Abstract
Over the past four decades, research has revealed that cells in the hippocampal formation provide an exquisitely detailed representation of an animal's current location and heading. These findings have provided the foundations for a growing understanding of the mechanisms of spatial cognition in mammals, including humans. We describe the key properties of the major categories of spatial cells: place cells, head direction cells, grid cells and boundary cells, each of which has a characteristic firing pattern that encodes spatial parameters relating to the animal's current position and orientation. These properties also include the theta oscillation, which appears to play a functional role in the representation and processing of spatial information. Reviewing recent work, we identify some themes of current research and introduce approaches to computational modelling that have helped to bridge the different levels of description at which these mechanisms have been investigated. These range from the level of molecular biology and genetics to the behaviour and brain activity of entire organisms. We argue that the neuroscience of spatial cognition is emerging as an exceptionally integrative field which provides an ideal test-bed for theories linking neural coding, learning, memory and cognition.
Keywords: hippocampus, entorhinal cortex, grid cells, place cells, head direction cells, boundary cells
1. Introduction
How does an animal or human being know where it is, and how does it remember distant goals, and then navigate efficiently towards them, while avoiding hazards and barriers? What kinds of representation underlie this kind of spatial ability? Over the past four decades, efforts to address such questions have provided the foundations for a richly productive field connecting different levels of neuroscientific investigation, from membrane potentials and synaptic currents to individual neurons, from neuronal networks to complex behaviour and human cognition.
In introducing the topic of ‘Space in the Brain’, we want to draw a distinction between spatial frameworks tied to a particular body part, object or action and those that are fixed with respect to the outside world, independent of particular actions and objects. The brain makes use of neural representations of both types. The first type of representation is crucial for behaviours such as catching a ball or picking a fruit from a tree. Behaviours such as navigating long distances over natural terrain or through a new city are also dependent on the second type of representation. This issue will focus primarily on the second type of spatial framework, which depends on a specialized system centred on the hippocampus, a phylogenetically ancient and well-preserved structure, which in humans is found deep in the medial temporal lobes.
To understand what is so special about the hippocampal formation, it is first instructive to consider how spatial parameters are reflected in the firing of neurons in other parts of the brain. Throughout the brain, individual neurons are often found to have spatially restricted firing fields, which carry spatial information about the source of sensory information or destination of planned actions. Thus, a neuron in primary visual cortex might respond to a stimulus in a particular part of the visual field [1], a neuron in primary somatosensory cortex might respond to a tactile stimulation of a particular body part [2], and the firing of a motor neuron might help to direct limb movements in a specific direction [3]. In each case, neural activity reflects the spatial relationship between a stimulus or response and a part of the body. Similar but somewhat more abstract forms of spatial coding are found beyond the primary sensory and motor cortices, notably in parietal cortex where individual neurons’ receptive fields may be fixed with respect to the hand, head or trunk, and may respond to multiple sensory modalities [4–6]. Such neural codes incorporate spatial information about stimuli and responses in terms of various egocentric reference frames (each anchored with respect to the body or part of the body). They are well suited to mediating spatial behaviour in the immediate environment and to computing transformations between visual and body-based reference frames in the online control of action [7]. They carry spatial information about stimuli and responses and can, in principle, perform spatial computations linking one with the other [8,9].
All of the above representations are ‘egocentric’ in terms of their spatial reference frame. It is debateable whether they represent space itself in an absolute sense, and when they do represent locations in the world, those locations must be updated as the various parts of the body, and the body itself moves.
By contrast, and as we explain in more detail in §2, cells in the hippocampal formation can represent an animal's current location or heading independently of individual sensory cues and particular actions. Their firing fields are anchored to the external environment (and thus termed ‘allocentric’ or world-centred), rather than to individual objects, actions or to the body. These cells appear to provide the basis for a cognitive map: a representation of the environment and the places and objects within it that is to some extent independent of bodily posture or orientation. As such it affords long-term memory for the spatial relationships between places, the routes between them, the resources, goals and hazards they contain, in that it does not require continuous updating as the animal goes about its daily life [10–13].
We briefly outline key aspects of the anatomy of the hippocampal formation and the properties of its spatial cells as characterized through in vivo extracellular unit recording in freely behaving animals, mainly rodents. These cells form the building blocks of spatial representation. Their fascinating properties provide detailed quantitative constraints on computational models which have been further supported by advances in optogenetics, juxtacellular recording and two-photon imaging in behaving animals, and human electrophysiology and neuroimaging. These developments have fuelled further discoveries, and we outline some of the themes of current research and the new avenues which have been opened up. The neuroscience of spatial cognition, we will argue, is emerging as an exceptionally integrative field which provides an ideal test-bed for theories linking neural coding, learning, memory and cognition.
2. Anatomy and spatial cells of the hippocampal formation
In this section, we outline the anatomy of the hippocampal formation and describe some of the spatial properties of the neurons within it. Much of the evidence we refer to is based on research in rodents, although as we explain later, there is mounting evidence that the critical spatial properties are maintained in other mammals, including humans. We should also note that although our focus on the hippocampal formation is justified by its central role in spatial cognition, cells with related spatial properties, notably head direction (HD) cells, are found in other brain regions.
(a). Anatomical sketch of the hippocampal formation
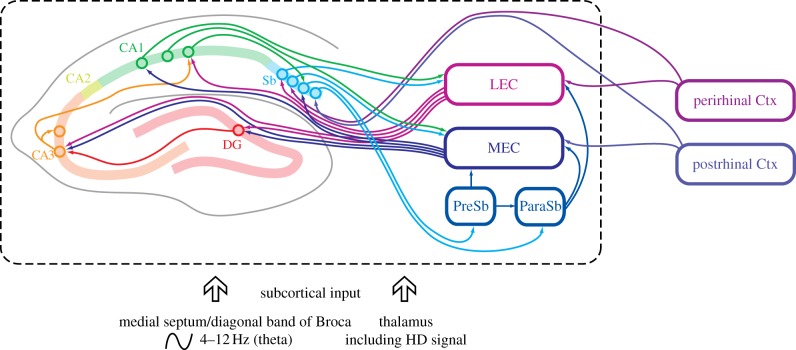
The hippocampal formation includes the hippocampus proper and the adjacent cortical areas to which it is connected. The hippocampus proper consists of the ‘cornu ammonis’ (CA) fields: the much-studied CA1 and CA3 fields and the smaller, little-studied CA2 field. The hippocampal formation thus consists of: the entorhinal cortex (divided into lateral and medial cortices), dentate gyrus, CA1, CA2, CA3, subiculum, presubiculum and parasubiculum (figure 1). Hippocampal regions and pathways were sufficiently distinct to allow the very early pioneers of neuroanatomy [16] to identify key elements of the circuitry (see left-hand side of figure 1). Indeed, the relative simplicity of the hippocampus, when compared with neocortex, strongly appealed to early researchers of memory, whether as physiologists demonstrating synaptic plasticity [17] or computational theorists modelling functional capacities [14,18]. Notably, this region contains several largely unidirectional projections, a crucial feature for early experiments on synaptic plasticity [19] (see figure 1 and legend). The superficial layers of the entorhinal cortex are typically regarded as the major conduit for neocortical information to enter the hippocampus, while its deep layers and the subiculum are thought to provide output from the hippocampal formation to the rest of the brain. CA1 also functions as an output (e.g. to prefrontal cortex). However, the presence of bidirectional and re-entrant pathways, and projections influencing both superficial and deep entorhinal layers, means that it is not entirely straightforward to define pure ‘input’ and ‘output’ pathways or feed-forward hierarchical structure (see [20] for further discussion).
Figure 1.
Schematic overview of major anatomical pathways in the hippocampal formation of the rat. Left-hand side of figure emphasizes gross morphology (rat brain) of cell layers in hippocampus and dentate gyrus and long-established unidirectional projections. Classic trisynaptic pathway consists of projection from entorhinal cortex (LEC: lateral entorhinal cortex; MEC: medial entorhinal cortex) to dentate gyrus (DG), from DG to CA3, and from CA3 to CA1. Entorhinal input also consists of direct monosynaptic LEC and MEC projections to CA3, to CA1, and to subiculum (Sb). CA1 projection to Sb and to LEC/MEC, and Sb projections to LEC/MEC, complete the circuit. Other circuits involve projections from subiculum to presubiculum (PreSb) and to parasubiculum (ParaSb), and projections from PreSb to MEC, and ParaSb to both LEC and MEC. Arrows indicate the direction of projection, and circles indicate cell bodies. For simplicity in this highly schematic figure, omissions include the following: dendrites and dendritic location of axonal termination zones; commissural projections connecting left and right hemispheres; CA2-involving projections. Additional guidance. The term ‘hippocampal formation’ applies to regions contained within dashed box. Entorhinal pathways to DG, CA3, CA1 and Sb known as perforant pathway, DG to CA3 pathway as mossy fibre projection, CA3 to CA1 pathway as Schaffer collaterals. As well as projecting in feed-forward manner to CA1, the CA3 pyramidal cells project to other CA3 pyramidal cells; these recurrent collaterals were proposed by Marr to underlie pattern completion (the ‘collaterals effect’ [14]). Postrhinal cortex is rat analogue of primate parahippocampal cortex (PHC), strongly implicated in visuospatial processing. In rodents, term ‘postsubiculum’ (containing many HD cells) refers to dorsal portion of presubiculum. Two parallel pathways formed by projections from postrhinal cortex and presubiculum to MEC, and perirhinal cortex to LEC, are not fully illustrated. Inspired by [15].
An important anatomical feature that the schematic figure 1 does not emphasize is the substantial projection of CA3 pyramidal cells to other CA3 pyramidal cells. The axons forming this projection are called the recurrent collaterals (as distinct from the CA3–CA1 projections called the Schaffer collaterals) and were proposed by Marr to underlie pattern completion (the ‘collaterals effect’ [14]). Pattern completion is the process by which the re-presentation of a subset of cues associated with an event gradually triggers reactivation of the full neural representation of the original event, and thereby enables retrieval of that event. (Marr's idea initiated the process of formally modelling hippocampal circuits using attractor dynamics; attractor models relevant to spatial coding are discussed in §3b.)
(i). Subcortical input and output and the theta oscillation
While the much-processed neocortical input to the hippocampus via the entorhinal cortex has often been emphasized in memory research, we note that many inputs crucial to spatial representation and behaviour are subcortical. For instance, cells with a robust HD signal (described in §2b(iii)) are found in the anterior thalamus, and this region projects strongly to the hippocampal formation (notably to the dorsal presubiculum, see [21] for review). Important subcortical outputs include projections to the mammillary bodies and ventral striatum. One perhaps under-emphasized subcortical projection is that from the hippocampus and subiculum to the lateral septum. Interestingly, there is a direct CA3–lateral septum projection. This projection is part of a polysynaptic CA3–ventral tegmental area (VTA) pathway which supports associations between reward and spatial contexts [22].
Importantly, all regions of the hippocampal formation receive direct projections from the medial septum and diagonal band of Broca (hereafter, medial septum), and these projections play a crucial role in generating and sculpting the 4–12 Hz theta oscillation, a quasi-sinusoidal fluctuation in the local field potential characteristically seen during locomotion [23]. This oscillatory medial septal input is a defining feature of the hippocampal formation in rodents and the theta rhythm has been hypothesized to play important roles in processing novelty (see citations in [24]), in scheduling memory encoding versus retrieval [25–27], and in spatial representation [28,29].
Regarding spatial representation specifically, several studies have shown that theta frequency and amplitude correlate with running speed [30–35]. These correlations, and the well-established relationship between theta phase of firing and distance coding in both place cells and grid cells, as further demonstrated by Jeewajee et al. in this issue [36], have led to the suggestion that the theta oscillation plays a role in coding for self-motion and can be used to estimate spatial translation [11,37,38]. We consider the phenomenon of phase precession in some detail in §2b(ii) and figure 3, discussing the properties of place cells and grid cells. Intriguingly, theta-modulated neurons have recently been discovered in the anterior ventral thalamus and medial septum which appear to combine the HD and theta self-motion signal, using a theta frequency code for locomotion speed in a specific allocentric direction. These have been called velocity-controlled oscillators [39,40]. In general, the view that spatial translation might be encoded using the theta oscillation remains influential and controversial. In this special issue, several authors explore this possibility [36,41,42], whereas others take issue with it [43]. Jacobs in this issue [44] considers the possibility that there is a navigation-related oscillation in humans which is homologous to rodent theta, but which occurs at a lower frequency [45,46].
Figure 3.
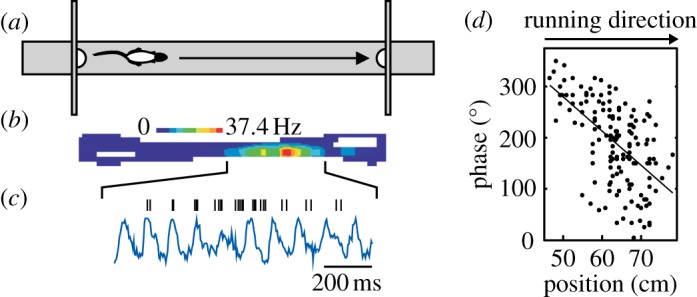
Theta phase precession of place cell firing. (a) As a rat runs along a linear track, a place cell in the hippocampus fires as the animal moves through the firing field (b). The firing rate code for location is also a temporal code (c): spikes (ticks) are fired at successively earlier phases of the theta rhythm of the local field potential (blue trace), referred to as ‘theta phase precession’. The theta phase of firing correlates with the distance travelled through the place field (d), even when pooled over runs that might be fast or slow. Adapted from [70].
We should emphasize that the hippocampal theta oscillation has multiple generators and is dependent upon much more than reciprocal anatomical connectivity with the medial septum and other subcortical regions, important as they are. Intrahippocampal connectivity and theta resonance characteristics conferred by intrinsic membrane properties are also crucial to normal functionality of hippocampal theta [47,48]. Here, we note that the rich functionality conferred by oscillatory systems in the hippocampal formation requires a vast supportive network of inhibitory neurons. Somogyi et al. in this issue [49] review important work on the classes of interneurons (currently numbering ca 20 in CA1) that support and control dominant hippocampal oscillations, such as theta, gamma and ripples, emphasizing the way in which pyramidal cell function in CA1 (i.e. place cell function) relies on a precise medley orchestrated by many spatio-temporally specific patterns of inhibition. Different classes of interneurons target different subcellular domains (e.g. the axon hillock or the distal or proximal apical dendrites) and/or provide inhibition at different phases of the theta cycle (e.g. the trough or the peak). How does spatio-temporally specific inhibition confer functionality? This will take considerable research effort to unravel, but some clues exist already. Mizuseki & Buzsaki in this issue [50] review evidence implicating interneurons in phase precession, including a study showing that silencing parvalbumin interneurons, which provide perisomatic inhibition to CA1 pyramidal cells, results in narrowed theta phase variance of pyramidal spikes, and thus disruption of the correlation between spike phase and location. The scheduling of encoding versus retrieval states by theta phase and acetylcholine in CA1 and CA3 place cells [25,27,51,52] may also depend upon spatio-temporally specific inhibition, in this case affecting the balance between feed-forward inputs supporting encoding versus recurrent inputs supporting retrieval.
(b). Spatial cells
In this section, we briefly discuss all the major types of spatial cells found in the hippocampal formation. Figure 2 provides an introductory overview, showing one example of each of the four fundamental spatial cells: a place cell (figure 2a), an HD cell (figure 2b), a grid cell (figure 2c) and a boundary cell (figure 2d).
Figure 2.
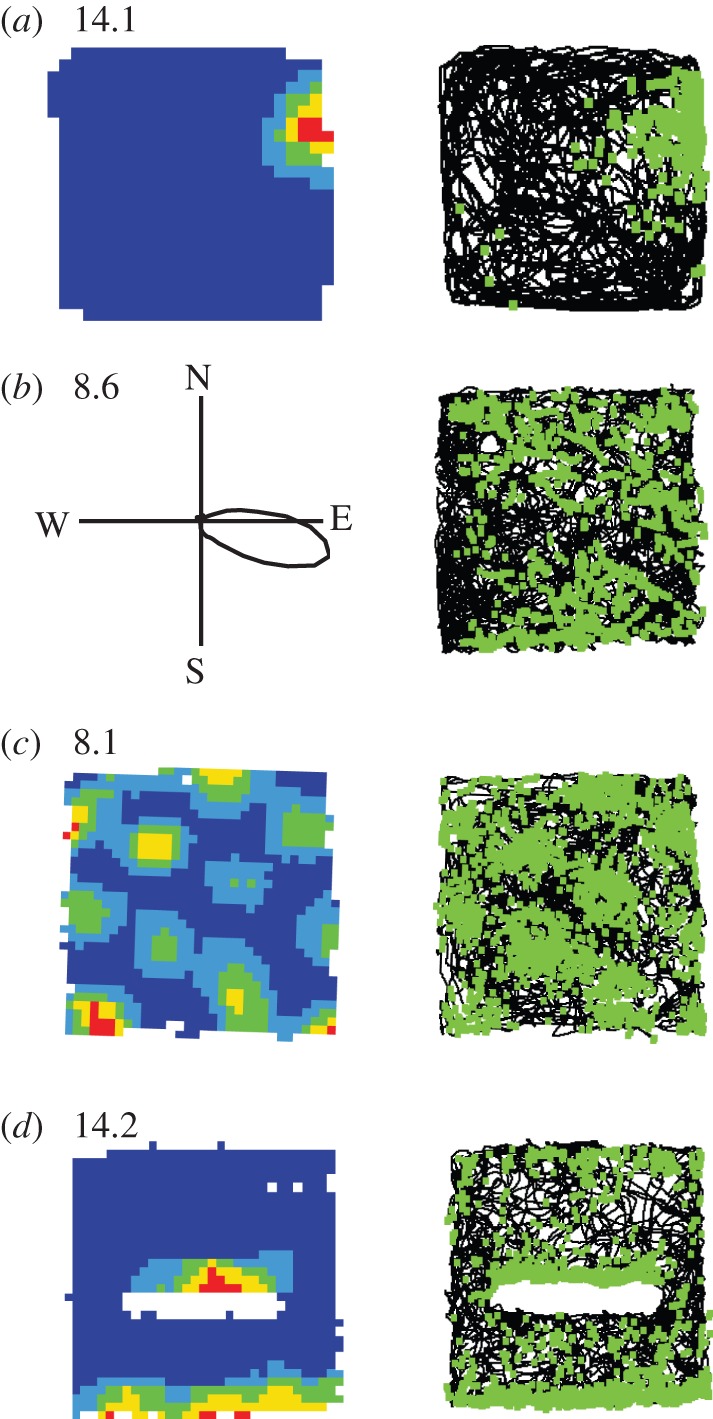
Four types of fundamental spatial cell. Figure shows one example of each type of fundamental spatial cell: (a) place cell; (b) HD cell; (c) grid cell; (d) boundary cell. For each cell: left-hand column shows locational firing ratemap (a,c,d) or directional firing polar plot (b), with peak firing rate in hertz shown top left of rate map/polar plot; right-hand column depicts path taken over whole trial (black line), on which are plotted the locations at which spikes were recorded (green squares). In firing rate maps, one of five colours in locational bin indicates spatially smoothed firing rate in that bin (autoscaled to firing rate peak; dark blue, 0–20%; light blue, 20–40%; green, 40–60%; yellow, 60–80%; red, 80–100%). HD, grid and boundary cell recorded in 1 × 1 m (place cell: 62 × 62 cm) square-walled box with 50 cm-high walls. For boundary cell, 50 cm-long barrier inserted into box elicits the second field along north side of barrier (as predicted by the boundary vector cell (BVC) model [53]) in addition to original field along south wall. Cells provided by Sarah Stewart and Colin Lever.
(i). Place cells
Place cells were discovered in the hippocampus of the freely behaving rat by O'Keefe & Dostrovsky [54]. They are principal cells in the hippocampus proper and dentate gyrus. Place cells characteristically fire at a low rate throughout most of the environment, but each cell shows increased firing when the animal is within a circumscribed region of the environment (its ‘place field’). The spatial pattern of firing including the place field(s) can be visualized using a firing rate map, where the average firing rate at each location is represented by the colour (see figure 2a, where the place field of the cell in the specific environment shown occurs in a restricted region towards the northern part of the east wall). Place cells recorded at dorsal sites tend to have smaller fields while those recorded at ventral sites are more broadly tuned [55,56]. The pattern of firing across different trials in the same environment can be very stable, but different patterns and different subsets of cells are seen in sufficiently different, or sufficiently experienced, environments (‘remapping’ [57–63]).
Different place cells recorded simultaneously in the same environment have different place fields, such that at any location only a small subset of place cells will be firing strongly. By monitoring the firing rates of a small population of cells, the animal's current location can be reconstructed very accurately [64,65]. Place fields can be remarkably robust to the removal of one or more individual cues [66], though some cues are more important than others (e.g. removing the bounding walls of an environment reliably elicits remapping [61]). In the open field, firing rates do not typically depend on the animal's orientation (HD or direction of travel [67]). Place fields are seen on first exposure to an environment and do not depend in a straightforward way on a particular task or action. Although place cells are defined by the prominent spatial correlates of their spiking activity, it should be noted that their firing rates are sensitive to changes in other environmental variables, such as odour and colour [68,69].
(ii). Phase precession and the theta oscillation
Two decades ago, O'Keefe & Recce [28] observed that the spatial code expressed by place cells extended beyond the rate-based code revealed by place field maps. Interestingly, within the place field, spikes occurred at progressively earlier phases of the local field potential (LFP) theta rhythm as the animal progressed across the field (figure 3). The theta phase precession phenomenon suggested that the locational firing of place cells might be causally linked to theta, and O'Keefe & Recce suggested that both phenomena might be understood in terms of interference between two velocity-sensitive theta-band oscillations, one occurring at a slightly higher frequency than the other, such as that of the LFP and the neuron's intrinsic oscillation. (We consider this issue further in §3b(ii) in discussion of grid cell models.)
Importantly, theta phase coding of distance-through-field observed in place cells, and also in grid cells [71], is cell-specific. At the same time, as place cell X fires at a late phase of pyramidal-layer theta as the rat enters cell X's place field, place cell Y will be firing at an intermediate phase of pyramidal-layer theta in the middle of cell Y's field, and place cell Z will be firing at an early phase of theta as the rat exits cell Y's place field. Thus, place cells firing at later, intermediate and early phases will have firing fields, respectively, centred ahead of, centred on and centred behind, the rat; the spatial order of firing fields on the track will be present in the temporal order of firing within each theta cycle. (While this effect is most clear on a linear track [28,72], the same pattern can also be seen in animals foraging in open environments [73,74].) Considerable information, then, exists in the potential of individual spatial cells to fire at different phases of the theta cycle. In this issue, Mizuseki & Buzsaki [50] argue for a more complex view of oscillations beyond providing synchrony and consider how theta increases information content by reducing redundancy, i.e. by reducing synchrony of firing. They find that the spike co-activation of principal cells (i.e. spike synchrony) in the hippocampus and entorhinal cortex is reduced under theta states (locomotion and rapid-eye-movement sleep), relative to under slow wave sleep and immobility-associated states. Rather, prominent synchrony is reserved for, and helps to define, the ‘cell assembly’ level of organization which operates at a shorter period than the theta cycle. In this case, the cell assembly refers to those co-actively spiking place cells whose place fields occur at similar track locations.
(iii). Head direction cells
Whereas place cells’ activity represents where the animal is, regardless of its orientation, HD cells, discovered by Ranck and co-workers [75], provide a representation of allocentric heading independent of location. HD cells are found in the dorsal presubiculum [75] and entorhinal cortex [76], but also, it should be noted, outside the hippocampal formation; for example, in anterior dorsal thalamic nucleus and retrosplenial cortex [77]. Each HD cell has a preferred direction corresponding to a compass direction. It fires rapidly whenever the animal is facing in the preferred direction and only weakly otherwise (see figure 2b, where the cell's preferred direction is east-south-east). The full range of directions is represented such that at any time a subset of HD cells will be firing. By monitoring the firing rates of a modest number of cells recorded concurrently, the animal's heading can be reconstructed with great accuracy [78,79]. When polarizing cues are held constant, the preferred directions of HD cells are stable, and the angular distance between two cells’ preferred directions remains constant. However, preferred tunings of the entire system can be rotated by moving prominent visual cues in an otherwise impoverished environment. Changes that affect the directional tuning of the HD system also affect the location of place fields. For example, in a cylindrical environment, rotations of a single salient cue that induce simultaneous rotation of HD cell tunings also induce similar rotations of place field locations. These and other data suggest that place cells rely on directional information from the HD system. Consistent with this view, lesioning the HD system disrupts the ability of visual cues to control the orientation of place fields within a cylinder [80]. Interestingly, the omnidirectionality of place cell firing also depends upon intact HD input. In an open environment, the direction that the rat travels through a given place field (e.g. from east to west versus from west to east) generally makes little difference to the cell's firing rate [67,81]. However, after lesioning the HD system, place fields become more directional [80]. In general, that spatial representations in the hippocampal formation have an allocentric map-like quality probably depends on intact HD function.
Several studies have examined the issue of sensory control over the HD system [77]. For instance, early studies considered to what extent one cue set (e.g. ‘idiothetic’ cues to self-motion derived from proprioceptive, motor and vestibular sources) might typically predominate over another (e.g. visual) [77,82,83]. Arguably, this question remains unresolved, though a common view is that self-motion cues control moment-to-moment firing, with periodic and rapid updating from distal cues at or beyond the boundaries of explorable space [77,84–86]. One approach to this question has been to explore influences on HD responses under cue conflict [77], showing that the influence of cues is not fixed by cue type, but is considerably plastic according to circumstances. For instance, Jeffery and colleagues had previously shown interesting plasticity in the sensory control of place field orientations (in effect a by-proxy study of the HD system): place cells increasingly ‘distrust’ a prominent visual landmark if that landmark is explicitly shown to be mobile with respect to other cues [87]. In this issue, Knight et al. [88] set up a conflict between visual cues and idiothetic-plus-background cues, and found that HD cells followed the visual cue when conflicts were small but ‘compromised’ between the two cue sets when conflicts were large. These studies provide important insights into the extent to which the HD system can adapt as different directional cues become available.
(iv). Grid cells
In 2005, the already very active field of spatial hippocampal research was electrified by the discovery of a new class of spatial cells, ‘grid cells’, by a group led by May-Britt and Edvard Moser [89]. Grid cells were first identified in medial entorhinal cortex (MEC) and have since been found in pre- and parasubiculum [90]. Like place cells, they fire at specific locations in the environment, but unlike place cells each grid cell has multiple firing fields which tessellate the environment with a strikingly regular triangular pattern (figure 2c).
The grid field can be characterized in terms of three properties: scale (determined by the distance between adjacent firing rate peaks), orientation (of grid axes relative to some reference direction) and spatial phase (i.e. the two-dimensional offset of the grid relative to an external reference point). Grid fields from cells recorded from the same site can have widely differing spatial phases, so that they may be offset with respect to one another, even when they share the same scale and orientation.
As in place cells, the scale tuning of grid cells varies systematically along the dorsal–ventral axis of the hippocampal formation, with fields recorded from cells in dorsal MEC being smaller and closer together, whereas fields recorded from ventral MEC are larger and more spread out [89,91]. This gradient parallels changes in the intrinsic temporal properties of the cell membrane that in turn appear to be governed by properties of a particular ion-channel (HCN1 [92,93]).
Initially, grid cells recorded from the same animal were thought to show the same orientation, and grids recorded from the same location in entorhinal cortex were thought to have the same scale [89], but accumulating evidence began to suggest that rather than forming a continuum, grid cells might form discrete subsets marked by abrupt jumps in scale [94]. The arrangement of grid scales has important consequences for the ability of the grid system to encode very large spatial scales [95,96], and this is explored further by Towse et al. in this issue [97]. Recent evidence from studies involving large numbers of grid cells recorded in the same animal [98] shows that grids form modules with distinct combinations of scale and orientation tunings. These are anatomically overlapping while still showing an overall tendency for scale to increase in the dorsal–ventral direction. Although across the entire population the distribution of orientations is far from uniformly distributed over their 60° range, orientations of grids vary more between than within modules, suggesting that different modules operate somewhat independently. Moser et al. [43] discuss these findings in terms of their implications for the underlying mechanisms of grid formation, arguing that they point to the existence of distinct subpopulations with dissimilar patterns of inhibitory interconnectivity, and that their independent responses to environmental change may have a functional role in driving place cell remapping and the formation of distinctive hippocampal codes for different environments.
The beautiful periodicity of grid fields has attracted widespread attention, and many papers in this issue are concerned with grid cells. Several explore how their characteristic firing patterns might be derived and maintained, including that in different environments [41,43,97,99]. What is the function of grid cells? The consensus has developed rapidly since their discovery in 2005 [89] that grid cells are involved in path integration. By path integration we mean the use of self-motion signals to estimate travelled distances and directions, which can in turn contribute to the maintenance of estimates of current location. Although the original picture of grid cells (e.g. that grid scale is invariant across environments) is being revised [94,100], these revisions will probably not undermine the widely held view that grid cells subserve path integration. Arguably, the significant change of view is the increasing appreciation that grid cells are not always crucial to place cell function and cognitive mapping in all situations (as §3a makes clear). Rather, their importance to other spatial cells and to behaviour may be restricted to those circumstances when path integration is dominant and adaptive. Thus, in this issue Poucet et al. [101] argue that the self-localizing properties of place cells will only be dependent upon grid cells when external sensory information is unavailable or degraded, as in the dark. These authors present an anatomically based model whereby the ventral MEC specifically supports navigation in the light, whereas the dorsal MEC, rich in grid cells, supports navigation in the dark. A synthesis may be that place cells and spatial behaviour can rely either on self-motion cues or external environmental cues but that precision, stability and adaptability are maximized by combining signals from both.
What, then, are the cues in the external environment that the hippocampal formation uses in spatial mapping, and how does it use them? One answer to this question is provided by boundary cells.
(v). Boundary cells
Early research on place cells often emphasized that the locational firing patterns were environment specific; that a cell might fire in the north-west of a rectangle but in a circle, fire at its centre or not at all [57]. However, it was later observed, with manipulations affecting only environmental geometry, that place cells typically fired at ‘corresponding’ locations in geometrically different environments—places that tended to maintain their distance to the nearer walls of each environment [61,102] (see also [103]). This suggested that distances to the boundaries of the environment might determine the spatial tunings of place cells, leading to the prediction [53,102,104,105] that the input to the hippocampus might include cells whose firing rates encoded preferred distances to environmental boundaries in specific allocentric directions (in turn determined by the HD system). The firing patterns of a given place cell under a variety of geometric manipulations were well modelled as the thresholded sum of a small number of the putative ‘boundary vector cell’ (BVC) inputs [53]. On the basis of this computational model, BVCs were predicted to have extended firing fields parallel to the edges of the environment and to have additional fields where new barriers were inserted. For example, a given BVC might fire whenever a wall or barrier is found approximately 5 cm to the south of the rat; this cell would be expected to fire along the southern perimeter of an enclosed environment and also along the northern side of a barrier introduced into the same environment (figure 2d). Cells with such characteristics were subsequently discovered in the subiculum [106,107], MEC [108,109] and presubiculum and parasubiculum [90]. In other words, boundary cells are found in all the regions of the hippocampal formation outside the hippocampus proper.
BVCs were hypothesized to have a wide range of distance tunings such that a significant proportion would be expected to fire at some remove from the environmental boundary. However, initial evidence suggests the large majority of boundary cells have firing fields very close to the edges of the environment (i.e. they encode short boundary vectors). Currently, the spatial properties of subicular ‘BVCs’ [106,107], entorhinal ‘boundary cells’ [109], entorhinal and presubicular and parasubicular ‘border cells’ [108] appear to show some overlap. It may be conservative to regard them as belonging to a common functional category which, to avoid any anatomical implication, one could label as ‘boundary cells’.
In this issue, Stewart et al. [110] further investigate the issue of what constitutes a boundary. They show that subicular boundary cells respond to two major types of environmental boundaries: vertical surfaces and drop edges. Both present interruptions to the ground plane but generate very different sensory perceptions. Stewart et al. show that a majority of boundary cells treat walls and drop edges similarly. For instance, a cell exhibiting an extra field in the location predicted by the BVC model in response to an inserted vertical barrier, as in figure 2d, will probably show the predicted extra field in response to a newly created drop boundary. They conclude that the cells they report are specialized to code environmental boundaries and are well described by the BVC model. They also report a subvariant of boundary cells (‘boundary-off cells’) which clearly look like ‘inverse’ boundary cells (they fire everywhere except where a short-range boundary cell might fire). Taken together with interneuron-like boundary cells, these results show that environmental boundaries can act in an inhibitory manner, and may provide proof-of-concept for some models of detour behaviour and place-input-dependent grid cell formation [111].
The existence of boundary cells suggests that cues derived from environmental geometry are among the more important sources of external sensory information supporting cognitive mapping in the hippocampal formation. That environmental boundaries influence place cell firing has been evident for some time; increasingly, we are beginning to understand that environmental boundaries influence grid cells too [94,111,112]. It is worth briefly mentioning, though it is beyond this review's scope to discuss in detail: (i) the hippocampal formation seems particularly necessary for boundary-based, rather than landmark-based, spatial learning in humans [113–115] and (ii) boundary-based learning may follow different learning rules than landmark-based learning, although this is controversial [113,114,116–118].
(vi). Other spatial cells
The discovery of grid cells led to the development of several computational models of grid formation [38,119,120] described at different levels of complexity and detail, but with the common property that grid fields result from the summation of three sets of band-like inputs. The firing fields of each set of inputs would resemble parallel bands occurring throughout an environment (i.e. stripes) and the orientation of each band would be separated by 60° from the other two sets.
The periodic structure of MEC and parasubicular cells was investigated by Krupic et al. [121] using two-dimensional Fourier analysis to decompose the spatial firing patterns of each cell into periodic components. Grid cells constituted 26% of the cells they recorded (characteristically showing three periodic components of similar wavelength (scale) orientated a 60° intervals), but a further 44% showed other spatially periodic responses. These included a subset of cells which could be described in terms of a single periodic component (showing a firing field with parallel band-like features, consistent with the models). Yet, across the population recorded in an individual animal, cells’ tunings tended to be clustered around a small set of orientations and scales (common to both grid and periodic non-grid cells), suggesting that input to each MEC/parasubicular cell might be composed of a common, discrete set of band-like inputs. Grid cells showed more stable periodic tunings than other cells and, intriguingly, some cells that manifested a grid-like field in one environment showed a less grid-like field in another, again suggesting a link between properties of the environment and the locus of grid cell firing. These observations raise the question as to whether grid cells form a distinct class of spatially periodic cell, or whether they might constitute an exceptionally ordered and stable extreme within a continuum of cells which might combine periodic inputs at different orientations. In this issue, Krupic et al. [111] draw on these data to present a model that addresses the issue of environmental dependence of grid fields by incorporating information about environmental boundaries.
(vii). Conjunctive cells, time cells and object cells
For completeness, we should also mention a number of classes of cells whose firing properties do not fall neatly into the categories discussed above, but which may prove to play an important role in the function of the hippocampal formation (for example, in integrating different forms of spatial and non-spatial information to form new memories). First, we note that many of the spatial cells in the entorhinal cortex show degrees of both locational (grid, boundary) and directional (HD) information [76,122]. Indeed, in tasks that constrain the animal's movement to a set path (for example, along a track) hippocampal place cells (normally insensitive to direction) often become directional and fire only or principally in one direction [35,67]. Second, and perhaps relatedly, in tasks involving repeated actions along a fixed path where the action must be delayed in time with respect to an event or location, a subset of hippocampal pyramidal cells (‘time cells’) become attuned to specific delays or in some cases jointly signal both time and location/distance along the path [123,124].
(viii). ‘Spatial’ versus ‘non-spatial’ pathways?
In the preceding discussion of spatial cells, we have not mentioned the lateral entorhinal cortex (LEC) which forms a substantial input to the hippocampal formation. So, what is its role in spatial cognition, if any? The HD signal arriving via the dorsal presubiculum appears to underpin the spatial processing of the entire hippocampal formation and it is notable that there are strong projections from the presubiculum to the MEC but not to LEC. This alone could suggest that the MEC is more involved in map-like spatial processing than the LEC. Furthermore, MEC is preferentially reciprocally connected with the postrhinal/parahippocampal cortex (PHC), strongly implicated in visuospatial processing, whereas LEC is preferentially reciprocally connected with the perirhinal cortex, strongly implicated in item/object processing. The MEC and LEC projections to the dentate, CA fields and subiculum, and the ensuing projections from those regions in turn, form two broadly parallel pathways. As the first approximation, these observations seem to imply is that the MEC-related pathway is spatial, whereas the LEC-related pathway is a ‘non-spatial’ or ‘what?’ pathway, perhaps.
The concept of spatial and non-spatial pathways may be a useful shorthand, but it may also require some refinement. In this issue, Knierim et al. [125] argue that the spatial MEC versus non-spatial LEC dichotomy is too simple and that the LEC can provide spatial information, but that this is spatial in a different sense, using local frames of reference in contrast to the global frame of reference used by MEC. Interestingly, one function of the LEC is to code for the remembered locations of objects [125–127].
(ix). Hippocampal replay and preplay
Because place cells fire so consistently at specific locations, it is possible to reconstruct an animal's location based on place cell firing [64]. As the animal moves along a given trajectory, place cells fire in a reliable order. Remarkably, these spatially constrained trajectories can also be detected in brief bursts of firing that occur when the animal is stationary and during quiet wakefulness and sleep—a phenomenon termed ‘replay’ or ‘reactivation’ [128–131]. Replay events are synchronized with electroencephalogram (EEG) features (‘sharp waves’, ‘ripples’) and may run in either forward or in reverse [132] directions. An individual ‘virtual’ replay trajectory (e.g. place 1 through to place 10 from the beginning to end of a track) takes one or two orders of magnitude less time than that required to move through the actual trajectory in physical space (say a 2 m track). However, it should be noted that such ‘compression’ of a longer trajectory is not unique to sharp wave/ripple replay events. Longer trajectories are also effectively seen in a single theta cycle (approx. 125 ms duration in a rat) owing to the phenomenon of theta phase precession (see discussion in §2b(ii)). A single theta cycle recapitulates in a temporal sequence the spatial sequence of locations centred behind, on, and then ahead of, the moving animal. (These spatial sequences occur for space ‘nearby’ the rat and will be shorter than whole-track trajectories.) Such temporal compression phenomena render learning spatial sequences more amenable to the associative rules underlying long-term synaptic plasticity, such as pre-before-postsynaptic neuron spiking within relatively short time windows (say up to approx. 50 ms). Consonant with plasticity-enhancing temporal firing sequences, the standard functional interpretation of replay events is that they reinstantiate activity that occurred during recent behaviour, and support a consolidation function in learning and memory. Rehearsing routes offline might aid the formation of behaviourally valuable spatial maps, or aid in the transfer of rapidly acquired hippocampal spatial memories to brain regions outside the hippocampus (systems consolidation; see [133,134] for reviews).
A recent, and very welcome, development in the replay literature has been to incorporate behavioural learning. In this issue, for instance, Csicsvari & Dupret [135] review their elegant work in a ‘cheeseboard’ task involving new spatial goals each day, in which they show that sharp wave/ripple replay of goal locations in sleep predicts subsequent memory performance. Csicsvari & Dupret [135] survey the replay literature as a whole to emphasize the importance of replay events during waking behaviour as well as sleep, and argue that replay events stabilize new cognitive maps and adaptive spatial memories. Their review also considers functions for replay that go beyond consolidation in the simplest sense. For instance, a recent study has shown that sharp wave/ripple reactivation sequences in the awake rat often predict the future trajectory of the rat towards a desired goal [136]. In this issue, Dragoi & Tonegawa [137] discuss ‘preplay’, a related phenomenon [138,139], in which cell assemblies fire in a constrained sequence in advance of any relevant spatial experience. They argue that preplay suggests a degree of preconfiguration within the hippocampal spatial network and that patterns of place cell activity during novel spatial experiences may involve selection from among a set of pre-existing cell assemblies. These intriguing findings are potentially challenging for a straightforward experience-consolidation account of replay. However, they are consistent with earlier observations that forward replay events tend to precede the corresponding actions [140] and with the idea that the hippocampus may be involved in planning future behaviour as well as in representing the present and storing past experiences [141].
3. Current themes
(a). The relationship between grid cells, head direction cells, place cells and boundary cells
An important theme of current work is to understand the interactions between the various classes of spatial cell in the hippocampal formation; where do these signals encoding location, heading and environmental geometry arise, and how are they combined? The standard view of the relationship between grid cells and place cells has been that a given place cell is formed by a linear summation of different grid cells; many studies have modelled this relationship [37,142,143]. A useful feature of the grid cell code is that, despite its periodicity at the level of individual cells, a suitable combination of grids with different scales can provide a highly specific code for a given location, because their spatial phases only coincide rarely; linear summation of such grids would thus lead to the highly circumscribed, aperiodic firing characteristic of place cells.
At the time of their discovery, there were strong a priori grounds to regard grid cells as key causal contributors to the locational signal in hippocampus: grid cells were first discovered in superficial layers of MEC which form the major spatial input to the hippocampus (within which, of the spatial cells described above, only place cells are encountered). Indeed, it was this well-established anatomical relationship that had encouraged investigation of the spatial properties of MEC cells [144]. However as noted by Witter et al. [20], the traditional hierarchical view of the system is giving way to a more sophisticated understanding as spatial cells with similar characteristics are discovered elsewhere in the hippocampal formation.
Recent work ([145], reviewed in [144]) uses a new optogenetic technique to directly investigate the spatial functional properties of cells immediately downstream of the hippocampus. Viral transduction is used to label entorhinal cells afferent to place cells at a hippocampal injection site. The infected entorhinal cells express channelrhodopsin, such that they can be selectively activated by a specific wavelength of laser light. Spatial properties of the light-activated entorhinal cells are identified using conventional extracellular recording techniques and their connectivity with hippocampal target cells is inferred from the latency with which postsynaptic spikes are observed in the hippocampus following light stimulation of MEC. The results indicate that all classes of spatial cells (i.e. grid, HD and boundary cells) provide input to hippocampal neurons.
These findings confirm that, in adult animals, grid cells provide an important input to hippocampus. However, it is also clear that other spatial cells contribute to this pathway. Thus, to the extent that grid cells do play a role in governing the locational signal in the hippocampus proper, they may not do so alone. Indeed, there is increasing evidence to suggest that the other spatial inputs to the hippocampus may be equally fundamental.
First, recent work ([146,147] reviewed by Wills et al. in the current issue [148]) has begun to address the development of spatial properties in different classes of spatial cell in the hippocampal formation. Wills et al. provide a wide-ranging review of the development of spatial representation and behaviour in rodents, posing several searching questions. For example, do hippocampal place cells’ spatial properties depend on the pre-existence of grid cells in the entorhinal cortex? In fact, the evidence indicates that place cells are already established before stable grid fields are apparent in MEC, suggesting that place cells must receive other, earlier, spatial inputs, possibly from HD and boundary cells.
Second, inactivation of the medial septum strongly disrupts grid cell firing patterns, while the properties of place cells and HD cells (and seemingly boundary cells) are relatively unperturbed [149]. Interestingly, inactivation of the hippocampus also makes grid cells lose their gridness [150]. To what extent this disruption is a specific consequence of the removal of place cell locational signals remains to be shown, but it is clearly evidence, if anything, in favour of the dependence of grid cells upon place cell firing.
In this issue, Krupic et al. [111] propose a speculative model of grid cell formation in which grid cell properties depend upon place-like signalling and upon inhibition from environmental boundaries; in other words, grid cells represent a form of place cell output. Another theoretical approach [29,151] has been to assume that boundary cells determine the location and shape of place fields in relation to environmental boundaries [53], and that place cells then anchor grid cells to the environment. In this model, therefore, the relationship between environmental boundaries and grid cells is indirect. An alternative view of boundary cells is that they directly anchor grid cells to environmental boundaries [108,112]. One of the reasons the boundary-to-grid relationship is important is that pure path integration processes rapidly accumulate error. External environmental boundaries potentially provide a valuable error-correction mechanism.
(b). Modelling mechanisms of spatial representation
(i). Attractor models of spatial representation
The spatial responses of individual neurons in the hippocampal formation potentially provide the basis for a neural code for variables such as location, heading and speed. Computational models are required to understand the way in which these spatial codes might be derived from more fundamental signals and how they interact with each other and with non-spatial information to form spatial memories and to guide complex spatial behaviour. The ordered anatomy of the hippocampus made it an early target for computational modelling and there was already an extensive literature, pre-dating the discovery of grid cells [89], addressing the mechanisms of spatial representation and memory in the hippocampus. However, the discovery of detailed and ordered spatial code in the grid cells of the MEC added important new impetus [152]. While it is beyond the scope of the current article to review the earlier modelling work in detail, we outline some key concepts (continuous attractors, ring attractors) derived from early modelling approaches that are necessary to follow current research in the area.
One of the most important insights originates with Marr [14], who pointed out that the recurrent collateral connections of the CA3 field provided an ideal substrate for an associative memory system, capable of storing patterns of cortical input and recovering such memories from degraded cues (pattern completion). CA3 pyramidal cells not only project to CA1 (Schaffer collaterals, the feed-forward projection), but also to themselves (the recurrent collaterals). Marr assumed synaptic plasticity between members of the set of CA3 neurons that fired together during a particular event (‘cells that fire together, wire together’). Under this assumption, the initial firing of only some members of that set (corresponding to the presentation of partial cues) would subsequently tend to trigger the firing of the other members of that set, such that eventually all the cells corresponding to the original event fired (corresponding to retrieval of the entire event). Thus, the network will tend to evolve towards one of these stable stored states, known as ‘attractors’. A special type of attractor is the continuous attractor, in which the stable states are not discrete but form a continuous manifold—that is, depending on its input (or potentially its internal connectivity) the network can move through a family of such stable states without encountering any ‘barrier’—it is argued (e.g. in [37,78]) that this type of representation is ideal for the representation of continuous variables (such as location and direction).
To date, one of the most promising applications of continuous attractors is in modelling the HD system [78]. Here, the periodicity of directional information, combined with the stability of the relative HD tunings of different cells under cue rotation are suggestive of a specific type of continuous attractor mechanism, known as the ring attractor. Neurons representing different headings can be visualized as if organized into a ring in which the strength of connections between any pair of neurons is a function of the angular difference between their preferred directions. With appropriately chosen symmetrical connections, short-range excitation establishes a stable ‘bump’ of activity at a particular location in the ring, representing the current heading. Because the attractor is continuous, the bump can be moved smoothly around the ring by asymmetric interactions whose strength relative to the symmetric interactions reflects the angular velocity of the head. The ring attractor has become the dominant model of the HD system and related periodic continuous attractors are incorporated into a number of models of the grid system.
In an empirical investigation in this issue, Knight et al. [88] set up a conflict between different sets of cues and tested predictions derived from a ring attractor model against those of an alternative Bayesian cue integration model. The results were consistent with attractor models when conflicts were small (‘follow visual cue’) and with Bayesian cue integration models when conflicts were large (‘follow weighted average of the two cue sets’). Importantly, experience altered the responses. In Knight et al. and the accompanying computational study [153], the authors suggest that the results can be explained by incorporating short-term and long-term plasticity effects into a ring attractor network. Interestingly, these results in HD cells follow similar findings in the orientation of the place cell representation [154].
(ii). Models of grid field formation
Much of the recent theoretical work on spatial representation has focused on the mechanism of grid field formation. Early models fell into two distinct categories, operating at different levels of description:
One type of model stressed the role of intercellular interactions in establishing the spatial pattern of grid cell firing and ensuring its subsequent stability by forming a continuous attractor network (CAN). CAN models had originally been proposed to explain place field firing [155,156]. In these models, a ‘bump’ of activity, this time representing the animal's two-dimensional location, is shifted by an asymmetric input determined by the animal's running speed. With the inclusion of periodic boundary conditions (analogous to those seen in ring attractors) [155], such mechanisms might account for the regularity and stability of grid fields [157,158].
With appropriately chosen lateral connections (forming a Mexican-hat function; local connections excite neighbouring cells while more distant connections are weakly inhibitory), a topographically organized population would spontaneously form a stable triangular grid-like pattern of activity across the cortex (the mechanisms of this self-organizing process are similar to those identified by Alan Turing in a seminal paper on the chemical basis of morphogenesis in biology [159], so this system is sometimes called a ‘Turing layer’). As in the earlier place cell models, this grid-like pattern could then be smoothly shifted across the cortical surface driven by velocity modulated input, with the result that the firing of any given neuron would peak at a grid of spatial locations [157]. One difficulty with this initial suggestion was that it suggests a cortical patterning of activity (neighbouring cells share similar spatial phases), which is not observed experimentally. However, this does not rule out the possibility that a continuous attractor could be established using non-local lateral connectivity. One proposal is that the appropriate non-local connectivity could be established by early postnatal learning [37]. The idea is that a ‘hard-wired’ Turing layer outside MEC provides a training input to the MEC. This patterned input enforces a periodic structure on the developing spatial representation in MEC. Competitive interactions between MEC cells ensure that different neurons come to represent different spatial phases, and Hebbian learning between MEC neurons then establishes long-range coupling between neurons sharing similar inputs from the Turing layer as required for continuous attractor dynamics.
While CAN models aim to explain the form of grid fields in terms of grid cell interactions, another type of model focuses on temporal properties of grid and place cell firing and the relationship between the timing of action potentials and the ongoing theta oscillation that dominates the hippocampal formation, especially during active motion. Several experimental phenomena link theta to functional properties of spatial cells in the hippocampal formation, including spatial representation, learning and memory. The most salient is the theta phase precession phenomenon [28,36]. Since its discovery in place cells, it had been speculated that spatial localization of action potentials might result from interference between theta oscillations in the local field potential and intrinsic oscillations within each place cell. Because the theta frequency is linearly dependent on running speed, the resulting ‘beating’ interference could produce localized fields. However, this beating activity would be expected to result in spatially periodic fields, in contrast to the typically aperiodic character of hippocampal place fields [160]. The discovery of (periodic) grid cells, naturally reinvigorated interest in oscillatory interference (OI); in order to account for the regularity of grid cells two-dimensional fields, they would require input from multiple velocity-controlled oscillators, each modulated by movement in a particular grid direction (i.e. at 60° intervals) [29].
The observation of grid cells had inspired two distinct computational accounts, and in turn the models’ predictions stimulated a burst of experimental research. In the past 5 years, remarkable technical advances have allowed researchers to record membrane potentials from isolated cells in behaving animals for the first time [161,162]. OI models received some support from a pioneering investigation of intracellular dynamics of place cells that showed the predicted interference of intrinsic oscillations in the theta band with those of the LFP [161]. However, results from a similar study in grid cells were more challenging for the OI account: grid fields were found to coincide with slow, ramping depolarizations [163], which it was argued were more consistent with the collective activity of a continuous attractor. So while spike timing in grid cells and place cells alike appeared to depend critically on intracellular theta, the signal driving the lattice-like location of grid fields appeared to have a different origin. CAN models appeared more consistent with the observed ramping depolarizations but did not address spike timing phenomena. By contrast, early OI models regarded theta phase precession as an essential element in the formation of grid fields, but did not simulate intercellular interactions, while noting that they might play a part in maintaining the stability of spatial responses across the population as a whole [38].
In the current issue, several authors point out that CAN and OI models are not incompatible and that a hybrid model which incorporates both mechanisms would address intercellular interactions and temporal properties simultaneously [99] (see also [164]). Indeed, Blair et al. [41] put forward one such model in which populations of interconnected theta cells form ring attractors within which the phases of theta bursts are modulated by the velocity of movement. The joint activity of these cells constitutes a synchrony code for location and, when they converge on a grid cell, both spatial periodicity and temporal properties are captured. More generally, recent studies investigating the part played by intracellular dynamics and temporal properties in generating and constraining spatial signals have brought a new, finer focus to models which are specified at increasingly detailed level. Hasselmo [42] investigates the phenomenon of theta phase skipping, wherein the activity of distinct subgroups of grid cells (and interneurons) alternates between theta cycles. The model provides a new network-level explanation of periodic spatial firing and theta phase skipping based on an OI-like mechanism and dependent upon cells’ intrinsic resonance, connecting with earlier work linking resonance to the spatial scale of grid fields [92]. Interestingly, the model provides an interference based explanation for spatial periodicity even in cells with low-frequency resonance (corresponding to slow, subtheta-band-intrinsic oscillations as reported in bats and humans [45,165,166]).
Continuous attractor and OI mechanisms provide some insight into how cellular and subcellular interactions could give rise to the remarkable regularity of grid fields, but they do not explain how the observed range of grid cells might come into existence and, in particular, how the distribution of grid cell tunings (scales, orientations) might emerge in a population of cells. Another strand of modelling work has stressed self-organizing processes that could underlie the development of network-level attractors and the emergence of specific tunings of grid cells. Grossberg & Pilly in this issue [167] review recent work which develops these themes, again exploiting velocity-driven ring attractor mechanisms, but focusing on self-organizing principles to provide an account for the formation of grid fields based on band-like inputs [38,121], the selection of particular grid scales and the development of grid modules. Linking functional, anatomical observations of grid cells to their computational properties, Brecht et al. [168] address the origin of grid field periodicity in a very different way by raising the intriguing hypothesis that it may arise from an isomorphic hexagonal organization of calbindin-positive patches in MEC.
To date, most modelling work has naturally been concerned with mechanisms underlying the settled periodicity of grid fields in fixed environments, but there is growing evidence that grid fields, and in particular their scales, orientations and angular symmetry are sensitive to geometric change [94], environmental novelty [100] and behavioural factors [112]. Ultimately, grid cell models will need to accommodate these findings. Towse et al. [97] investigate the idea that the expansion in grid scale observed when animals encounter new environments may be explained as an optimal response to spatial uncertainty, minimizing the effects of spatial inconsistency between the locations represented by different modules of grid cells.
The grid cell phenomenon may have wider implications for neuroscience. The strikingly periodic representation of non-periodic variables (two-dimensional spatial location) is as yet unparalleled elsewhere in the nervous system. This type of code has some powerful and distinctive computational properties [95–97,169]. In several current models, periodicity is seen as a robust property of a dynamic CAN, which may have some functional value in forming a coherent and stable population response in the face of diverse multi-modal inputs of varying reliability. In particular, periodicity means that grid cells can form a continuous and isotropic representation of space while extending over an arbitrary interval. It seems possible that such properties may have applications to other continuous variables, and one wonders whether non-spatial ‘grids’ may be detected elsewhere in the brain.
(iii). Novel methods and increasing integration
Understanding of the detailed functional properties of the hippocampal formation has benefitted greatly from recent technical advances which have allowed manipulation and measurement of cellular activity at an unprecedentedly microscopic resolution. Recording neural activity in awake behaving animals is necessary in order to expose the spatial correlates of activity in the hippocampal formation, but until fairly recently this was only possible using extracellular techniques where the location of the electrodes can only be coarsely estimated through (postmortem) histology. Now, however, whole cell recording techniques can be used in vivo to isolate and target identifiable cells with specific anatomical and electrophysiological characteristics and can also be carried out in awake behaving, and even freely moving, animals [170,171]. Drawing on an approach first used to facilitate human neuroimaging studies of spatial behaviour, virtual reality can be used to present realistic visual–spatial cues as an experimental animal runs on a rollerball [172,173]. This allows for complex spatial behaviours (and, potentially, drastic environmental manipulations) while maintaining the stability of the head in the recording apparatus. Studies combining virtual reality with intracellular electrophysiology are generating new insights into the detailed anatomy and subcellular structure of spatial representation in the hippocampal formation [161,163,164]. Such methods can, in principle, be combined with optical imaging and microscopy to resolve correlates of subcellular activity at the level of individual dendrites and synapses [174]. This will allow characterization of physical growth processes involved in learning and memory.
Another very important technical advance has been the development of optogenetics. This exciting technique [175] permits neurons to be selectively labelled, and then reversibly activated or inactivated using laser application of specific wavelengths of light. As noted earlier, the technique is already being applied to the characterization of functional connections within the hippocampal formation [144], but it opens up many more possibilities for research into the causal role of different parts of the hippocampal formation in supporting specific spatial behaviours and memories. Work by Tonegawa and co-workers [176] indicates that optogenetics may be used to selectively label and later reactivate the sparse ensemble of hippocampal neurons involved in the encoding of a specific fear memory, eliciting ‘freezing’ behaviour (normally specific to the spatial context in which it was acquired) at a new environment. There is great potential to build on this approach in understanding the basis for spatial and episodic memory (or its putative analogue in animals, ‘episodic-like’ memory [177]). Our growing understanding of spatial representations in the hippocampal formation should make it possible to target and manipulate specific spatial-context-specific memories and behaviours, and show how different cell types, ensembles and brain structures are causally involved in episodic encoding, storage and retrieval.
(iv). Spatial representation in non-rodent species
Most of our current understanding of the neuronal representations of space in the hippocampal formation are based on experiments in rats and mice. Cells with spatial properties corresponding to those of place cells, HD cells and grid cells have now all been reported in primates [178–180]. However, methods established in rodents for extracellular recording during free movement around an enclosure or along a track are not practical in primates. As a result, these studies tend to produce sparser data which make the unambiguous characterization of detailed spatial correlates of spiking activity and intercellular interactions much more difficult. There may also be significant differences in the forms of sensory information available to different mammalian groups. For instance, primates can perform a lot of useful spatial exploration purely visually, using eye movements as an alternative to locomotion. This has implications for primate processing based upon ‘spatial view’ [180,181].
A more recent suggestion of a species difference concerns theta, and thus, potentially, self-motion processing. In rats and mice, active navigation is often associated with a strong theta oscillation, a phenomenon linked to spatial representation through the phase precession effect and theoretically important in a class of models of grid cell formation. However, grid cells can be identified in bats in the absence of such oscillations [182] (but see also [183]). It will be important to establish cross-species similarities and differences in the relationship between locomotion speed and theta. One way to achieve this will be to compare theta oscillations across different species in similar circumstances, for example, comparing theta power during navigation in virtual reality in mice [173] and in humans [44,166,184].
(v). Human spatial cognition: functions of the hippocampal formation and wider navigation network
One of the central motivations for animal research on the hippocampal formation is to shed light on its function in the human brain. Although this issue focuses on the spatial functions of the system, the hippocampus has a more general role in memory. For example, atrophy in the hippocampal formation is often an early feature in the progression of Alzheimer's disease [185], and patients with damaged hippocampi often have difficulty in forming new, enduring memories of personally experienced events [186,187]. This general episodic amnesia coexists with marked deficits in spatial orientation and navigation [188]. Consistent with the omnidirectional nature of place cell firing in the open field in rats and humans [67,189] the hippocampus particularly supports view-independent and allocentric spatial memory [190,191]. Spatial function is also dependent on those medial temporal and parietal regions through which the hippocampus receives its input: damage to the posterior part of the parahippocampal gyrus (known as parahippocampal cortex or PHC) leading to topographical disorientation, a specific impairment of spatial memory and behaviour [192], while damage to the retrosplenial/medial parietal region also leads to navigational problems [193–195].
Understanding the basis of spatial cognition in the intact human brain has been advanced through functional neuroimaging. Techniques such as functional magnetic resonance imaging (fMRI) have limited temporal and spatial resolution, but do allow for the imaging of the whole brain, so that the role of different structures can be investigated on the millimetre scale.
Responses to static visual stimuli (spatial scenes including images of rooms, buildings, landscapes and so on) have proved revealing, with regions of the ventral, medial parietal and lateral occipital neocortex found to be selectively responsive to different forms of visual stimulus. An area of PHC lying close to the posterior collateral sulcus (the ‘parahippocampal place area’ (PPA) [196]) is characteristically active in the processing of static spatial scenes and, for example, in sections of a movie which depict places [197]. Scene selective activity is also observed on the lateral surface of the occipital cortex and on the medial surface in the region of retrosplenial cortex (RSC), often extending into adjacent medial parietal, medial temporal and posterior parietal cortex. Although these regions were initially identified in studies of high-level visual processing, early evidence indicated that they were specifically involved in spatial processing. For example, the PPA is sensitive to spatially organized scenes but not to visually similar scenes in which the same elements have been scrambled [196]. Interestingly, although the PPA is normally unresponsive to images of isolated objects, it is more active for ‘landmark’ objects which have previously been seen at navigationally significant locations [198].
Neuroimaging investigations of spatial cognition have also explored more active spatial tasks, implicating the hippocampal formation, consistent with the cell-level literature outlined above. For example, activity during a wayfinding task, which requires participants to find new accurate routes within a familiar virtual environment, can be compared to the activity seen during a task where participants follow a visible trail. Here, activity in the hippocampus is associated with accurate navigation, with more accurate navigators showing greater hippocampal activity during a wayfinding task [199,200]. These studies characteristically also show navigation-related activation encompassing posterior and medial regions (including RSC) and PHC (including PPA). It has been argued that this ‘core network’, centred on the hippocampus, is not unique to navigation but is also active during tasks that involve spatial imagery (for example, imagining another person's point of view, retrieving episodic memories), and thus subserving a more general ‘self-projection’ or ‘scene construction’ function [12,201–205].
Early fMRI studies focused on univariate task comparisons, which reveal regions that are more active during one task than another, and on correlations between behaviour and voxel-level neural activity. More recent advanced techniques, such as fMR-adaptation and multi-voxel pattern analysis, now permit the computational properties of to be probed in more detail. For example, one study showed that scene categories (for example, beach and building) can be decoded from patterns of activity in the PPA in response to static images [206]. One promising avenue for current research is to determine whether spatial parameters, such as heading and location, can be decoded from the activity within different parts of the core spatial network. Epstein & Vass [207] review some recent work from the Epstein laboratory using these techniques to investigate the complementary functional roles of regions within and beyond the hippocampal formation. For example, they show that the retrosplenial/medial parietal region might encode information about locations and directions within well-learned, familiar environments.
The non-invasive character of fMRI makes it an ideal technique for investigating function in healthy human participants, but the spatial resolution of the technique is limited to (at best) around 1 mm, making it difficult to directly investigate the cell-level properties which have been so critical to the study of spatial representation in animals. However, the organization of the grid cell system may make some of these properties accessible at the macroscopic level, rather as molecular structures are revealed in analyses of macroscopic properties of crystals. Preliminary evidence came from a study by Doeller et al. [208]. They investigated the grid system in humans using fMRI by analysing activity during a virtual spatial memory task in which participants explored a circular arena locating objects. In entorhinal cortex, the degree of activation was modulated by the direction of movement, with peak activity observed when participants moved parallel to six axes separated by 60°, similar to the axes of grid fields which appear to be clustered throughout entorhinal cortex in rats [94,98]. An advantage of fMRI over cellular electrophysiology is that it allows activity to be imaged across the whole brain. Interestingly, for instance, the sixfold directional signature suggestive of grid system activity was seen beyond the hippocampal formation in a network typically associated with autobiographical memory retrieval.
More direct evidence of spatial representation in the human hippocampal formation comes from studies involving intracranial electrophysiology. The implantation of depth or surface electrodes is sometimes required prior to surgery for the treatment of pharmacologically intractable epilepsy in order to identify the origin of the seizure. When microelectrodes are implanted within the brain, it is possible to record not only EEG but also the spiking activity of individual neurons as participants undertake spatial tasks. Place responsive cells with properties that resemble rats’ place cells have been found in the human hippocampal formation [189]. A proportion of cells were also found to be modulated according to the current goal in a virtual navigation task, and some cells were sensitive to specific objects or views. In a separate virtual navigation study [209], cells in entorhinal cortex (termed ‘path cells’) showed spatially distributed firing patterns which were modulated by the direction of movement (clockwise or anticlockwise). Excitingly, Jacobs et al. [165] have recently reported cells with grid-like firing in humans during virtual navigation. While many characteristics of spatial cells seen in animals have been observed in humans, these studies also point up potential differences. For instance, in this issue, Jacobs [44] argues that the dominant oscillation in humans is at a lower frequency than that in rodents. A similar view has also been argued by Watrous et al. [166], who find that human hippocampal rhythmicity is centred around ca 3 Hz while that of rats is centred around ca 8 Hz.
If something akin to the rodent grid system exists in humans, it will be interesting to see to what extent its properties (such as scale, orientation coherence and graded topographical organization and modularity) are preserved. Practical limitations to electrophysiology in humans may make detailed population-level analyses impractical, but the unique macroscopic properties of the grid system may make them accessible to fMRI [208]. More generally, we might expect human neuroimaging, with its larger scope, to contribute further to the bigger picture, helping to reveal the modular and topographical structure, macroscopic functions and interactions of spatial systems within and beyond the hippocampal formation. It seems likely that, as with other classes of spatial cell [165,189], boundary cells may exist in humans, but this remains to be demonstrated. Establishing the existence and properties of boundary, geometry and distance-related processing in humans is a potential target for future neuroimaging research [114,115], where it may make contact with the growing literature which addresses related processes from the standpoint of human and computer vision [206,210–213].
Overall, results from human neuropsychology, neuroimaging and electrophysiology strongly suggest an evolutionary continuity spanning mammalian species and implicating the hippocampal formation and its cortical inputs in allocentric spatial processing in rodents, primates and humans alike.
4. Conclusion
The study of spatial cognition and the function of the hippocampal formation are proving to be a particularly successful example of integrative neuroscience. It is a field in which we can point to substantive empirical and theoretical links at every stage between the most elemental mechanisms and their most macroscopic manifestations in behaviour and cognition. What is the secret of this successful integration? First, the critical central phenomena, the observation and characterization of the spatial cells of the hippocampal formation, provide a well-established and theoretically grounded coupling of neural firing to behaviour [54,75,89,107–109]. Second, the field benefits from a solid foundation in anatomy [16,214]. Third, there is an extensive, solid literature connecting anatomy to learning, memory and behaviour [17,215]. Fourth, spatial neuroscience has an unusually mature, quantitative and mechanistic theoretical basis in computational modelling [14,37,38,78,85,95,120,155,216] which acts to connect empirical phenomena at one level to those at another and to generate new testable predictions. Perhaps most important of all, in the analysis of the brain basis of complex behaviour, the field of spatial cognition benefits from a focus on functions which appear to be largely preserved across vertebrate species including humans. Insights with their bases in the fundamental neuroscience of the hippocampal formation in animals have proved to generalize to the mechanisms of human cognition in demanding and naturalistic tasks such as navigation. These foundations have provided the theoretical and empirical basis for targeted and connected investigations of spatial cognition ranging from the level of molecular biology and genetics to the behaviour and brain activity of entire organisms, including humans.
If anything, the future looks even more exciting than the past: the combination of well-established techniques, such as single unit recording with powerful new approaches in optical imaging, optogenetics, intracellular electrophysiology and human neuroimaging, should lead to even stronger links between molecular, anatomical, neural, systems, behavioural and cognitive neuroscience. In turn, this process is already driving new discoveries, invigorating the field with fresh questions which will continue to contribute, we believe, not just to a deeper understanding of spatial cognition, but also towards integrated theories of the mechanisms of learning, memory and neural representation more generally.
Acknowledgements
We thank the Royal Society, the Wellcome Trust and Gatsby Foundation for supporting the Theo Murphy meeting which led to this special issue.
Funding statement
The authors acknowledge the support of the BBSRC, the Wellcome Trust, EU SpaceCog and MRC for funding research described here. Work leading to this issue was supported by a University of York Anniversary Lectureship award to T.H.
References
- 1.Hubel DH, Wiesel TN. 1962. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaas JH, Nelson RJ, Sur M, Lin C-S, Merzenich MM. 1979. Multiple representations of the body within the primary somatosensory cortex of primates. Science 204, 521–523. ( 10.1126/science.107591) [DOI] [PubMed] [Google Scholar]
- 3.Georgopoulos AP, Schwartz AB, Kettner RE. 1986. Neuronal population coding of movement direction. Science 233, 1416–1419. ( 10.1126/science.3749885) [DOI] [PubMed] [Google Scholar]
- 4.Colby CL. 1998. Action-oriented spatial reference frames in cortex. Neuron 20, 15–24. ( 10.1016/S0896-6273(00)80429-8) [DOI] [PubMed] [Google Scholar]
- 5.Andersen RA. 1997. Multimodal integration for the representation of space in the posterior parietal cortex. Phil. Trans. R. Soc. Lond. B 352, 1421–1428. ( 10.1098/rstb.1997.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess N, Jeffery KJ, O'Keefe J. 1999. Intergrating hippocampal and parietal functions: a spatial point of view. In The hippocampal and parietal foundations of spatial cognition (eds N Burgess, KJ Jeffery, J O'Keefe), pp. 3–29. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Milner AD, Goodale MA. 1995. The visual brain in action. New York, NY: Oxford University Press. [Google Scholar]
- 8.Salinas E, Abbott L. 1996. A model of multiplicative neural responses in parietal cortex. Proc. Natl Acad. Sci. USA 93, 11 956–11 961. ( 10.1073/pnas.93.21.11956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouget A, Deneve S, Duhamel J-R. 2002. A computational perspective on the neural basis of multisensory spatial representations. Nat. Rev. Neurosci. 3, 741–747. ( 10.1038/nrn914) [DOI] [PubMed] [Google Scholar]
- 10.Tolman EC. 1948. Cognitive maps in rats and men. Psychol. Rev. 55, 189–208. ( 10.1037/h0061626) [DOI] [PubMed] [Google Scholar]
- 11.O'Keefe J, Nadel L. 1978. The hippocampus as a cognitive map. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Burgess N, Becker S, King JA, O'Keefe J. 2001. Memory for events and their spatial context: models and experiments. Phil. Trans. R. Soc. Lond. B 356, 1493–1503. ( 10.1098/rstb.2001.0948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner AD, Dijkerman HC, Carey DP. 1999. Visuospatial processing in a case of visual form agnosia. In The hippocampal and parietal foundations of spatial cognition (eds N Burgess, KJ Jeffery, J O'Keefe), pp. 443–466. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Marr D. 1971. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B 262, 23–81. ( 10.1098/rstb.1971.0078) [DOI] [PubMed] [Google Scholar]
- 15.Doherty A. 1999. MRC Centre for Synaptic Plasticity, University of Bristol available at http://www.bristol.ac.uk/synaptic/pathways/ (accessed 27 August 2013).
- 16.Cajal SRY. 1955. Histologie du systeme nerveux de l'homme & des vertebres. Consejo Superior de Investigaciones Cientificas, Instituto Ramon y Cajal. [Google Scholar]
- 17.Bliss TV, Lomo T. 1973. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner-Medwin AR. 1976. The recall of events through the learning of associations between their parts. Proc. R. Soc. Lond. B 194, 375–402. ( 10.1098/rspb.1976.0084) [DOI] [PubMed] [Google Scholar]
- 19.Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J. 2006. The hippocampus book. Oxford, UK: Oxford University Press. [Google Scholar]
- 20.Witter MP, Canto CB, Couey JJ, Koganezawa N, O'Reilly KC. 2014. Architecture of spatial circuits in the hippocampal region. Phil. Trans. R. Soc. B 369, 20120515 ( 10.1098/rstb.2012.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube JS. 1998. Head direction cells and the neuropsychological basis for a sense of direction. Prog. Neurobiol. 55, 225–256. ( 10.1016/S0301-0082(98)00004-5) [DOI] [PubMed] [Google Scholar]
- 22.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. 2013. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333, 353–357. ( 10.1126/science.1204622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderwolf CH. 1969. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 26, 407–418. ( 10.1016/0013-4694(69)90092-3) [DOI] [PubMed] [Google Scholar]
- 24.Jeewajee A, Lever C, Burton S, O'Keefe J, Burgess N. 2008. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 18, 340–348. ( 10.1002/hipo.20394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasselmo ME, Bodelon C, Wyble BP. 2002. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817. ( 10.1162/089976602317318965) [DOI] [PubMed] [Google Scholar]
- 26.Easton A, Douchamps V, Eacott M, Lever C. 2012. A specific role for septohippocampal acetylcholine in memory? Neuropsychologia 50, 3156–3168. ( 10.1016/j.neuropsychologia.2012.07.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douchamps V, Jeewajee A, Blundell P, Burgess N, Lever C. 2013. Evidence for encoding versus retrieval scheduling in the hippocampus by theta phase and acetylcholine. J. Neurosci. 33, 8689–8704. ( 10.1523/JNEUROSCI.4483-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. ( 10.1002/hipo.450030307) [DOI] [PubMed] [Google Scholar]
- 29.Burgess N, O'Keefe J. 2011. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 21, 734–744. ( 10.1016/j.conb.2011.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivas J, Gaztelu J, Garcia-Austt E. 1996. Changes in hippocampal cell discharge patterns and theta rhythm spectral properties as a function of walking velocity in the guinea pig. Exp. Brain Res. 108, 113–118. ( 10.1007/BF00242908) [DOI] [PubMed] [Google Scholar]
- 31.Slawinska U, Kasicki S. 1998. The frequency of rat's hippocampal theta rhythm is related to the speed of locomotion. Brain Res. 796, 327–331. ( 10.1016/S0006-8993(98)00390-4) [DOI] [PubMed] [Google Scholar]
- 32.Lever C, Jeewajee A, Burton S, O'Keefe J, Burgess N. 2009. Hippocampal theta frequency, novelty, and behavior. Hippocampus 19, 409–410. ( 10.1002/hipo.20557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinman JR, Penley SC, Long LL, Escabi MA, Chrobak JJ. 2011. Septotemporal variation in dynamics of theta: speed and habituation. J. Neurophysiol. 105, 2675–2686. ( 10.1152/jn.00837.2010) [DOI] [PubMed] [Google Scholar]
- 34.Wells CE, Amos DP, Jeewajee A, Douchamps V, Rodgers J, O'Keefe J, Burgess N, Lever C. 2013. Novelty and anxiolytic drugs dissociate two components of hippocampal theta in behaving rats. J. Neurosci. 33, 8650–8667. ( 10.1523/JNEUROSCI.5040-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNaughton BL, Barnes CA, O'Keefe J. 1983. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp. Brain. Res 52, 41–49. ( 10.1007/BF00237147) [DOI] [PubMed] [Google Scholar]
- 36.Jeewajee A, Barry C, Douchamps V, Manson D, Lever C, Burgess N. 2014. Theta phase precession of grid and place cell firing in open environments. Phil. Trans. R. Soc. B 369, 20120532 ( 10.1098/rstb.2012.0532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B. 2006. Path integration and the neural basis of the 'cognitive map’. Nat. Rev. Neurosci. 7, 663–678. ( 10.1038/nrn1932) [DOI] [PubMed] [Google Scholar]
- 38.Burgess N, Barry C, O'Keefe J. 2007. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812. ( 10.1002/hipo.20327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welday AC, Shlifer IG, Bloom ML, Zhang K, Blair HT. 2011. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16 157–16 176. ( 10.1523/JNEUROSCI.0712-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgess N. 2008. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus 18, 1157–1174. ( 10.1002/hipo.20518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair HT, Wu A, Cong J. 2014. Oscillatory neurocomputing with ring attractors: a network architecture for mapping locations in space onto patterns of neural synchrony. Phil. Trans. R. Soc. B 369, 20120526 ( 10.1098/rstb.2012.0526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasselmo ME. 2014. Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex. Phil. Trans. R. Soc. B 369, 20120523 ( 10.1098/rstb.2012.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser EI, Moser M-B, Roudi Y. 2014. Network mechanisms of grid cells. Phil. Trans. R. Soc. B 369, 20120511 ( 10.1098/rstb.2012.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs J. 2014. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Phil. Trans. R. Soc. B 369, 20130304 ( 10.1098/rstb.2013.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heys JG, MacLeod KM, Moss CF, Hasselmo ME. 2013. Bat and rat neurons differ in theta-frequency resonance despite similar coding of space. Science 340, 363–367. ( 10.1126/science.1233831) [DOI] [PubMed] [Google Scholar]
- 46.Barry C, Doeller CF. 2013. 3D mapping in the brain. Science 340, 279–280. ( 10.1126/science.1237569) [DOI] [PubMed] [Google Scholar]
- 47.Buzsaki G. 2002. Theta oscillations in the hippocampus. Neuron 33, 325–340. ( 10.1016/S0896-6273(02)00586-X) [DOI] [PubMed] [Google Scholar]
- 48.Buzsaki G. 2009. Rhythms of the brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 49.Somogyi P, Katona L, Klausberger T, Lasztóczi B, Viney TJ. 2014. Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus. Phil. Trans. R. Soc. B 369, 20120518 ( 10.1098/rstb.2012.0518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuseki K, Buzsaki G. 2014. Theta oscillations decrease spike synchrony in the hippocampus and entorhinal cortex. Phil. Trans. R. Soc. B 369, 20120530 ( 10.1098/rstb.2012.0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. 2007. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol. Learn. Mem. 87, 9–20. ( 10.1016/j.nlm.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 52.Jezek K, Henriksen EJ, Treves A, Moser EI, Moser M-B. 2011. Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246–249. ( 10.1038/nature10439) [DOI] [PubMed] [Google Scholar]
- 53.Hartley T, Burgess N, Lever C, Cacucci F, O'Keefe J. 2000. Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369–379. () [DOI] [PubMed] [Google Scholar]
- 54.O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. ( 10.1016/0006-8993(71)90358-1) [DOI] [PubMed] [Google Scholar]
- 55.Jung MW, Wiener SI, McNaughton BL. 1994. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 14, 7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser M-B. 2008. Finite scale of spatial representation in the hippocampus. Science 321, 140–143. ( 10.1126/science.1157086) [DOI] [PubMed] [Google Scholar]
- 57.Muller RU, Kubie JL. 1987. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 7, 1951–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bostock E, Muller RU, Kubie JL. 1991. Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1, 193–205. ( 10.1002/hipo.450010207) [DOI] [PubMed] [Google Scholar]
- 59.Sharp PE. 1997. Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav. Brain Res. 85, 71–92. ( 10.1016/S0166-4328(96)00165-9) [DOI] [PubMed] [Google Scholar]
- 60.Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. 1998. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280, 2121–2126. ( 10.1126/science.280.5372.2121) [DOI] [PubMed] [Google Scholar]
- 61.Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J. 2002. Long-term plasticity in the hippocampal place cell representation of environmental geometry. Nature 416, 90–94. ( 10.1038/416090a) [DOI] [PubMed] [Google Scholar]
- 62.Knierim JJ. 2002. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J. Neurosci. 22, 6254–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. 2004. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305, 1295–1298. ( 10.1126/science.1100265) [DOI] [PubMed] [Google Scholar]
- 64.Wilson MA, McNaughton BL. 1993. Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058. ( 10.1126/science.8351520) [DOI] [PubMed] [Google Scholar]
- 65.Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ. 1998. Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J. Neurophysiol. 79, 1017–1044. [DOI] [PubMed] [Google Scholar]
- 66.O'Keefe J, Conway DH. 1978. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp. Brain Res. 31, 573–590. ( 10.1007/BF00239813) [DOI] [PubMed] [Google Scholar]
- 67.Muller RU, Bostock E, Taube JS, Kubie JL. 1994. On the directional firing properties of hippocampal place cells. J. Neurosci. 14, 7235–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson MI, Jeffery KJ. 2003. Heterogeneous modulation of place cell firing by changes in context. J. Neurosci. 23, 8827–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser M-B. 2005. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science 309, 619–623. ( 10.1126/science.1114037) [DOI] [PubMed] [Google Scholar]
- 70.Huxter J, Burgess N, O'Keefe J. 2003. Nature 425, 828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hafting T, Fyhn M, Bonnevie T, Moser M-B, Moser EI. 2008. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252. ( 10.1038/nature06957) [DOI] [PubMed] [Google Scholar]
- 72.Dragoi G, Buzsaki G. 2006. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50, 145–157. ( 10.1016/j.neuron.2006.02.023) [DOI] [PubMed] [Google Scholar]
- 73.Burgess N, Recce M, O'Keefe J. 1994. A model of hippocampal function. Neural Netw. 7, 1065–1081. ( 10.1016/S0893-6080(05)80159-5) [DOI] [Google Scholar]
- 74.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172. () [DOI] [PubMed] [Google Scholar]
- 75.Taube JS, Muller RU, Ranck JB., Jr 1990. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser M-B, Moser EI. 2006. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762. ( 10.1126/science.1125572) [DOI] [PubMed] [Google Scholar]
- 77.Taube JS. 2007. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181–207. ( 10.1146/annurev.neuro.29.051605.112854) [DOI] [PubMed] [Google Scholar]
- 78.Zhang K. 1996. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci. 16, 2112–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson A, Seeland K, Redish AD. 2005. Reconstruction of the postsubiculum head direction signal from neural ensembles. Hippocampus 15, 86–96. ( 10.1002/hipo.20033) [DOI] [PubMed] [Google Scholar]
- 80.Calton JL, Stackman RW, Goodridge JP, Archey WB, Dudchenko PA, Taube JS. 2003. Hippocampal place cell instability after lesions of the head direction cell network. J. Neurosci. 23, 9719–9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burgess N, Cacucci F, Lever C, O'Keefe J. 2004. Characterizing multiple independent behavioral correlates of cell firing in freely moving animals. Hippocampus 15, 149–153. ( 10.1002/hipo.20058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knierim JJ, Kudrimoti HS, McNaughton BL. 1998. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J. Neurophysiol. 80, 425–446. [DOI] [PubMed] [Google Scholar]
- 83.Jeffery KJ. 1998. Learning of landmark stability and instability by hippocampal place cells. Neuropharmacology 37, 677–687. ( 10.1016/S0028-3908(98)00053-7) [DOI] [PubMed] [Google Scholar]
- 84.Zugaro MLB, Arleo A, Berthoz A, Wiener SI. 2003. Rapid spatial reorientation and head direction cells. J. Neurosci. 23, 3478–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNaughton BL, et al. 1996. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 199, 173–185. [DOI] [PubMed] [Google Scholar]
- 86.Cressant A, Muller RU, Poucet B. 1997. Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci. 17, 2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeffery KJ, Donnett JG, Burgess N, O'Keefe J. 1997. Directional control of hippocampal place fields. Exp. Brain Res. 117, 131–142. ( 10.1007/s002210050206) [DOI] [PubMed] [Google Scholar]
- 88.Knight R, Piette CE, Page H, Walters D, Marozzi E, Nardini M, Stringer S, Jeffery KJ. 2014. Weighted cue integration in the rodent head direction system. Phil. Trans. R. Soc. B 369, 20120512 ( 10.1098/rstb.2012.0512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. 2005. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. ( 10.1038/nature03721) [DOI] [PubMed] [Google Scholar]
- 90.Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser M-B. 2010. Grid cells in pre-and parasubiculum. Nat. Neurosci. 13, 987–994. ( 10.1038/nn.2602) [DOI] [PubMed] [Google Scholar]
- 91.Fyhn M, Hafting T, Witter MP, Moser EI, Moser MB. 2008. Grid cells in mice. Hippocampus 18, 1230–1238. ( 10.1002/hipo.20472) [DOI] [PubMed] [Google Scholar]
- 92.Giocomo LM, Hasselmo ME. 2008. Time constants of h current in layer II stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex. J. Neurosci. 28, 9414–9425. ( 10.1523/JNEUROSCI.3196-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giocomo LM, Hussaini SA, Zheng F, Kandel ER, Moser M-B, Moser EI. 2011. Grid cells use HCN1 channels for spatial scaling. Cell 147, 1159–1170. ( 10.1016/j.cell.2011.08.051) [DOI] [PubMed] [Google Scholar]
- 94.Barry C, Hayman R, Burgess N, Jeffery K. 2007. Experience-dependent rescaling of entorhinal grids. Nat. Neurosci. 10, 682–684. ( 10.1038/nn1905) [DOI] [PubMed] [Google Scholar]
- 95.Fiete IR, Burak Y, Brookings T. 2008. What grid cells convey about rat location. J. Neurosci. 28, 6858–6871. ( 10.1523/JNEUROSCI.5684-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorchetchnikov A, Grossberg S. 2007. Space, time and learning in the hippocampus: how fine spatial and temporal scales are expanded into population codes for behavioral control. Neural Netw. 20, 182–193. ( 10.1016/j.neunet.2006.11.007) [DOI] [PubMed] [Google Scholar]
- 97.Towse BW, Barry C, Bush D, Burgess N. 2014. Optimal configurations of spatial scale for grid cell firing under noise and uncertainty. Phil. Trans. R. Soc. B 369, 20130290 ( 10.1098/rstb.2013.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stensola H, Stensola T, Solstad T, Froland K, Moser M-B, Moser EI. 2012. The entorhinal grid map is discretized. Nature 492, 72–78. ( 10.1038/nature11649) [DOI] [PubMed] [Google Scholar]
- 99.Schmidt-Hieber C, Häusser M. 2014. How to build a grid cell. Phil. Trans. R. Soc. B 369, 20120520 ( 10.1098/rstb.2012.0520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barry C, Ginzberg LL, O'Keefe J, Burgess N. 2012. Grid cell firing patterns signal environmental novelty by expansion. Proc. Natl Acad. Sci. USA 109, 17 687–17 692. ( 10.1073/pnas.1209918109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poucet B, Sargolini F, Song EY, Hangya B, Fox S, Muller RU. 2014. Independence of landmark and self-motion-guided navigation: a different role for grid cells. Phil. Trans. R. Soc. B 369, 20130370 ( 10.1098/rstb.2013.0370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Keefe J, Burgess N. 1996. Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428. ( 10.1038/381425a0) [DOI] [PubMed] [Google Scholar]
- 103.Muller RU, Kubie JL, Ranck JB., Jr 1987. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J. Neurosci. 7, 1935–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burgess N, Jackson A, Hartley T, O'Keefe J. 2000. Predictions derived from modelling the hippocampal role in navigation. Biol. Cybern. 83, 301–312. ( 10.1007/s004220000172) [DOI] [PubMed] [Google Scholar]
- 105.Lever C, Burgess N, Cacucci F, Hartley T, O'Keefe J. 2002. What can the hippocampal representation of environmental geometry tell us about Hebbian learning? Biol. Cybern. 87, 356–372. ( 10.1007/s00422-002-0360-z) [DOI] [PubMed] [Google Scholar]
- 106.Barry C, Lever C, Hayman R, Hartley T, Burton S, O'Keefe J, Jeffery K, Burgess N. 2006. The boundary vector cell model of place cell firing and spatial memory. Rev. Neurosci. 17, 71–97. ( 10.1515/REVNEURO.2006.17.1-2.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lever C, Burton S, Jeewajee A, O'Keefe J, Burgess N. 2009. Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 29, 9771–9777. ( 10.1523/JNEUROSCI.1319-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. 2008. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868. ( 10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 109.Savelli F, Yoganarasimha D, Knierim JJ. 2008. Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 18, 1270–1282. ( 10.1002/hipo.20511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stewart S, Jeewajee A, Wills TJ, Burgess N, Lever C. 2014. Boundary coding in the rat subiculum. Phil. Trans. R. Soc. B 369, 20120514 ( 10.1098/rstb.2012.0514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krupic J, Bauza M, Burton S, Lever C, O'Keefe J. 2014. How environment geometry affects grid cell symmetry and what we can learn from it. Phil. Trans. R. Soc. B 369, 20130188 ( 10.1098/rstb.2013.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser M-B, Moser EI. 2009. Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci. 12, 1325–1332. ( 10.1038/nn.2396) [DOI] [PubMed] [Google Scholar]
- 113.Doeller CF, Burgess N. 2008. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proc. Natl Acad. Sci. USA 105, 5909–5914. ( 10.1073/pnas.0711433105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doeller CF, King JA, Burgess N. 2008. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl Acad. Sci. USA 105, 5915–5920. ( 10.1073/pnas.0801489105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bird CM, Capponi C, King JA, Doeller CF, Burgess N. 2010. Establishing the boundaries: the hippocampal contribution to imagining scenes. J. Neurosci. 30, 11 688–11 695. ( 10.1523/JNEUROSCI.0723-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Horne MR, Iordanova MD, Pearce JM. 2010. Spatial learning based on boundaries in rats is hippocampus-dependent and prone to overshadowing. Behav. Neurosci. 124, 623–632. ( 10.1037/a0020824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee SA, Spelke ES. 2010. Two systems of spatial representation underlying navigation. Exp. Brain Res. 206, 179–188. ( 10.1007/s00221-010-2349-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cheng K, Newcombe NS. 2005. Is there a geometric module for spatial orientation? Squaring theory and evidence. Psychon. Bull. Rev. 12, 1–23. ( 10.3758/BF03196346) [DOI] [PubMed] [Google Scholar]
- 119.Mhatre H, Gorchetchnikov A, Grossberg S. 2012. Grid cell hexagonal patterns formed by fast self-organized learning within entorhinal cortex. Hippocampus 22, 320–334. ( 10.1002/hipo.20901) [DOI] [PubMed] [Google Scholar]
- 120.Hasselmo ME, Brandon MP. 2012. A model combining oscillations and attractor dynamics for generation of grid cell firing. Front. Neural Circuits 6, 30 ( 10.3389/fncir.2012.00030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krupic J, Burgess N, O'Keefe J. 2012. Neural representations of location composed of spatially periodic bands. Science 337, 853–857. ( 10.1126/science.1222403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cacucci F, Lever C, Burgess N, O'Keefe J. 2000. Topodirectional cells in the hippocampal formation of the rat. Eur. J. Neurosci. 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. 2011. Hippocampal ‘time cells’ bridge the gap in memory for discontiguous events. Neuron 71, 737–749. ( 10.1016/j.neuron.2011.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kraus BJ, Robinson RJ, II, White JA, Eichenbaum H, Hasselmo ME. 2013. Hippocampal ‘time cells’: time versus path integration. Neuron 78, 1090–1101. ( 10.1016/j.neuron.2013.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knierim JJ, Neunuebel JP, Deshmukh SS. 2014. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local–global reference frames. Phil. Trans. R. Soc. B 369, 20130369 ( 10.1098/rstb.2013.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsao A, Moser M-B, Moser EI. 2013. Traces of experience in the lateral entorhinal cortex. Curr. Biol. 23, 399–405. ( 10.1016/j.cub.2013.01.036) [DOI] [PubMed] [Google Scholar]
- 127.Deshmukh SS, Knierim JJ. 2011. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front. Behav. Neurosci. 5, 69 ( 10.3389/fnbeh.2011.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. ( 10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 129.Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873. ( 10.1126/science.271.5257.1870) [DOI] [PubMed] [Google Scholar]
- 130.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. ( 10.1016/S0896-6273(02)01096-6) [DOI] [PubMed] [Google Scholar]
- 132.Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683. ( 10.1038/nature04587) [DOI] [PubMed] [Google Scholar]
- 133.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. 2010. Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–229. ( 10.1016/j.tins.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 134.Girardeau G, Zugaro M. 2011. Hippocampal ripples and memory consolidation. Curr. Opin. Neurobiol. 21, 452–459. ( 10.1016/j.conb.2011.02.005) [DOI] [PubMed] [Google Scholar]
- 135.Csicsvari J, Dupret D. 2014. Sharp wave/ripple network oscillations and learning-associated hippocampal maps. Phil. Trans. R. Soc. B 369, 20120528 ( 10.1098/rstb.2012.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dragoi G, Tonegawa S. 2014. Selection of preconfigured cell assemblies for representation of novel spatial experiences. Phil. Trans. R. Soc. B 369, 20120522 ( 10.1098/rstb.2012.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401. ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dragoi G, Tonegawa S. 2013. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl Acad. Sci. USA 110, 9100–9105. ( 10.1073/pnas.1306031110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diba K, Buzsaki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242. ( 10.1038/nn1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Buckner RL. 2010. The role of the hippocampus in prediction and imagination. Annu Rev. Psychol. 61, 27–34. ( 10.1146/annurev.psych.60.110707.163508) [DOI] [PubMed] [Google Scholar]
- 142.Solstad T, Moser EI, Einevoll GT. 2006. From grid cells to place cells: a mathematical model. Hippocampus 16, 1026–1031. ( 10.1002/hipo.20244) [DOI] [PubMed] [Google Scholar]
- 143.Rolls ET, Stringer SM, Elliot T. 2006. Entorhinal cortex grid cells can map to hippocampal place cells by competitive learning. Netw. Comput. Neural Syst. 17, 447–465. ( 10.1080/09548980601064846) [DOI] [PubMed] [Google Scholar]
- 144.Zhang S-J, Ye J, Couey JJ, Witter M, Moser EI, Moser M-B. 2014. Functional connectivity of the entorhinal–hippocampal space circuit. Phil. Trans. R. Soc. B 369, 20120516 ( 10.1098/rstb.2012.0516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang S-J, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser M-B, Moser EI. 2013. Optogenetic dissection of entorhinal–hippocampal functional connectivity. Science 340, 1232627 ( 10.1126/science.1232627) [DOI] [PubMed] [Google Scholar]
- 146.Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser M-B. 2010. Development of the spatial representation system in the rat. Science 328, 1576–1580. ( 10.1126/science.1188210) [DOI] [PubMed] [Google Scholar]
- 147.Wills TJ, Cacucci F, Burgess N, O'Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328, 1573–1576. ( 10.1126/science.1188224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wills TJ, Muessig L, Cacucci F. 2014. The development of spatial behaviour and the hippocampal neural representation of space. Phil. Trans. R. Soc. B 369, 20130409 ( 10.1098/rstb.2013.0409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koenig J, Linder AN, Leutgeb JK, Leutgeb S. 2011. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595. ( 10.1126/science.1201685) [DOI] [PubMed] [Google Scholar]
- 150.Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser M-B. 2013. Grid cells require excitatory drive from the hippocampus. Nat. Neurosci. 16, 309–317. ( 10.1038/nn.3311) [DOI] [PubMed] [Google Scholar]
- 151.O'Keefe J, Burgess N. 2005. Dual phase and rate coding in hippocampal place cells: theoretical significance and relationship to entorhinal grid cells. Hippocampus 15, 853–866. ( 10.1002/hipo.20115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zilli EA. 2012. Models of grid cell spatial firing published 2005–2011. Front. Neural Circuits 6, 16 ( 10.3389/fncir.2012.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Page HJI, Walters DM, Knight R, Piette CE, Jeffery KJ, Stringer SM. 2014. A theoretical account of cue averaging in the rodent head direction system. Phil. Trans. R. Soc. B 369, 20130283 ( 10.1098/rstb.2013.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rotenberg A, Muller RU. 1997. Variable place-cell coupling to a continuously viewed stimulus: evidence that the hippocampus acts as a perceptual system. Phil. Trans. R. Soc. Lond. B 352, 1505–1513. ( 10.1098/rstb.1997.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Samsonovich A, McNaughton BL. 1997. Path integration and cognitive mapping in a continuous attractor neural network model. J. Neurosci. 17, 5900–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Conklin J, Eliasmith C. 2005. A controlled attractor network model of path integration in the rat. J. Comput. Neurosci. 18, 183–203. ( 10.1007/s10827-005-6558-z) [DOI] [PubMed] [Google Scholar]
- 157.Fuhs MC, Touretzky DS. 2006. A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276. ( 10.1523/JNEUROSCI.4353-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guanella A, Verschure PF. 2006. A model of grid cells based on a path integration mechanism. In Artificial Neural Networks—ICANN 2006, Part I, LNCS4131 (eds S Kollias, A Stafylopatis, W Duch, E Oja), pp. 740–749. Berlin, Germany: Springer. [Google Scholar]
- 159.Turing AM. 1990. The chemical basis of morphogenesis. Bull. Math. Biol. 52, 153–197. ( 10.1007/BF02459572) [DOI] [PubMed] [Google Scholar]
- 160.Fenton AA, Kao H-Y, Neymotin SA, Olypher A, Vayntrub Y, Lytton WW, Ludvig N. 2008. Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly arranged, and expanded place fields in the larger space. J. Neurosci. 28, 11 250–11 262. ( 10.1523/JNEUROSCI.2862-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harvey CD, Collman F, Dombeck DA, Tank DW. 2009. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941–946. ( 10.1038/nature08499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chorev E, Epsztein J, Houweling AR, Lee AK, Brecht M. 2009. Electrophysiological recordings from behaving animals—going beyond spikes. Curr. Opin. Neurobiol. 19, 513–519. ( 10.1016/j.conb.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 163.Domnisoru C, Kinkhabwala AA, Tank DW. 2013. Membrane potential dynamics of grid cells. Nature 495, 199–204. ( 10.1038/nature11973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schmidt-Hieber C, Hausser M. 2013. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nat. Neurosci. 16, 325–331. ( 10.1038/nn.3340) [DOI] [PubMed] [Google Scholar]
- 165.Jacobs J, et al. 2013. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat. Neurosci. 16, 1188–1190. ( 10.1038/nn.3466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. 2013. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 23, 656–661. ( 10.1002/hipo.22124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grossberg S, Pilly PK. 2014. Coordinated learning of grid cell and place cell spatial and temporal properties: multiple scales, attention and oscillations. Phil. Trans. R. Soc. B 369, 20120524 ( 10.1098/rstb.2012.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brecht M, Ray S, Burgalossi A, Tang Q, Schmidt H, Naumann R. 2014. An isomorphic mapping hypothesis of the grid representation. Phil. Trans. R. Soc. B 369, 20120521 ( 10.1098/rstb.2012.0521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burak Y, Fiete IR. 2009. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 5, e1000291 ( 10.1371/journal.pcbi.1000291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lapray D, et al. 2012. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 15, 1265–1271. ( 10.1038/nn.3176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Epsztein J, Brecht M, Lee AK. 2011. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120. ( 10.1016/j.neuron.2011.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Holscher C, Schnee A, Dahmen H, Setia L, Mallot HA. 2005. Rats are able to navigate in virtual environments. J. Exp. Biol. 208, 561–569. ( 10.1242/jeb.01371) [DOI] [PubMed] [Google Scholar]
- 173.Chen G, King JA, Burgess N, O'Keefe J. 2013. How vision and movement combine in the hippocampal place code. Proc. Natl Acad. Sci. USA 110, 378–383. ( 10.1073/pnas.1215834110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. 2010. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci. 13, 1433–1440. ( 10.1038/nn.2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. 2005. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268. ( 10.1038/nn1525) [DOI] [PubMed] [Google Scholar]
- 176.Ramirez S, Liu X, Lin P-A, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. 2013. Creating a false memory in the hippocampus. Science 341, 387–391. ( 10.1126/science.1239073) [DOI] [PubMed] [Google Scholar]
- 177.Eacott MJ, Easton A. 2010. Episodic memory in animals: remembering which occasion. Neuropsychologia 48, 2273–2280. ( 10.1016/j.neuropsychologia.2009.11.002) [DOI] [PubMed] [Google Scholar]
- 178.Hori E, Tabuchi E, Matsumura N, Tamura R, Eifuku S, Endo S, Nishijo H, Ono T. 2003. Representation of place by monkey hippocampal neurons in real and virtual translocation. Hippocampus 13, 190–196. ( 10.1002/hipo.10062) [DOI] [PubMed] [Google Scholar]
- 179.Robertson RG, Rolls ET, Georges-Francois P, Panzeri S. 1999. Head direction cells in the primate pre-subiculum. Hippocampus 9, 206–219. () [DOI] [PubMed] [Google Scholar]
- 180.Killian NJ, Jutras MJ, Buffalo EA. 2012. A map of visual space in the primate entorhinal cortex. Nature 491, 761–764. ( 10.1038/nature11587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rolls ET, Robertson RG, Georges-Francois P. 1997. Spatial view cells in the primate hippocampus. Eur. J. Neurosci. 9, 1789–1794. ( 10.1111/j.1460-9568.1997.tb01538.x) [DOI] [PubMed] [Google Scholar]
- 182.Yartsev MM, Witter MP, Ulanovsky N. 2011. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107. ( 10.1038/nature10583) [DOI] [PubMed] [Google Scholar]
- 183.Barry C, Bush D, O'Keefe J, Burgess N. 2012. Models of grid cells and theta oscillations. Nature 488, E1 ( 10.1038/nature11276) [DOI] [PubMed] [Google Scholar]
- 184.Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N. 2012. Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biol. 10, e1001267 ( 10.1371/journal.pbio.1001267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fox NC, Warrington EK, Stevens JM, Rossor MN. 1996. Atrophy of the hippocampal formation in early familial Alzheimer's disease. A longitudinal MRI study of at-risk members of a family with an amyloid precursor protein 717Val-Gly mutation. Ann. NY Acad. Sci. 777, 226–232. ( 10.1111/j.1749-6632.1996.tb34423.x) [DOI] [PubMed] [Google Scholar]
- 186.Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. ( 10.1136/jnnp.20.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Spiers HJ, Maguire EA, Burgess N. 2001. Hippocampal amnesia. Neurocase 7, 357–382. ( 10.1076/neur.7.5.357.16245) [DOI] [PubMed] [Google Scholar]
- 188.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. 1997. Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277, 376–380. ( 10.1126/science.277.5324.376) [DOI] [PubMed] [Google Scholar]
- 189.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. 2003. Cellular networks underlying human spatial navigation. Nature 425, 184–188. ( 10.1038/nature01964) [DOI] [PubMed] [Google Scholar]
- 190.King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. 2002. The human hippocampus and viewpoint dependence in spatial memory. Hippocampus 12, 811–820. ( 10.1002/hipo.10070) [DOI] [PubMed] [Google Scholar]
- 191.Hartley T, Harlow R. 2012. An association between human hippocampal volume and topographical memory in healthy young adults. Front. Hum. Neurosci. 6, 338 ( 10.3389/fnhum.2012.00338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Habib M, Sirigu A. 1987. Pure topographical disorientation: a definition and anatomical basis. Cortex 23, 73–85. ( 10.1016/S0010-9452(87)80020-5) [DOI] [PubMed] [Google Scholar]
- 193.Aguirre GK, D'Esposito M. 1999. Topographical disorientation: a synthesis and taxonomy. Brain 122, 1613–1628. ( 10.1093/brain/122.9.1613) [DOI] [PubMed] [Google Scholar]
- 194.Epstein RA. 2008. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn. Sci. 12, 388–396. ( 10.1016/j.tics.2008.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vann SD, Aggleton JP, Maguire EA. 2009. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802. ( 10.1038/nrn2733) [DOI] [PubMed] [Google Scholar]
- 196.Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature 392, 598–601. ( 10.1038/33402) [DOI] [PubMed] [Google Scholar]
- 197.Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. 2004. Intersubject synchronization of cortical activity during natural vision. Science 303, 1634–1640. ( 10.1126/science.1089506) [DOI] [PubMed] [Google Scholar]
- 198.Janzen G, Van Turennout M. 2004. Selective neural representation of objects relevant for navigation. Nat. Neurosci. 7, 673–677. ( 10.1038/nn1257) [DOI] [PubMed] [Google Scholar]
- 199.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. 1998. Knowing where and getting there: a human navigation network. Science 280, 921–924. ( 10.1126/science.280.5365.921) [DOI] [PubMed] [Google Scholar]
- 200.Hartley T, Maguire EA, Spiers HJ, Burgess N. 2003. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888. ( 10.1016/S0896-6273(03)00095-3) [DOI] [PubMed] [Google Scholar]
- 201.Buckner RL, Carroll DC. 2007. Self-projection and the brain. Trends Cogn. Sci. 11, 49–57. ( 10.1016/j.tics.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 202.Hassabis D, Maguire EA. 2007. Deconstructing episodic memory with construction. Trends Cogn. Sci. 11, 299–306. ( 10.1016/j.tics.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 203.Burgess N, Maguire EA, Spiers HJ, O'Keefe J. 2001. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14, 439–453. ( 10.1006/nimg.2001.0806) [DOI] [PubMed] [Google Scholar]
- 204.Byrne P, Becker S, Burgess N. 2007. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340–375. ( 10.1037/0033-295X.114.2.340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schacter DL, Addis DR. 2007. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Phil. Trans. R. Soc. B 362, 773–786. ( 10.1098/rstb.2007.2087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Walther DB, Caddigan E, Fei-Fei L, Beck DM. 2009. Natural scene categories revealed in distributed patterns of activity in the human brain. J. Neurosci. 29, 10 573–10 581. ( 10.1523/JNEUROSCI.0559-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Epstein RA, Vass LK. 2014. Neural systems for landmark-based wayfinding in humans. Phil. Trans. R. Soc. B 369, 20120533 ( 10.1098/rstb.2012.0533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Doeller CF, Barry C, Burgess N. 2010. Evidence for grid cells in a human memory network. Nature 463, 657–661. ( 10.1038/nature08704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I. 2010. A sense of direction in human entorhinal cortex. Proc. Natl Acad. Sci. USA 107, 6487–6492. ( 10.1073/pnas.0911213107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Oliva A, Torralba A. 2006. Building the gist of a scene: the role of global image features in recognition. Prog. Brain Res. 155, 23–36. [DOI] [PubMed] [Google Scholar]
- 211.Henderson JM, Zhu DC, Larson CL. 2011. Functions of parahippocampal place area and retrosplenial cortex in real-world scene analysis: an fMRI study. Vis. Cogn. 19, 910–927. [Google Scholar]
- 212.Kravitz DJ, Peng CS, Baker CI. 2011. Real-world scene representations in high-level visual cortex: it's the spaces more than the places. J. Neurosci. 31, 7322–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vass LK, Epstein RA. 2013. Abstract representations of location and facing direction in the human brain. J. Neurosci. 33, 6133–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amaral D, Witter M. 1989. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591. ( 10.1016/0306-4522(89)90424-7) [DOI] [PubMed] [Google Scholar]
- 215.Morris RGM, Garrud P, Rawlins JN, O'Keefe J. 1982. Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. ( 10.1038/297681a0) [DOI] [PubMed] [Google Scholar]
- 216.Rolls ET, Treves A. 1997. Neural networks and brain function. Oxford, UK: Oxford University Press. [Google Scholar]