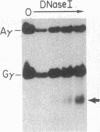
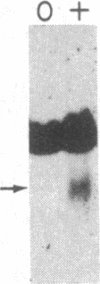
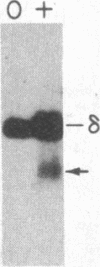
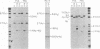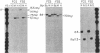Abstract
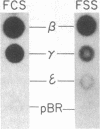
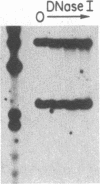
Fetal hemoglobin production can be reactivated in vivo in adult persons with various hemoglobinopathies and other hemopoietic disorders, and in cultures of adult erythroid progenitors. We show that the activation of Hb F in adult cells is transcriptional in nature and is accompanied by the appearance of DNase I-hypersensitive sites and undermethylation of Hpa II sites 5' to the gamma-globin genes. Production of Hb F in culture is strongly modulated by the environment, and the repression of Hb F synthesis by specific culture conditions has been reported. By nuclear runoff, chromatin, and methylation analyses, we show that this inhibition of Hb F production in vitro is at the level of transcription with the concomitant loss of characteristic gamma hypersensitive sites and methylation of gamma Hpa II sites. These data indicate, first, that the organization of globin chromatin of adult cells that produce fetal hemoglobin resembles that of fetal erythroid cells and, second, that this organization switches from a fetal to an adult pattern in response to changes in the environment of the erythroid cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busslinger M., Hurst J., Flavell R. A. DNA methylation and the regulation of globin gene expression. Cell. 1983 Aug;34(1):197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Giampaolo A., Carè A., Migliaccio G., Calandrini M., Russo G., Pagliardi G. L., Mastroberardino G., Marinucci M., Peschle C. Molecular mechanisms of human hemoglobin switching: selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6907–6911. doi: 10.1073/pnas.80.22.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Bunn H. F., Stamatoyannopoulos G. Cellular distribution of hemoglobin F in a clonal hemopoietic stem-cell disorder. N Engl J Med. 1978 Jan 12;298(2):72–75. doi: 10.1056/NEJM197801122980203. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Kurachi S., Nakamoto B., Zanjani E. D., Stamatoyannopoulos G. Hemoglobin switching in culture: evidence for a humoral factor that induces switching in adult and neonatal but not fetal erythroid cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6579–6583. doi: 10.1073/pnas.79.21.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Rosse W., Stamatoyannopoulos G. Fetal hemoglobin in paroxysmal nocturnal hemoglobinuria (PNH): evidence for derivation of HbF-containing erythrocytes (F cells) from the PNH clone as well as from normal hemopoietic stem cell lines. Blood. 1978 Oct;52(4):740–749. [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Kurnit D. M., Papayannopoulou T. Stochastic expression of fetal hemoglobin in adult erythroid cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7005–7009. doi: 10.1073/pnas.78.11.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Nakamoto B., Kurachi S., Papayannopoulou T. Direct evidence for interaction between human erythroid progenitor cells and a hemoglobin switching activity present in fetal sheep serum. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5650–5654. doi: 10.1073/pnas.80.18.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]