Abstract
Collagen tripeptide (CTP) is a functional food material with several biological effects such as improving dry skin and wound and bone fracture healing. This study focused on the anti-photoaging effects of CTP on a hairless mouse model. To evaluate the effects of CTP on UVB-induced skin wrinkle formation in vivo, the hairless mice were exposed to UVB radiation with oral administration of CTP for 14 weeks. Compared with the untreated UVB control group, mice treated with CTP showed significantly reduced wrinkle formation, skin thickening, and transepidermal water loss (TEWL). Skin hydration and hydroxyproline were increased in the CTP-treated group. Moreover, oral administration of CTP prevented UVB-induced MMP-3 and -13 activities as well as MMP-2 and -9 expressions. Oral administration of CTP increased skin elasticity and decreased abnormal elastic fiber formation. Erythema was also decreased in the CTP-treated group. Taken together, these results strongly suggest that CTP has potential as an anti-photoaging agent.
Keywords: collagen tripeptide (CTP), photoaging, matrix metalloproteinases (MMPs), transepidermal water loss (TEWL), wrinkle formation
INTRODUCTION
Human skin can age from many factors such as genetics exposure to ultraviolet (UV) radiation, mechanical stress, hormonal changes (1). Therefore, the skin aging process can be either due to intrinsic aging or photoaging. Photoaging refers to premature skin aging caused by chronic exposure to UV radiation, especially its UVB component, which is regarded as the primary cause of skin damage (2). The characteristics of photoaging include fine and coarse wrinkles, dryness, roughness, shallowness, mottled dyspigmentation, histological changes, and a variety of cutaneous changes including altered skin barrier function (3).
MMPs (matrix metalloproteinases) are a family of 24 zinc-dependent proteolytic enzymes, sub-classified into functional groups based on substrate preference and structural homology. MMPs play important roles as structurally related matrix-degrading enzymes in various destructive processes, including inflammation, tumor invasion, and skin aging. Since MMPs participate in the skin photoaging process, regulating their activities may be a strategy for the prevention and treatment of UV-induced skin damage (4,5). Once human or mouse skin is exposed to UV radiation, the synthesis of MMP-1 (interstitial collagenase) is induced, which then degrades type I and III collagens; MMP-3 (stromelysin-1) degrades type IV collagen of the basement membrane and MMP-9 (gelatinase) further degrades the collagen fragments generated by MMP-1. Rodents lack the gene for MMP-1, which is instead functionally replaced by MMP-13 (6).
Collagen has been used as a skin function ingredient for its efficacy in moisturizing and enhancing elasticity. Recent studies have shown that peptides obtained from protein hydrolysis, such as collagen hydrolysate, are effective skin moisturizing and anti-wrinkle agents (7). As previously reported, the oral intake of collagen or collagen peptides are effective antioxidants for skin protection and beneficial against osteoarthritis and osteoporosis (8,9). In this research, we estimated the effects of CTP, obtained from the skin of sutchi catfish, on UV-induced skin damage, using a hairless mouse model, in order to uncover mechanisms underlying its skin moisturizing and anti-wrinkle effects and also a potentially new functional dietary ingredient and beauty food material (10,11).
MATERIALS AND METHODS
Materials
CTP, which was obtained by the enzymatic degradation of collagens derived from the skin of sutchi catfish (Pangasius hypophthalmus), was supplied from Jellice Co., Ltd. (product name HACP-CF). CTP contained 15.0% of tripeptides Gly-Xaa-Yaa (Xaa and Yaa are arbitrary but are often occupied by proline, hydroxyproline, and alanine) as bioactive compounds.
Animal experiments
Five-week-old female hairless mice (SKH-1) were purchased from Deahan Biolink Ltd. (Eumsung, Korea). The mice were housed in temperature- (23±2°C), humidity- (55±10%), and light- (12 hr day/12 hr night) controlled conditions at Yonsei Laboratory Animal Research Center (YLARC, Seoul, Korea). Five mice were allocated to each group (total four groups): 1) normal group (Control), 2) UVB-irradiated group (UVB control), 3) UVB-irradiated and 167 mg/kg/day CTP-treated group (CTP-167), 4) UVB-irradiated and 333 mg/kg/day CTP-treated group (CTP-333). The mice in treated groups received oral CTP daily for 14 weeks with concurrent exposure to UVB radiation three times per week. The starting dose of UVB radiation was 75 mJ/cm2 during the first week, and the dose was increased weekly by 1 minimal erythema dose (MED) until reaching 3 MED, which was maintained until 14 weeks. After 14 weeks, mice were sacrificed under anesthetization by intraperitoneal injection of a mixture of zoletil (Virbac, Carros, France) and rompun (Bayer Korea Ltd., Seoul, Korea). Samples obtained from the dorsal skin were rapidly frozen in liquid nitrogen and stored at −70°C. Skin biopsy samples for histological analysis were fixed in 10% buffered formalin for optical microscopy.
Reverse transcription-polymerase chain reaction (RT-PCR)
PCR amplification of the cDNA products (5 μL) was performed with PCR premix (ELPIS-Biotech, Daejeon, Korea) and the primer pairs (Bioneer, Daejeon, Korea) shown in Table 1. Before PCR amplification, products were denatured at 94°C for 5 min. Amplification consisted of 25 cycles: denaturation at 94°C for 30 s, annealing at 56°C for 1 min, and extension at 72°C for 1 min, followed by a final 5 min extension at 72°C. PCR was performed in a Gene Amp PCR System 2700 (Applied Biosystems, Foster City, CA, USA). PCR products were separated by 1.5% agarose gel electrophoresis and visualized by 6X Loading STAR solution (Dyne-bio, Sungnam, Korea) and UV illumination.
Table 1.
The nucleotide sequences of forward and reverse primer sets for mouse genes
| Host | Gene | Primer | Sequence |
|---|---|---|---|
| Mouse | MMP-3 | Forward | 5′-TAG CAG GTT ATC CTA AAA GCA-3′ |
| Reverse | 5′-CCA GCT ATT GCT CTT CAA T-3′ | ||
| MMP-13 | Forward | 5′-CAT CCA TCC CGT GAC CTT AT-3′ | |
| Reverse | 5′-GCA TGA CTC TCA CAA TGC GA-3′ | ||
| β-Actin | Forward | 5′-CCA GCC AGC CAC CAT CGC TC-3′ | |
| Reverse | 5′-TGA CCT TGG CCA GGG GTG CA-3′ |
Western blot analysis
Homogenized skin sections were lysed in Protein Extraction Solution (NP40, ELPIS-Biotech) with protease inhibitor cocktail (Sigma-Aldrich Co., St. Louis, MO, USA). The protein concentrations were determined by Bradford protein assay (12). For Western blotting, equal amounts of proteins (30 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blocked with 5% skim milk in Tris-buffered saline with Tween-20 (TBST). The membranes were then detected with primary antibodies against MMP-3, MMP-13, and α-tubulin (Cell Signaling, Beverly, MA, USA). Bound antibodies were detected with a horse-radish peroxidase-linked secondary antibody (Bethyl Laboratories, Inc., Montgomery, TX, USA). Proteins were detected with the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Little Chalfont, UK) and visualized with LuminoImager (LAS 3000 Bio Imaging Analysis System, Fuji Film, Tokyo, Japan).
Gelatin zymography
MMP-2 and -9 activities were assessed by gelatin zymography. Samples were mixed with standard gel loading buffer containing 2% SDS without β-mercapto-ethanol and loaded on the gel without prior heating. Samples were separated by electrophoresis on a 10% polyacrylamide gel containing SDS and 1% gelatin at 85 V for 2 hr in a Bio-Rad Mini PROTEAN 3 Cell electrophoretic apparatus (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, the gel was washed with 2.5% Triton X-100 for 1 hr and incubated in reaction buffer (50 mM Tris-HCl [pH 7.5] containing 10 mM CaCl2 and 0.15 M NaCl) at 37°C. After 24 hr, the gel was stained with Coomassie® brilliant blue R-250 (Fluka, Buchs, Switzerland) and then destained with 30% methanol and 10% acetic acid. MMP-2 and MMP-9 were detected at 72 kDa and 92 kDa, respectively, as a clear zone against the dark background.
Evaluation of skin wrinkle formation
After 14 weeks of treatment with CTP, the dorsal skin of anesthetized hairless mice was photographed with a digital camera (Coolpix P80, Nikon, Tokyo, Japan). Skin surface impressions were measured using Replica (Epigem, Seoul, Korea) and analyzed with Visioline VL650 (CK Electronics GmbH, Cologne, Germany).
Histological analysis
After sacrifice, the skin samples were fixed in 10% formalin for 24 hr and stained with hematoxylin and eosin (H&E), Masson’s trichrome, and Verhoeffvan Gieson’s stain. The stained sections were analyzed using an Eclipse TE2000U Inverted Microscope with twin CCD cameras (Nikon).
Evaluation of erythema
Skin erythema was measured photometrically using a Mexameter® 18 apparatus (CK Electronics GmbH), based on a remission principle (12).
Evaluation of skin hydration and transepidermal water loss (TEWL)
Skin hydration was measured with a Corneometer® 825, and TEWL as a marker of epidermal skin barrier function was measured by Tewameter® TM300, which was mounted on a Multi Probe Adapter® MPA5 (CK Electronics GmbH).
Hydroxyproline assay
Skin tissues (600 mg) were homogenized with glass beads in 1 mL of 6 N HCl and hydrolyzed at 105°C for 18 hr (14). The hydroxyproline content was measured using a hydroxyproline assay kit (Quickzyme Bioscience, Leiden, Netherlands).
Evaluation of skin elasticity
Skin elasticity was evaluated using a Cutometer® MPA580 (CK Electronics GmbH). Physical parameters were as follows: immediate distention (Ue), final distension (Uf), immediate retraction (Ur), delayed distention (Uv), and final retraction (Ua). Skin elasticity was calculated with the gross elasticity (Ua/Uf), representing the rate of recovery to the initial state (15).
Statistical analysis
Results are expressed as mean±standard deviation (SD). Groups were compared by the Scheffé test and Duncan test (SPSS 12.0, SPSS Inc., Chicago, IL, USA). ##p<0.01, #p<0.05, **p<0.01, and *p<0.05 were considered to be statistically significant.
RESULTS AND DISCUSSION
Effects of oral administration of CTP on UVB-induced wrinkle formation
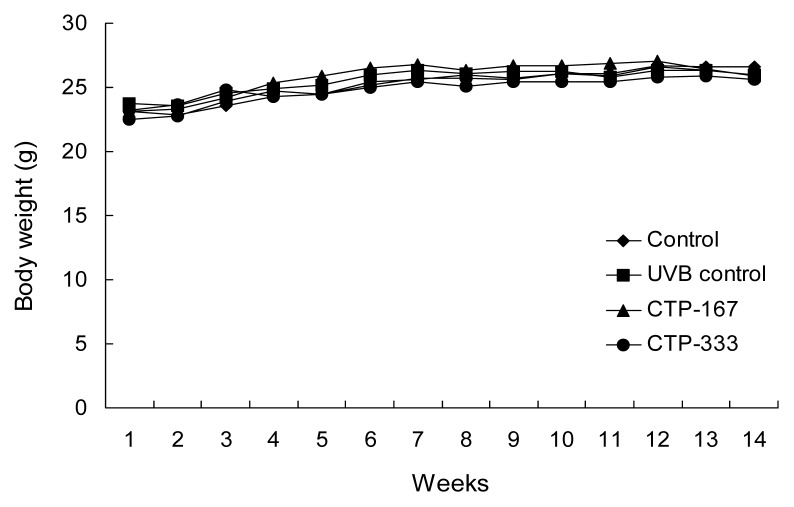
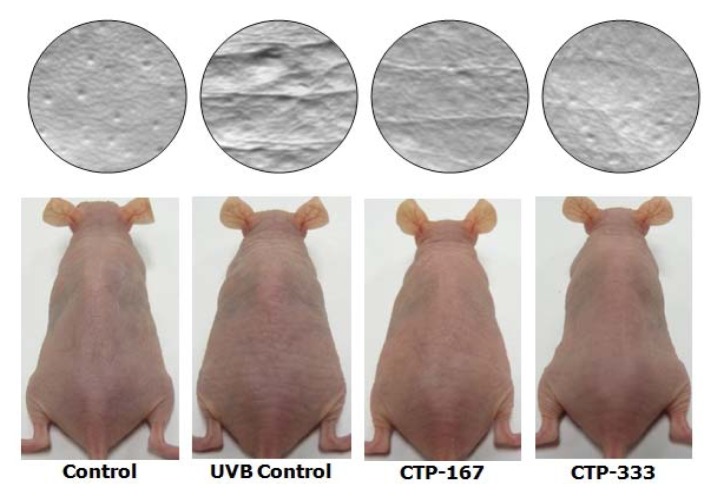
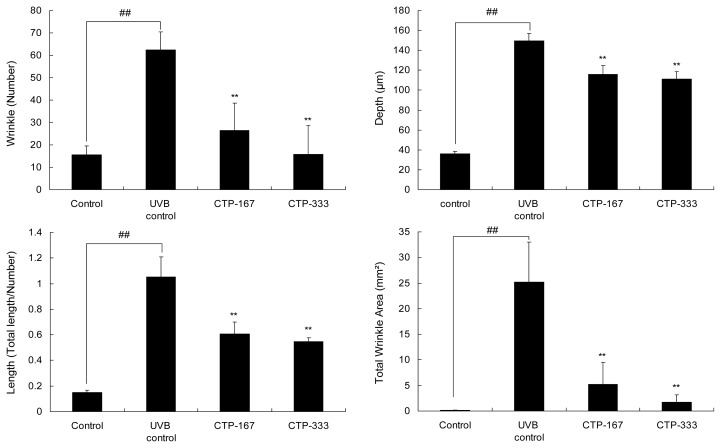
Hairless SKH-1 mice have been reported to be a suitable animal model that recapitulates key anatomic and cellular responses observed in human skin during acute UV exposure (16). To examine in vivo anti-photoaging effects of CTP, hairless SKH-1 mice were used to access biomarkers of photoaging such as skin thickness, skin hydration, TEWL, MMPs, collagen, and elastic fibers. During the experiment period, body weights of mice were measured weekly and showed no difference between the groups (Fig. 1). Therefore, UVB-irradiation and treatment with CTP had no effect on body weight. Repetitive UVB irradiation was associated with UV-induced epidermal changes such as deep wrinkles and roughening (17). Therefore, effects of CTP on UVB-induced epidermal changes were investigated by analysis of photography and skin replica (Fig. 2). UVB irradiation induced significant wrinkle formation on the dorsal skin of the UVB control group. However, oral administration of CTP reduced wrinkle formation induced by UVB irradiation (Fig. 3). CTP inhibited MMPs such as collagenase (MMP- 3 and -13) and gelatinase (MMP-2 and -9). These results indicate that CTP attenuates collagen degradation in the extracellular matrix (ECM) by reducing MMP expression, thereby decreasing wrinkle formation in vivo.
Fig. 1.
Body weight changes in UVB-irradiated hairless mice. Mice were administered CTP for 14 weeks and concurrently exposed to UVB radiation three times a week. Control, vehicle +non-UVB irradiated; UVB control, vehicle+UVB irradiated; CTP-167, 167 mg/kg/day CTP+UVB irradiated; CTP- 333, 333 mg/kg/day CTP+UVB irradiated. Data are expressed as mean±SD of five mice in each group.
Fig. 2.
Effects of orally administered CTP on wrinkle formation in UVB-irradiated hairless mice (photographs and gross appearance). The dorsal skin surface of hairless mice was exposed to UVB three times a week for 14 weeks. Before sacrifice, photographs were taken from the dorsal areas of hairless mice.
Fig. 3.
Effects of oral administration of CTP on wrinkle formation in UVB-irradiated hairless mice (wrinkle values). The dorsal skin surface of hairless mice was exposed to UVB three times a week for 14 weeks. Wrinkle values were obtained from the surface of skin replica. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
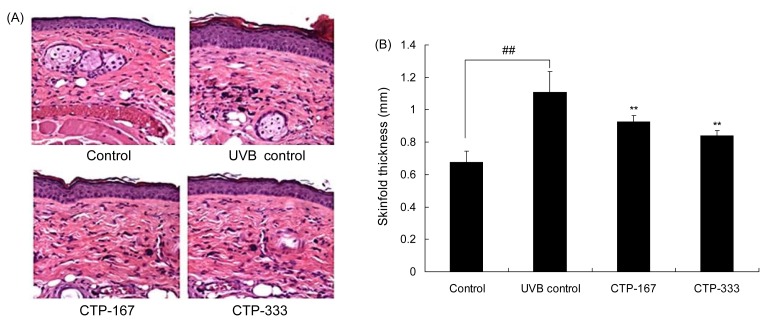
Effects of oral administration of CTP on skin thickness
Skin thickness of the hairless mice was measured by calipers before sacrifice. Histopathological changes were visualized by H&E staining. UVB irradiation significantly increased skinfold thickness by 1.1 mm. However, CTP-treated groups (167 or 333 mg/kg/day) had decreased skinfold thickness by 0.93 mm and 0.84 mm, respectively, compared with the UVB control group (Fig. 4A). UVB-induced epidermal thickness was visualized in histological sections stained with H&E. Similar to the results of skinfold thickness, skin thickness of CTP-treated groups was thinner than the UVB control group (Fig. 4B). Taken together, the results show that the skinfold thickness is significantly decreased in CTP-treated groups.
Fig. 4.
Effects of CTP administered orally on skin thickness in UVB-irradiated hairless mice. (A) Skin tissue sections were stained with H&E. (B) Skinfold thickness was measured with a caliper mid-way between the neck and hips at 14 weeks. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
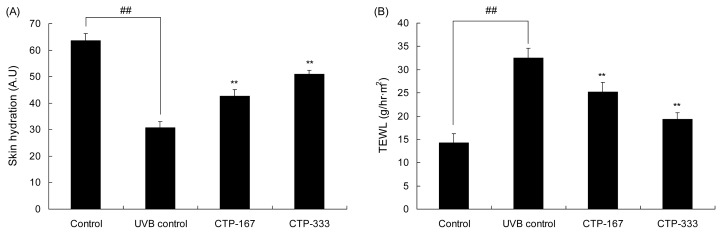
Effects of oral administration of CTP on skin barrier function
UVB irradiation causes skin damage, leading to skin dehydration and an increase in TEWL. The mechanisms of wrinkle formation are related to reduced water content, ceramide, and hyaluronan (18). As shown in Fig. 5A, skin hydration of the UVB control group declined by 51.7% compared with the control group. However, skin hydration of the CTP-treated groups (167 or 333 mg/kg/day) increased as much as 38.7% and 65.8%, respectively, compared to the UVB control group. The TEWL level of the UVB control group increased by 127.2% compared with the non-irradiated group, but the TEWL level of the CTP-treated groups (167 or 333 mg/kg/day) was reduced by as much as 22.7% and 40.5%, respectively, compared with the UVB control group (Fig. 5B). Skin hydration, maintained by preventing TEWL, affects skin barrier function. Enhanced skin barrier function can improve skin wrinkling (19,20). Therefore, CTP has a beneficial effect on the improvement of skin barrier function by decreasing TEWL and increasing skin hydration, thereby reducing skin wrinkling.
Fig. 5.
Effects of orally administrated CTP on skin barrier function in UVB-irradiated hairless mice. (A) Skin hydration and (B) TEWL as markers of skin barrier function were measured by Corneometer® and Tewameter® three days before the animals were sacrificed. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
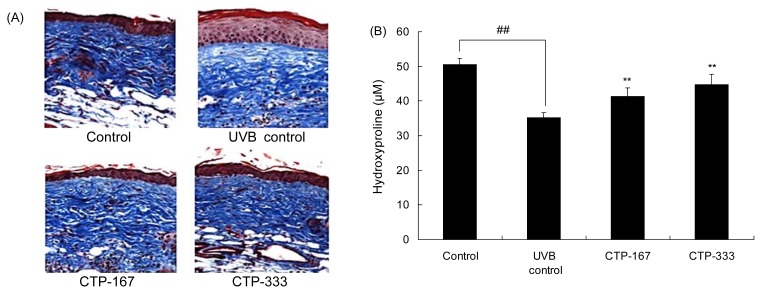
Effects of oral administration of CTP on collagen fibers
During skin aging, the spatial density of collagen bundles is reduced by decreased dermal collagens (21). In addition, degradation of collagen fibers is observed in both intrinsically aged and photoaged skin. Total collagen was estimated by measuring the content of hydroxyproline. Hydroxyproline is a specific amino acid of collagen and is used as a factor to estimate collagen contents. To observe the change in collagen fibers, dorsal skins were stained with Masson’s trichrome stain, and hydroxyproline contents were measured with the hydroxyproline assay kit. As shown in Fig. 6, UVB-irradiated collagen fibers were decreased but recovered with CTP treatment.
Fig. 6.
Effects of oral administration of CTP on collagen fibers in UVB-irradiated hairless mice. (A) Skin tissue sections were stained with Masson’s trichrome stain for collagen fibers. (B) Amount of hydroxyproline was estimated after UVB irradiation for 14 weeks. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
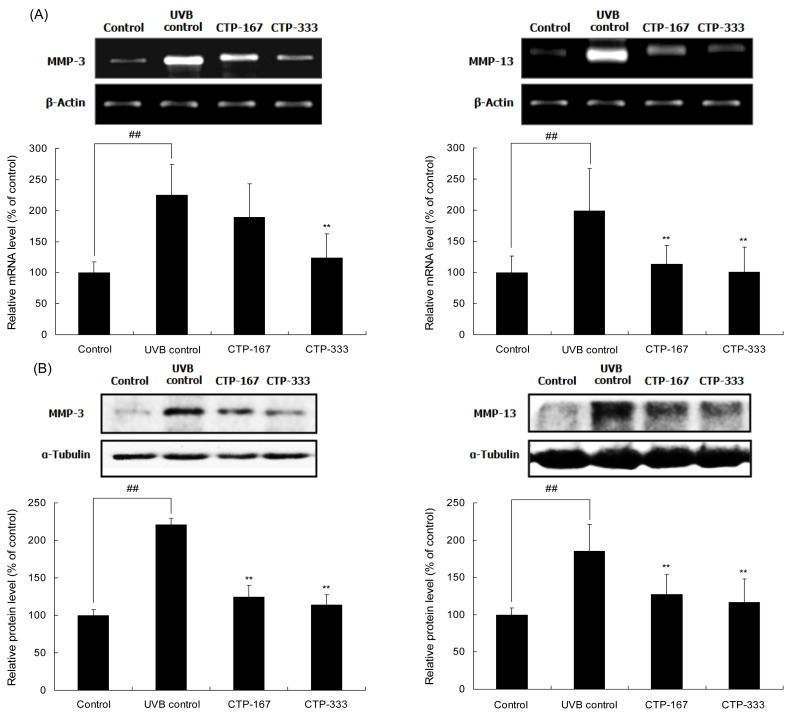
Effects of oral administration of CTP on UVB-induced MMP-3 and -13 activities
Deep wrinkles are caused by degradation of dermal collagen digested by MMPs. To understand how CTP inhibited UVB-induced wrinkle formation, MMP expression was determined by using RT-PCR and Western blotting analysis. MMP-3 and -13 mRNA expressions in the CTP-treated group (333 mg/kg/day) were reduced by as much as 44.7% and 42.9%, respectively, compared with the UVB control group (Fig. 7A). Additionally, CTP treatment with 333 mg/kg/day reduced UVB-induced MMP-3 and -13 protein expressions by 48.5% and 36.8 %, respectively (Fig. 7B). UVB-induced MMP-3 and -13 are related to the degradation of types I, III, and IV collagen. UVB irradiation increased MMP-3 and -13 expressions in hairless mouse skin, whereas oral treatment with CTP significantly abrogated this effect. Therefore, CTP inhibited dermal collagen breakdown and reduced wrinkle formation by suppressing MMP expression.
Fig. 7.
Effects of CTP administered orally on UVB-induced MMP-3 and -13 expressions in UVB-irradiated hairless mice. The mRNA levels (A) and protein levels (B) of MMP-3 and -13 were determined by RT-PCR and Western Blot analysis, respectively. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
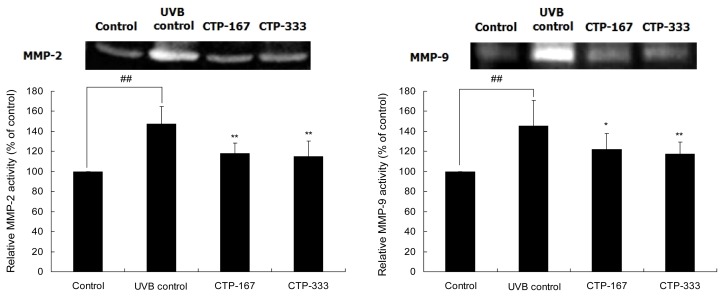
Effects of oral administration of CTP on UVB-induced MMP-2 and -9 activities
UVB-induced MMP-2 and -9 (gelatinase) activities may play an important role in the processes of UVB-induced skin damage, including skin thickening and wrinkle formation (4). To determine whether CTP could inhibit UVB-induced MMP-2 and -9 activities, gelatin zymography assay was assessed using dorsal skin of hairless mice. MMP-2 and -9 activities in the CTP-treated group (167 or 333 mg/kg/day) were reduced as much as 22.18% and 19.18%, respectively, compared with the UVB control group (Fig. 8). Oral administration of CTP significantly inhibited UVB-induced MMP-2 and -9 activation. Therefore, by suppressing MMP-2 and -9 activation, CTP inhibited the breakdown of dermal collagen and reduced wrinkle formation.
Fig. 8.
Effects of oral administration of CTP on UVB-induced MMP-2 and -9 in UVB-irradiated hairless mice. The amounts of MMP-2 and -9 activities in equal amounts of protein lysates were analyzed by gelatin zymography. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; *p<0.05, **p<0.01 compared with UVB-irradiated mice.
Effects of oral administration of CTP on elastic fibers
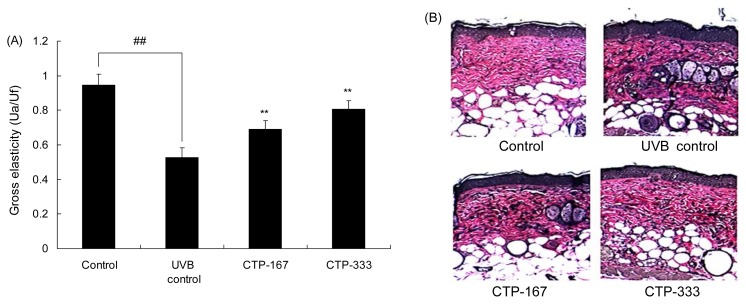
In vivo studies show that UVB irradiation activates elastin promoter, thereby increasing elastin production and accumulation of abnormal elastic fibers in photoaged skin (22). In addition, UVB irradiation reduced skin elasticity, decreased the linearity of dermal elastic fibers and collagen loss, and increased wrinkle formation (23). To examine the association of CTP with elastin, skin elasticity was measured by Cutometer®, and elastic fibers were stained by Verhoeffvan Gieson’s staining.
Administration of CTP increased the skin elasticity (Fig. 9A) and decreased abnormal elastic fiber formation (Fig. 9B). Elastin and collagen combine to give skin tensile strength with elasticity (24). Therefore, elasticity is enhanced by inhibiting production of abnormal elastic fibers and collagen degradation (25). These results suggest that CTP treatment inhibits accumulation of abnormal elastic fibers and degradation of collagen, leading to increased skin elasticity. CTP also prevented degradation of collagen by suppressing production of MMPs, thus enhancing skin elasticity related to synthesis of collagen and elastin.
Fig. 9.
Effects of orally administered CTP on elasticity in UVB-irradiated hairless mice. (A) Effects of CTP on gross elasticity (Ua/Uf) of skin were measured by Cutometer®. (B) Elastic fibers in the dermis were stained with Verhoeff’s stain. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
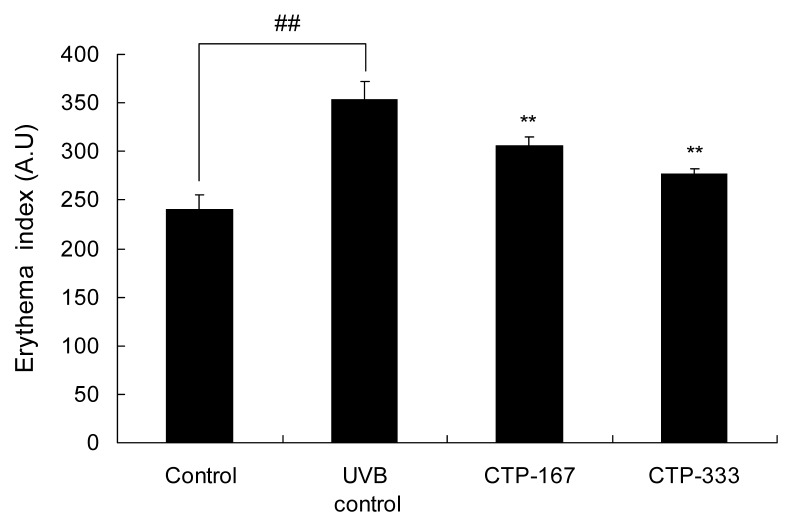
Effects of oral administration of CTP on UVB-induced erythema
UV irradiation causes erythema, which is characterized clinically by reticular hyperpigmentation and histologically by the degeneration of elastic fiber alteration (26). Erythema by UVB irradiation induces wrinkle formation on the dorsal skin of mice, and thus UVB-induced erythema is a suitable factor to assess wrinkle formation (27). In the UVB control group, erythema formation increased by 46.94% compared with the non-UVB irradiated group. Erythema of CTP-treated groups (167 or 333 mg/kg/day) was inhibited as much as 13.32% and 21.55%, respectively, compared with the UVB control group (Fig. 10). Therefore, the effects of oral administration of CTP on UVB-induced erythema may be associated with anti-wrinkle effects.
Fig. 10.
Effects of oral administration of CTP on erythema formation in UVB-irradiated hairless mice. After UV irradiation for 14 weeks, erythema was determined by Mexameter®. Data are expressed as mean±SD of five mice in each group. ##p<0.01 compared with non-UVB irradiated mice; **p<0.01 compared with UVB-irradiated mice.
Because CTP inhibits UVB-induced MMP expression and prevents wrinkle formation, oral CTP has potential to be an anti-photoaging agent. However, the molecular mechanisms of how CTP affects UVB-induced skin aging remain to be studied. In conclusion, the present study shows that CTP inhibits MMP-2, -3, -9 and -13 expressions, thereby reducing UVB-induced skin thickness and wrinkle formation. These findings demonstrate the potential of CTP for alleviating UVB-induced skin aging.
REFERENCES
- 1.Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2003;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung JH. Photoaging in Asians. Photodermatol Photoimmunol Photomed. 2003;19:109–121. doi: 10.1034/j.1600-0781.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 4.Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 5.Inomata S, Matsunaga Y, Amano S. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Invest Dermatol. 2003;120:128–134. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- 6.Mariani TJ, Sandefur S, Roby JD, Pierce RA. Collagenase-3 induction in rat lung fibroblasts requires the combined effects of tumor necrosis factor-alpha and 12-lipoxygenase metabolites: a model of macrophage-induced, fibroblast-driven extracellular matrix remodeling during inflammatory lung injury. Mol Biol Cell. 1998;9:1411–1424. doi: 10.1091/mbc.9.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci Biotechnol Biochem. 2009;73:930–932. doi: 10.1271/bbb.80649. [DOI] [PubMed] [Google Scholar]
- 8.Pei M, Yu C, Qu M. Expression of collagen type I, II, and III in loose body of osteoarthritis. J Orthop Sci. 2000;5:288–293. doi: 10.1007/s007760050165. [DOI] [PubMed] [Google Scholar]
- 9.Adam M, Spacek P, Hulejova H, Galianova A, Blahos J. Postmenopausal osteoporosis treatment with calcitonin and a diet rich in cartilage proteins. Cas Lek Ces. 1996;135:74–78. [PubMed] [Google Scholar]
- 10.Shigemura Y, Iwai K, Morimatsu F, Iwamoto T, Mori T, Oda C, Taira T, Park EY, Nakamura Y, Sato K. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J Agric Food Chem. 2009;57:444–449. doi: 10.1021/jf802785h. [DOI] [PubMed] [Google Scholar]
- 11.Naoki T, Rumiko Y, Yasuo S, Yoshino Y, Hideto Y. Promotion by collagen tripeptide of type I collagen gene expression in human osteoblastic cells and fracture healing of rat femur. Biosci Biotechnol Biochem. 2007;71:2680–2687. doi: 10.1271/bbb.70287. [DOI] [PubMed] [Google Scholar]
- 12.Noble JE, Bailey MJ. Quantitation of protein. Methods Enzymol. 2009;463:73–95. doi: 10.1016/S0076-6879(09)63008-1. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz E, Borchert HH. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema – an in vivo study. Int J Pharm. 2006;307:232–238. doi: 10.1016/j.ijpharm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein EF, Uitto J. The effect of photodamage on dermal extracellular matrix. Clin Dermatol. 1996;14:143–151. doi: 10.1016/0738-081x(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 16.Sharma MR, Werth B, Werth VP. Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH1 and Balb/c mice. Photochem Photobiol. 2011;87:690–698. doi: 10.1111/j.1751-1097.2011.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21:610–614. doi: 10.1016/s0190-9622(89)70227-9. [DOI] [PubMed] [Google Scholar]
- 18.Kambayashi H, Odake Y, Takada K, Funasaka Y, Ichihashi M. Involvement of changes in stratum corneum keratin in wrinkle formation by chronic ultraviolet irradiation in hairless mice. Exp Dermatol. 2003;12:22–27. doi: 10.1034/j.1600-0625.12.s2.4.x. [DOI] [PubMed] [Google Scholar]
- 19.Draelos ZD. The latest cosmeceutical approaches for anti-aging. J Cosmet Dermatol. 2007;6:2–6. doi: 10.1111/j.1473-2165.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 21.Moragas A, García-Bonafé M, Sans M, Torán N, Huguet P, Martín-Plata C. Image analysis of dermal collagen changes during skin aging. Anal Quant Cytol Histol. 1998;20:493–499. [PubMed] [Google Scholar]
- 22.Bernstein EF, Brown DB, Urbach F, Forbes D, Del Monaco M, Wu M, Katchman SD, Uitto J. Ultraviolet radiation activates the human elastin promoter in transgenic mice: a novel in vivo and in vitro model of cutaneous photoaging. J Invest Dermatol. 1995;105:269–273. doi: 10.1111/1523-1747.ep12318419. [DOI] [PubMed] [Google Scholar]
- 23.Imokawa G, Takema Y, Yorimoto Y, Tsukahara K, Kawai M, Imayama S. Degree of ultraviolet-induced tortuosity of elastic fibers in rat skin is age dependent. J Invest Dermatol. 1995;105:254–258. doi: 10.1111/1523-1747.ep12317607. [DOI] [PubMed] [Google Scholar]
- 24.Kim BS, Baez CE, Atala A. Biomaterials for tissue engineering. World J Urol. 2000;18:2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- 25.Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987–1000. doi: 10.1002/ptr.1363. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz RM, Tisserand ME. Erythema ab igne. Arch Dermatol. 1987;123:21–23. [PubMed] [Google Scholar]
- 27.Takema Y, Fujimura T, Ohsu H, Imokawa G. Unusual wrinkle formation after temporary skin fixation followed by UVB irradiation in hairless mouse skin. Exp Dermatol. 1996;5:145–149. doi: 10.1111/j.1600-0625.1996.tb00109.x. [DOI] [PubMed] [Google Scholar]