Abstract
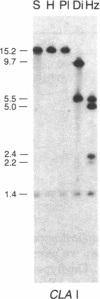
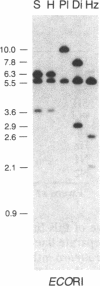
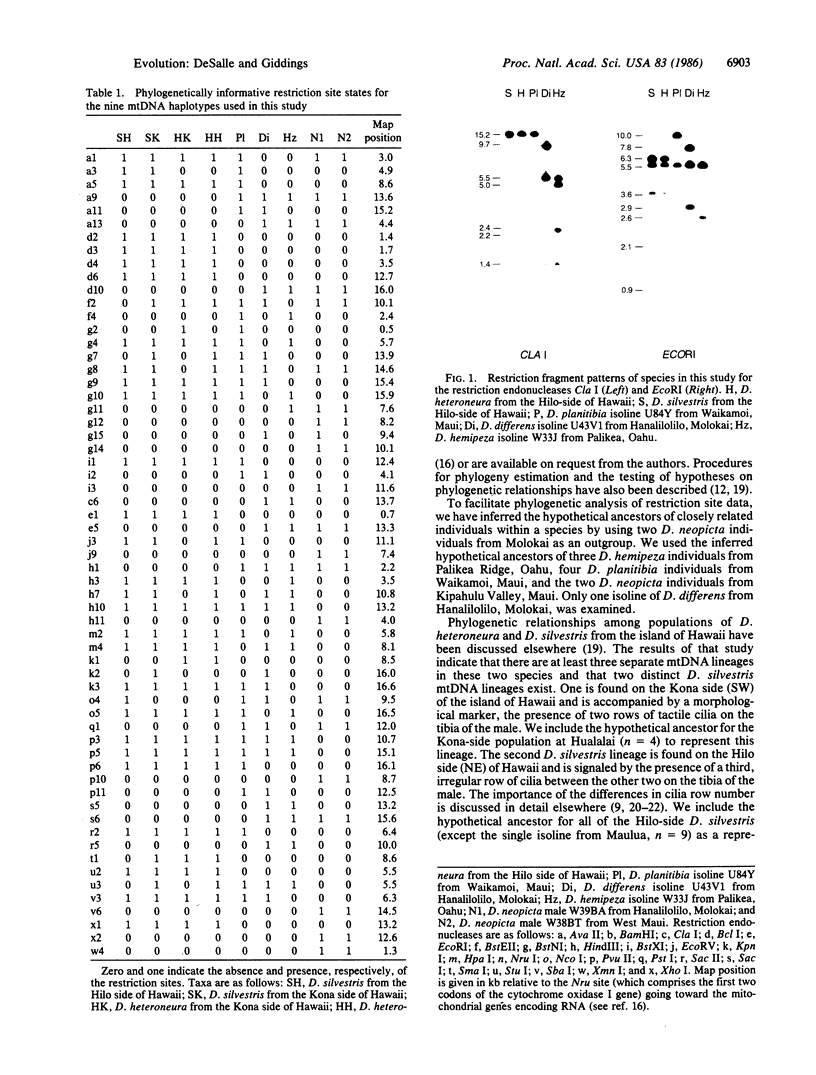
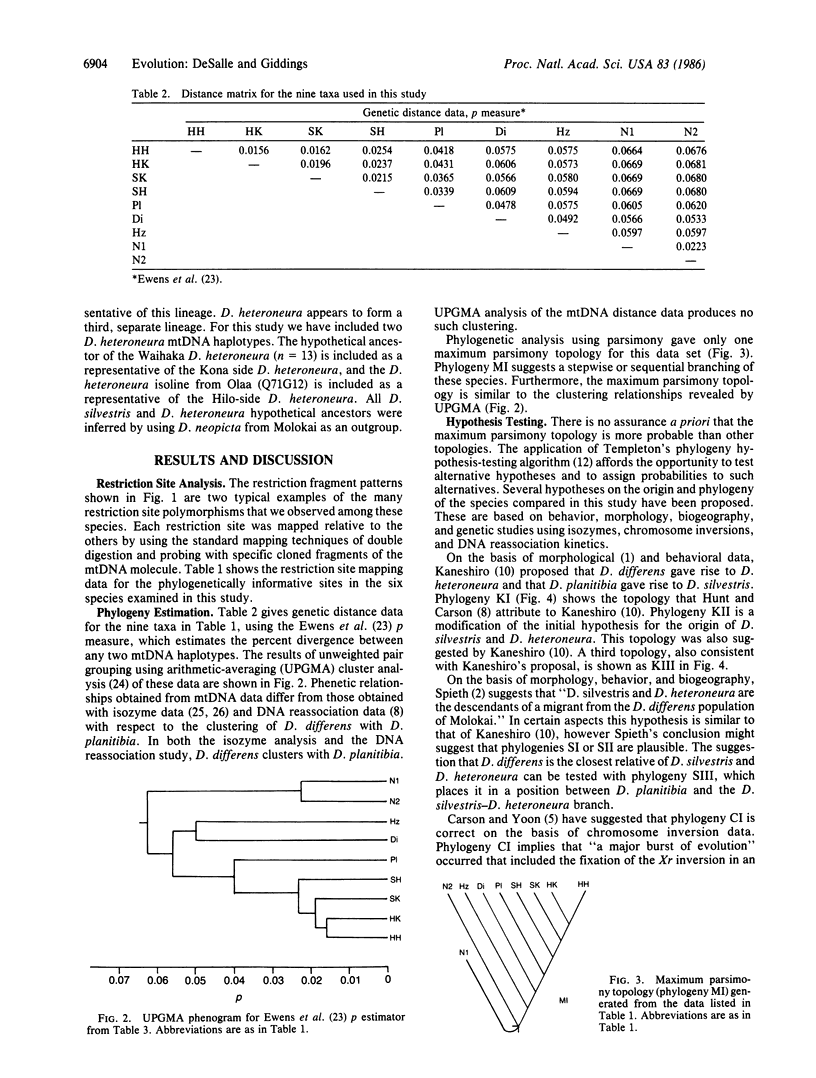
Drosophila differens, endemic to Molokai, Drosophila planitibia of Maui, and Drosophila silvestris and Drosophila heteroneura from the island of Hawaii are chromosomally homosequential species that presumably have colonized the newer islands of the Hawaiian archipelago by sequential founder events. We have examined the phylogenetic relationships of these four species by using mitochondrial DNA restriction site data for 23 enzymes. Both distance and character-state analyses indicate that a sequential or monotonic branching relationship exists for mtDNA restriction site data from the four species. The mtDNA data suggest that the maternal lineage that gave rise to D. differens is ancestral to the D. planitibia maternal lineage, which in turn shares the most recent common ancestor with the D. silvestris and D. heteroneura maternal lineages [with Drosophila hemipeza (Oahu) and Drosophila neopicta (Molokai and Maui) as outside references]. We also discuss the phylogenetic implications of the mtDNA data in comparison with other sources of phylogenetic data. We conclude that hybridization of the species in this group has been an important factor in the evolution of the nuclear genomes. Because of small population sizes and mating asymmetries, it is possible that the nuclear genetic distance of species that are physically capable of hybridizing (e.g., on the same island or island complex) is depressed. Consequently the mtDNA genetic distance appears to be more sensitive in establishing the sequence of evolutionary events responsible for the present distribution and population structure of these species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Neigel J. E., Arnold J. Demographic influences on mitochondrial DNA lineage survivorship in animal populations. J Mol Evol. 1984;20(2):99–105. doi: 10.1007/BF02257369. [DOI] [PubMed] [Google Scholar]
- Avise J. C., Shapira J. F., Daniel S. W., Aquadro C. F., Lansman R. A. Mitochondrial DNA differentiation during the speciation process in Peromyscus. Mol Biol Evol. 1983 Dec;1(1):38–56. doi: 10.1093/oxfordjournals.molbev.a040301. [DOI] [PubMed] [Google Scholar]
- Birky C. W., Jr, Maruyama T., Fuerst P. An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics. 1983 Mar;103(3):513–527. doi: 10.1093/genetics/103.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson H. L. Ancient chromosomal polymorphism in Hawaiian Drosophila. Nature. 1973 Jan 19;241(5386):200–202. doi: 10.1038/241200a0. [DOI] [PubMed] [Google Scholar]
- Carson H. L. Chromosomal sequences and interisland colonizations in hawaiian Drosophila. Genetics. 1983 Mar;103(3):465–482. doi: 10.1093/genetics/103.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson H. L. Chromosome tracers of the origin of species. Science. 1970 Jun 19;168(3938):1414–1418. doi: 10.1126/science.168.3938.1414. [DOI] [PubMed] [Google Scholar]
- Carson H. L. Evolution of Drosophila on the newer Hawaiian volcanoes. Heredity (Edinb) 1982 Feb;48(Pt 1):3–25. doi: 10.1038/hdy.1982.2. [DOI] [PubMed] [Google Scholar]
- Carson H. L. Inference of the time of origin of some Drosophila species. Nature. 1976 Feb 5;259(5542):395–396. doi: 10.1038/259395a0. [DOI] [PubMed] [Google Scholar]
- Carson H. L., Lande R. Inheritance of a secondary sexual character in Drosophila silvestris. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6904–6907. doi: 10.1073/pnas.81.21.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson H. L., Teramoto L. T. Artificial selection for a secondary sexual character in males of Drosophila silvestris from Hawaii. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3915–3917. doi: 10.1073/pnas.81.12.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalle R., Giddings L. V., Kaneshiro K. Y. Mitochondrial DNA variability in natural populations of Hawaiian Drosophila. II. Genetic and phylogenetic relationships of natural populations of D. silvestris and D. heteroneura. Heredity (Edinb) 1986 Feb;56(Pt 1):87–96. doi: 10.1038/hdy.1986.12. [DOI] [PubMed] [Google Scholar]
- DeSalle R., Giddings L. V., Templeton A. R. Mitochondrial DNA variability in natural populations of Hawaiian Drosophila. I. Methods and levels of variability in D. silvestris and D. heteroneura populations. Heredity (Edinb) 1986 Feb;56(Pt 1):75–85. doi: 10.1038/hdy.1986.11. [DOI] [PubMed] [Google Scholar]
- Ewens W. J., Spielman R. S., Harris H. Estimation of genetic variation at the DNA level from restriction endonuclease data. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3748–3750. doi: 10.1073/pnas.78.6.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Huang C. M., Nielsen J. T., Ritte U., Wilson A. C. Flow of mitochondrial DNA across a species boundary. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2290–2294. doi: 10.1073/pnas.80.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L. R., Beckenbach A. T. Mitochondrial DNA variation in Drosophila pseudoobscura and related species in Pacific northwest populations. Can J Genet Cytol. 1985 Jun;27(3):357–364. doi: 10.1139/g85-053. [DOI] [PubMed] [Google Scholar]
- Hunt J. A., Bishop J. G., 3rd, Carson H. L. Chromosomal mapping of a middle-repetitive DNA sequence in a cluster of five species of Hawaiian Drosophila. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7146–7150. doi: 10.1073/pnas.81.22.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. A., Carson H. L. Evolutionary relationships of four species of hawaiian Drosophila as measured by DNA reassociation. Genetics. 1983 Jun;104(2):353–364. doi: 10.1093/genetics/104.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. R. Interspecific cytoplasmic gene flow in the absence of nuclear gene flow: evidence from Drosophila. Proc Natl Acad Sci U S A. 1983 Jan;80(2):492–495. doi: 10.1073/pnas.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N., Slatkin M. Mitochondrial gene flow. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1764–1767. doi: 10.1073/pnas.81.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]