Abstract
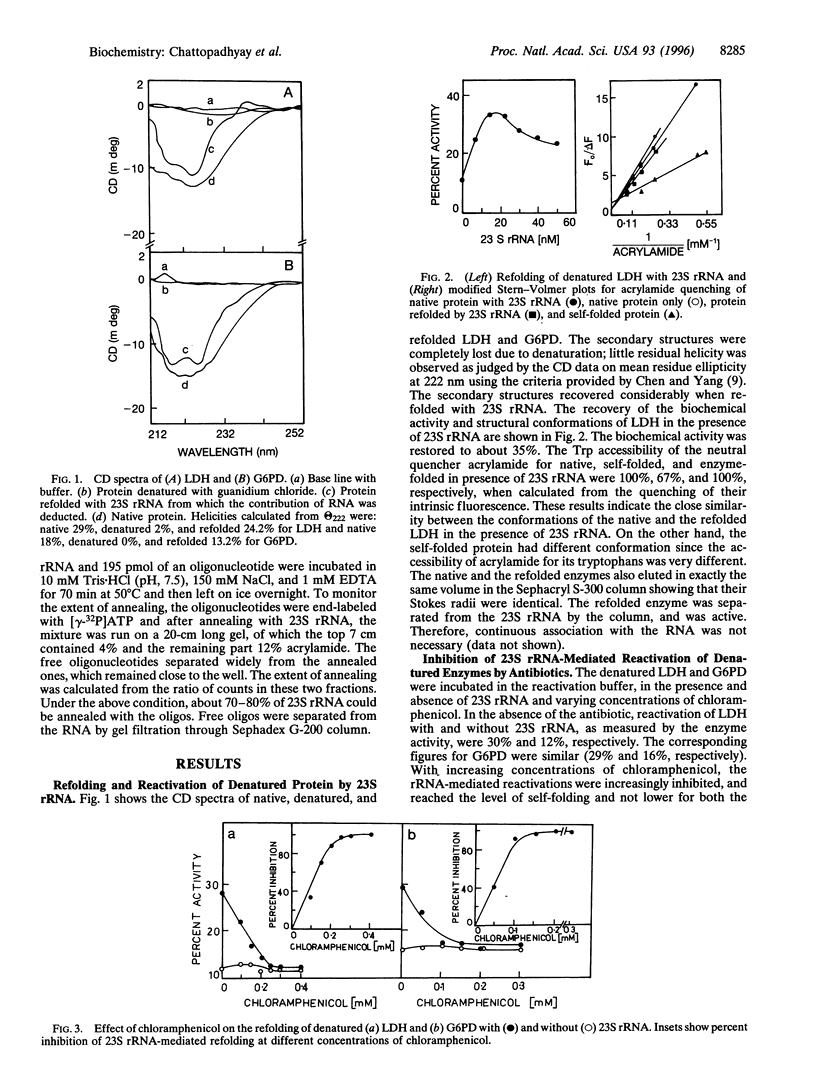
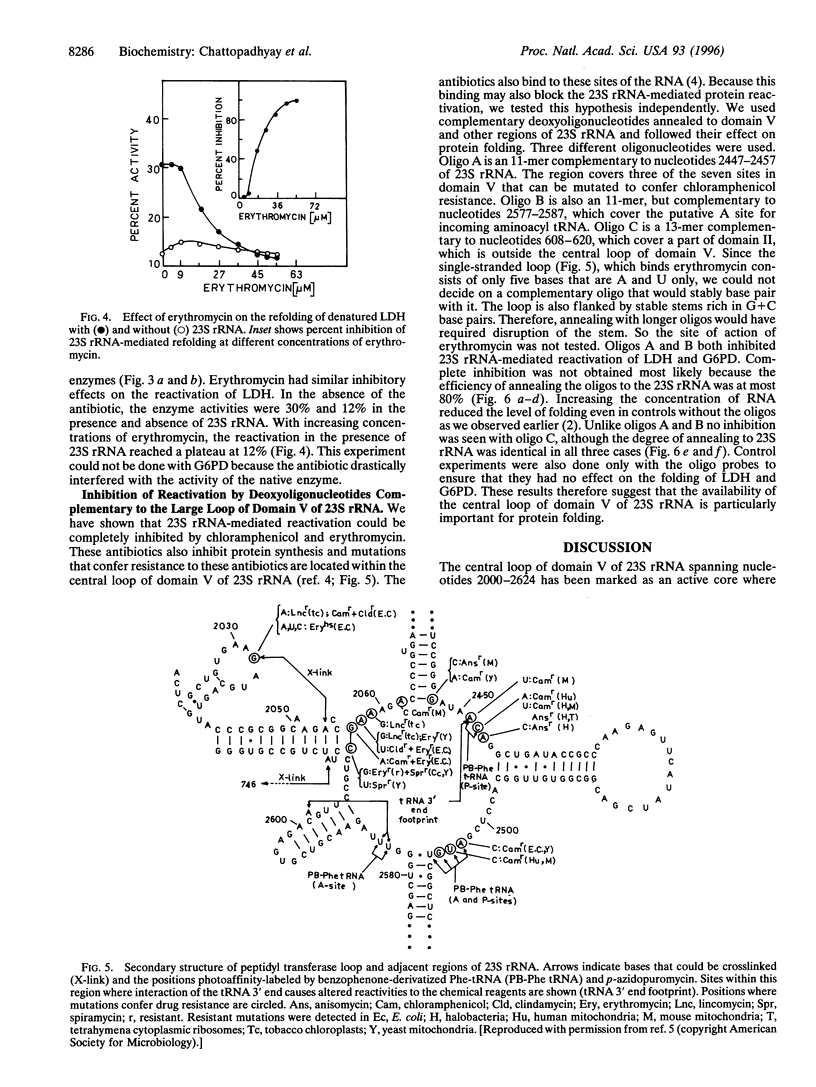
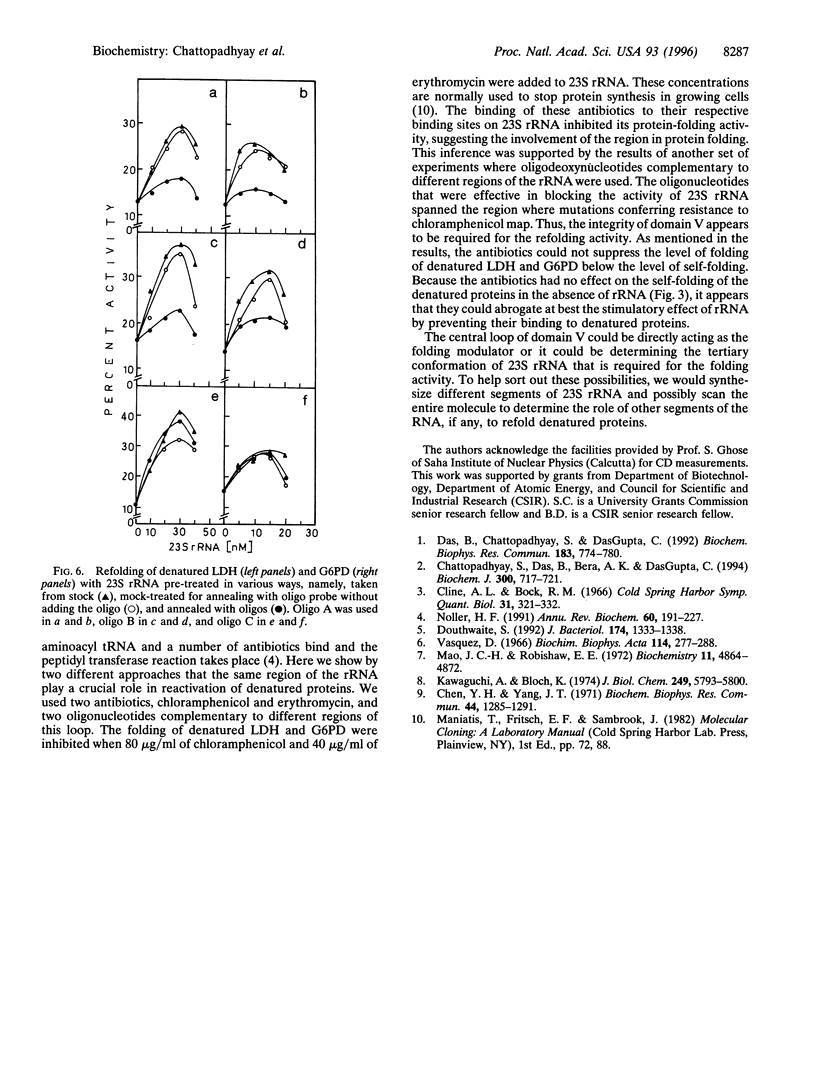
Escherichia coli ribosome, its 50S subunit, or simply the 23S rRNA can reactivate denatured proteins in vitro. Here we show that protein synthesis inhibitors chloramphenicol and erythromycin, which bind to domain V of 23S rRNA of E. coli, can inhibit reactivation of denatured pig muscle lactate dehydrogenase and fungal glucose-6-phosphate dehydrogenase by 23S rRNA completely. Oligodeoxynucleotides complementary to two regions within domain V (which cover sites of chloramphenicol resistant mutations and the putative A site of the incoming aminoacyl tRNA), but not to a region outside of domain V, also can inhibit the activity. Domain V of 23S rRNA, therefore, appears to play a crucial role in reactivation of denatured proteins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chattopadhyay S., Das B., Bera A. K., Dasgupta D., Dasgupta C. Refolding of denatured lactate dehydrogenase by Escherichia coli ribosomes. Biochem J. 1994 Jun 15;300(Pt 3):717–721. doi: 10.1042/bj3000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1285–1291. doi: 10.1016/s0006-291x(71)80225-5. [DOI] [PubMed] [Google Scholar]
- Cline A. L., Bock R. M. Translational control of gene expression. Cold Spring Harb Symp Quant Biol. 1966;31:321–333. doi: 10.1101/sqb.1966.031.01.042. [DOI] [PubMed] [Google Scholar]
- Das B., Chattopadhyay S., Das Gupta C. Reactivation of denatured fungal glucose 6-phosphate dehydrogenase and E. coli alkaline phosphatase with E. coli ribosome. Biochem Biophys Res Commun. 1992 Mar 16;183(2):774–780. doi: 10.1016/0006-291x(92)90550-5. [DOI] [PubMed] [Google Scholar]
- Douthwaite S. Functional interactions within 23S rRNA involving the peptidyltransferase center. J Bacteriol. 1992 Feb;174(4):1333–1338. doi: 10.1128/jb.174.4.1333-1338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A., Bloch K. Inhibition of glucose 6-phosphate dehydrogenase by palmitoyl coenzyme A. J Biol Chem. 1974 Sep 25;249(18):5793–5800. [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Erythromycin, a peptidyltransferase effector. Biochemistry. 1972 Dec 5;11(25):4864–4872. doi: 10.1021/bi00775a035. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Vazquez D. Binding of chloramphenicol to ribosomes. The effect of a number of antibiotics. Biochim Biophys Acta. 1966 Feb 21;114(2):277–288. doi: 10.1016/0005-2787(66)90309-1. [DOI] [PubMed] [Google Scholar]


