foreign body response, and the arrowheads indicate vacuolated macrophages. D, Inflammation score 3 in Ventralight ST/SorbaFix specimen. The arrows indicate a moderate foreign body response, and the arrowhead indicates a minimal foreign body response. M = Monocryl, P = polypropylene.
Both groups exhibited similar amounts of type I and type III collagen deposition, with scores of 3.3 ± 0.2 and 0.7 ± 0.2, respectively, for Ventralight ST/SorbaFix and scores of 3.5 ± 0.2 and 0.5 ± 0.2, respectively, for Physiomesh/Securestrap (Table 2). Type I collagen scores in the range of 3.3 to 3.5 (on a scale of 0–4) indicated moderate to marked amounts of type I collagen that were easily detectable in the stained sections. Type III collagen scores in the range of 0.5 to 0.7 (on a scale of 0–4) indicated minimal/barely detectable amounts of type III collagen in the stained sections. Statistically, there were no significant differences between the two mesh-fixation combinations for type I collagen (P = .3736) or type III collagen (P = .3736).
Ventralight ST/SorbaFix produced fibrosis at the surface of the mesh with a score of 0.0 ± 0.0 compared with 1.2 ± 0.3 for Physiomesh/Securestrap (Table 2). Fibrosis scores in this range indicated the absence of fibrosis in Ventralight ST/SorbaFix and minimal/barely detectable fibrosis in Physiomesh/Securestrap. Statistically, Physiomesh/Securestrap produced a significantly greater fibrotic response compared with Ventralight ST/SorbaFix (P = .0017). Fibroplasia was associated with active or ongoing tissue ingrowth and granulation tissue formation in Physiomesh/Securestrap. Active tissue ingrowth was already complete for Ventralight ST/SorbaFix, and more advanced features of mature tissue integration were observed.
Similarly, Ventralight ST/SorbaFix exhibited no hemorrhage, with a score of 0.0 ± 0.0, compared with mild/slightly detectable hemorrhage, with a score of 2.0 ± 0.3, for Physiomesh/Securestrap (Table 2). Statistically, Physiomesh/Securestrap produced significantly greater hemorrhage than Ventralight ST/SorbaFix (P = .0001). The microscopic observations of hemorrhage for Physiomesh/Securestrap corresponded with the macroscopic observations of widespread to diffuse red discoloration at the time of explantation and were associated with active and ongoing granulation tissue ingrowth, incomplete integration of the mesh, and incomplete tissue coverage of Physiomesh/Securestrap.
No evidence of necrosis was observed for either Ventralight ST/SorbaFix (0.0 ± 0.0) or Physiomesh/Securestrap (0.0 ± 0.0) (Table 2). Statistically, there was no significant difference in necrosis between the two mesh-fixation systems (P > .99).
Ventralight ST/SorbaFix exhibited angiogenesis with a score of 2.8 ± 0.1 compared with 3.6 ± 0.2 for Physiomesh/Securestrap (Figure 5, Table 2). Angiogenesis scores in this range indicated moderate angiogenesis in Ventralight ST/SorbaFix specimens and moderate to marked angiogenesis in Physiomesh/Securestrap specimens. Specimens in both mesh groups displayed regularly distributed vascular channels that supported fibrous connective tissue ingrowth. Statistically, Physiomesh/Securestrap produced significantly greater angiogenesis than Ventralight ST/SorbaFix (P = .0032). Physiomesh/Securestrap exhibited very dense arborizations of newly formed vessels at the leading edge of the granulation tissue as it progressed into the mesh (Figures 5A and 5B), whereas Ventralight ST/SorbaFix showed an even distribution of regularly spaced vascular channels throughout the mesh (Figures 5C and 5D).
Figure 5.
von Willebrand factor stain, original magnification ×10. A, Angiogenesis score 3 in Physiomesh/Securestrap specimen. The arrows indicate moderate density. B, Angiogenesis score 4 in Physiomesh/Securestrap specimen. The arrows indicate very high density. C, Angiogenesis score 2 in Ventralight ST/SorbaFix specimen. Arrows indicate mild angiogenesis. D, Angiogenesis score 3 in Ventralight ST/SorbaFix specimen. The arrows indicate moderate density. M = Monocryl.
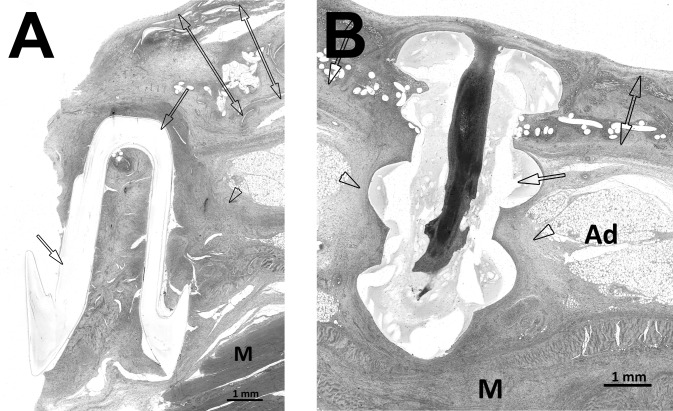
Finally, the SorbaFix and Securestrap fixation devices exhibited a mild foreign body response associated specifically with structural features of the fixation devices, with inflammation scores of 1.8 ± 0.2 and 1.8 ± 0.2, respectively; fibrosis scores of 2.0 ± 0.3 and 1.8 ± 0.2, respectively; and no evidence of erosion or degradation of the fixation devices (ie, scores of 0.0 ± 0.0 for both) after the 14-day implantation period (Figures 6A and 6B).
Figure 6.
Hematoxylin-eosin stain, original magnification ×10. A, Physiomesh/Securestrap. The single-headed arrows indicate Securestrap, the arrowhead indicates chronic inflammatory response and fibrosis along Securestrap, and the double-headed arrows indicate Physiomesh. B, Ventralight ST/SorbaFix. The single-headed arrow indicates SorbaFix fixation device, the arrowheads indicate chronic inflammatory response and fibrosis along the SorbaFix fixation device, and the double-headed arrows indicate Ventralight ST. Ad = adipose tissue, M = abdominal muscle.
DISCUSSION
The objective of this study was to compare mesh contracture, adhesion characteristics (area covered by adhesions and tenacity of adhesions), strength of tissue ingrowth, and host tissue response associated with Ventralight ST/SorbaFix compared with Physiomesh/Securestrap after implantation for 14 days in a porcine model of LVHR.
The results of this study are comparable with those of another study previously reported by our group, in which Proceed, Sepramesh IP Composite, Ventrio, and Ventrio ST materials were evaluated for mesh contracture, adhesion characteristics, tissue ingrowth, and histologic response after implantation for 28 days in a porcine model of LVHR.12 Aside from the implantation time and the fact that SorbaFix was used for all devices, all other methods were identical to those in the current study. All meshes except Ventrio have absorbable barriers, providing relevant comparisons with the absorbable barrier meshes evaluated in this study. For instance, the percent mesh contracture associated with Ventralight ST/SorbaFix (17%) and Physiomesh/Securestrap (17%) in this study are comparable with that of Ventrio (15%), slightly greater than that of Sepramesh IP Composite (9%) and Ventrio ST (9%), and much lower than that of Proceed (27%) reported in our previous study.12 In addition, the percentage of mesh covered by adhesions (1.2%) and adhesion tenacity (0.4) associated with Ventralight ST/SorbaFix in this study are comparable with those of Ventrio (0.3% and 0.4, respectively) and Sepramesh IP Composite (0.1% and 0.4, respectively) in the previous study. However, the percentage of mesh covered by adhesions (6.0%) and adhesion tenacity (1.1) associated with Physiomesh/Securestrap in this study more closely approximate those of Proceed (5% and 1.5, respectively) and Ventrio ST (4% and 1.3, respectively) in the previous study. Finally, the tissue ingrowth strength associated with Ventralight ST/SorbaFix (1.4 N/cm) in this study is comparable with that of Ventrio (1.4 N/cm), Proceed (1.3 N/cm), and Ventrio ST (1.3 N/cm) in the previous study, whereas the tissue ingrowth strength associated with Physiomesh/Securestrap in this study (0.9 N/cm) is more comparable with that of Sepramesh IP Composite (1.0 N/cm) in the previous study.
It should be noted that the difference in implantation time between this study (14 days) and our previous study (28 days) may impact these comparisons.12 A neoperitoneum is expected to form within the first postoperative week, so a 14-day implantation period provides adequate time for evaluation of adhesion formation during/after this critical period yet before full resorption of the barrier layer.7 A 14-day implantation period was also chosen for evaluation of the initial phase of active barrier degradation and resorption. Because most barrier layers require a resorption time of at least 30 days, it is possible that the inflammatory response to these materials is still fairly active throughout the entire first postoperative month. At least one preclinical model (intraperitoneal placement of mesh in Wistar rats) has shown an increase in the formation of adhesions between 7 and 30 days, perhaps because of increased inflammation caused by the increased activity of macrophages and fibroblasts as the barrier layer is actively degraded and resorbed.20 Thus future studies should investigate implantation times on the order of 14, 30, and 90 days to better describe the progression of adhesions, tissue ingrowth, and inflammation associated with these materials over time. In addition, it is likely that the chemical composition of the barrier layer also plays a role in the associated inflammatory response and that this response may vary with longer implantation times. Thus it is extremely important to evaluate multiple time points in future studies to capture these differences. Trends observed at a single, isolated time point such as 14 days may not be representative of the overall trend that may occur over the course of 30 and 90 days during and after the active resorption of the barrier layer. In addition, future studies should also incorporate other commonly used absorbable barrier meshes for a more complete comparison of all available materials. Of the 7 currently available absorbable barrier hernia repair materials, only C-QUR and Parietex Composite were not represented in this study or our previous study.12 However, these materials have been represented in several other preclinical5,13,14 and clinical11 studies that have measured parameters such as mesh contracture, adhesion characteristics, tissue ingrowth, and histologic response in a similar manner. It is difficult to make direct comparisons among these studies and our results because these studies often involved different hosts (ie, rabbits or humans) or slightly different surgical techniques (ie, abrasion of the bowel to induce adhesion formation).
In summary, Ventralight ST/SorbaFix and Physiomesh/Securestrap exhibited similar mesh contracture, percent adhesion coverage, adhesion tenacity, collagen deposition, and levels of necrosis (P > .05 in all cases) after a 14-day implantation period in a porcine model of LVHR. However, Ventralight ST/SorbaFix exhibited significantly less inflammation (P = .0001), fibrosis (P = .0017), hemorrhage (P = .0001), and angiogenesis (P = .0032) and significantly greater strength of tissue ingrowth (P = .0003) than Physiomesh/Securestrap after the 14-day implantation period in this preclinical porcine study.
CONCLUSIONS
Ventralight ST/SorbaFix exhibited similar mesh contracture and adhesion characteristics and more favorable strength of tissue ingrowth and histologic response compared with Physiomesh/Securestrap over the short-term 14-day implantation period in this preclinical porcine study.
Contributor Information
Corey R. Deeken, Section of Minimally Invasive Surgery, Department of Surgery, Washington University School of Medicine, St Louis, MO, USA..
Brent D. Matthews, Section of Minimally Invasive Surgery, Department of Surgery, Washington University School of Medicine, St Louis, MO, USA..
References:
- 1. Deeken CR, Abdo MS, Frisella MM, Matthews BD. Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair. Surg Endosc. 2011;25:1541–1552 [DOI] [PubMed] [Google Scholar]
- 2. Deeken CR, Faucher KM, Matthews BD. A review of the composition, characteristics, and effectiveness of barrier mesh prostheses utilized for laparoscopic ventral hernia repair. Surg Endosc. 2012;26:566–575 [DOI] [PubMed] [Google Scholar]
- 3. Proceed Surgical Mesh Instructions for Use [package insert]. Somerville, NJ: Ethicon Inc; 2007 [Google Scholar]
- 4. Sepramesh IP. Composite Instructions for Use [package insert]. Warwick, RI: Bard, Inc; 2005 [Google Scholar]
- 5. Pierce RA, Perrone J, Nimeri A, et al. 120-Day comparative analysis of adhesion grade and quantity, mesh contraction, and tissue response to a novel omega-3 fatty acid bioabsorbable barrier macroporous mesh after intraperitoneal placement. Surg Innov. 2009;16:46–54 [DOI] [PubMed] [Google Scholar]
- 6. Physiomesh Instructions for Use [package insert]. Somerville, NJ: Ethicon, Inc; 2011 [Google Scholar]
- 7. Tingstedt B, Isaksson K, Andersson E, Andersson R. Prevention of abdominal adhesions-–-present state and what's beyond the horizon? Eur Surg Res. 2007;39:259–268 [DOI] [PubMed] [Google Scholar]
- 8. Melman L, Jenkins ED, Deeken CR, et al. Evaluation of acute fixation strength for mechanical tacking devices and fibrin sealant versus polypropylene suture for laparoscopic ventral hernia repair. Surg Innov. 2010;17:285–290 [DOI] [PubMed] [Google Scholar]
- 9. Lerdsirisopon S, Frisella MM, Matthews BD, Deeken CR. Biomechanical evaluation of potential damage to hernia repair materials due to fixation with helical titanium tacks. Surg Endosc. 2011;25:3890–3897 [DOI] [PubMed] [Google Scholar]
- 10. Karahasanoglu T, Onur E, Baca B, et al. Spiral tacks may contribute to intra-abdominal adhesion formation. Surg Today. 2004;34:860–864 [DOI] [PubMed] [Google Scholar]
- 11. Jenkins ED, Yom V, Melman L, et al. Prospective evaluation of adhesion characteristics to intraperitoneal mesh and adhesiolysis-related complications during laparoscopic re-exploration after prior ventral hernia repair. Surg Endosc. 2010;24:3002–3007 [DOI] [PubMed] [Google Scholar]
- 12. Deeken CR, Matthews BD. Comparison of contracture, adhesion, tissue ingrowth, and histologic response characteristics of permanent and absorbable barrier meshes in a porcine model of laparoscopic ventral hernia repair. Hernia. 2012;16:69–76 [DOI] [PubMed] [Google Scholar]
- 13. Duffy AJ, Hogle NJ, LaPerle KM, Fowler DL. Comparison of two composite meshes using two fixation devices in a porcine laparoscopic ventral hernia repair model. Hernia. 2004;8:358–364 [DOI] [PubMed] [Google Scholar]
- 14. McGinty JJ, Hogle NJ, McCarthy H, Fowler DL. A comparative study of adhesion formation and abdominal wall ingrowth after laparoscopic ventral hernia repair in a porcine model using multiple types of mesh. Surg Endosc. 2005;19:786–790 [DOI] [PubMed] [Google Scholar]
- 15. Jacob BP, Hogle NJ, Durak E, Kim T, Fowler DL. Tissue ingrowth and bowel adhesion formation in an animal comparative study: polypropylene versus Proceed versus Parietex Composite. Surg Endosc. 2007;21:629–633 [DOI] [PubMed] [Google Scholar]
- 16. Ventralight ST. Instructions for Use [package insert]. Warwick, RI: Bard, Inc; 2010 [Google Scholar]
- 17. Product Sheet for SorbaFix Absorbable Fixation. Available at: http://www.davol.com/default/assets/File/MMESSS.pdf. Published 2012 Accessed February 2, 2012
- 18. Product Sheet for Securestrap. Available at: http://www. ethicon360.com/products/ethicon-securestrap. Published 2012 Accessed February 2, 2012
- 19. Report of the American Veterinary Medical Association (AVMA) Panel on Euthanasia. J Am Vet Med Assoc. 2001;218:668–69611280395 [Google Scholar]
- 20. Schreinemacher MH, Emans PJ, Gijbels MJ, Greve JW, Beets GL, Bouvy ND. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg. 2009;96:305–313 [DOI] [PubMed] [Google Scholar]