Abstract
Over the past decade, nanopores have rapidly emerged as stochastic biosensors. This protocol describes the cloning, expression, and purification of the channel of bacteriophage phi29 DNA packaging nanomotor and its subsequent incorporation into lipid membranes for single-pore sensing of dsDNA and chemicals. The membrane-embedded phi29 nanochannels remain functional and structurally intact under a range of conditions. When ions and macromolecules translocate through these nanochannels, reliable fingerprint changes in conductance are observed. Compared with other well studied biological pores, the phi29 nanochannel has a larger cross-sectional area, which enables the translocation of dsDNA. Furthermore, specific amino acids can be introduced by site-directed mutagenesis within the large cavity of the channel to conjugate receptors that are able to bind specific ligands or analytes for desired applications. The lipid membrane embedded nanochannel system has immense potential nanotechnological and biomedical applications in bioreactors, environmental sensing, drug monitoring, controlled drug delivery, early disease diagnosis, and high-throughput DNA sequencing. The total time required for completing one round of this protocol is around one month.
Keywords: bacteriophage phi29, connector, nanopore, liposomes, ion channel, single channel conductance, single pore, DNA packaging motor, membrane channel, bionanotechnology, nanobiotechnology, high throughput DNA sequencing
INTRODUCTION
DNA, RNA and proteins have intrinsically defined features at the nanometer scale and can serve as building blocks for bottom-up fabrication of nanostructures in materials engineering and synthetic biology. The DNA packaging motor of bacteriophage phi29 (Fig. 1a, b) uses a hexameric packaging RNA (pRNA) ring and an ATP-dependent enzyme as the motor gearing components. The motor contains a so-called connector that forms a channel serving as a path for dsDNA translocation. The phi29 nanomotor was reconstituted in a defined system over twenty years ago1 and remains one of the most well-studied systems. It is also one of the most powerful molecular motors, generating forces up to 57–110 pN2, 3. A number of biophysical and structural studies of the phi29 motor have been carried out over the past three decades in Peixuan Guo's laboratory; results from these studies include the construction of the bacteriophage phi29 DNA packaging motor in vitro;1 discovery of phi29 motor pRNA;4 assembly of infectious viruses of phi29 in vitro using purified components of the DNA packaging motor;5 and elucidation of the formation of the pRNA hexamer,6-9 an achievement that laid the foundation for RNA nanotechnology.10-13
Figure 1. Structure of phi29 DNA packaging motor and connector nanochannel.
(a, b) An illustration of DNA translocation through the phi29 DNA packaging motor. (c) Side view of the phi29 connector showing the acidic (red), basic (blue), and other (white) amino acids. (d) Top view of the connector showing the diameter of the narrow part and wide part of the channel. Figures reproduced with permissions from: (a) Ref. 22, © American Chemical Society; (b–d) Ref. 20, © Nature Publishing Group.
Explicit protein engineering via site-directed mutagenesis of the phi29 connector is possible because the crystal structure of the wild-type connector has been elucidated.14-16 It is therefore feasible to reengineer the connector by introducing specific binding elements at strategic positions (at the C- or N-termini or in the interior of the channel) for desired applications17-19. The phi29 connector consists of 12 subunits of the gp10 protein that form a central channel shaped like a hollow truncated cone. The native connector's wider C-terminal end is buried within the procapsid, while its narrow N-terminal end partially protrudes out of the procapsid. The central pore has a cross-sectional area of 10 nm2 (3.6 nm in diameter) at the narrow end, and 28 nm2 (6 nm in diameter) at the wide end14-16(Fig. 1c, d).
Surface charge analysis of the connector revealed that its central region is slightly hydrophobic compared to the flanking hydrophilic C- and N-termini.20 The wild-type connector was reengineered to include a his-tag (for affinity purification) downstream of a six glycine linker (for affinity tag flexibility) at the N- or C-terminus. The modified connector retained its 12-fold symmetric structure and its ability to package DNA after incorporation into the procapsid. The reengineered phi29 connector was then inserted into a lipid bilayer.20 The resulting bilayer-embedded nanopore system is robust and generates extremely reliable, precise, and sensitive current signatures, when ions or DNA pass through the channel, as revealed by single-channel conductance measurements.20-24 The pore sizes of reengineered channels are nearly identical from sample to sample, which demonstrates the extreme reproducibility of the approach at the atomic level20, 21, 23. Within the large cavity of the pore, substrate-specific functionalities can be introduced by chemical conjugation with relative ease.25 The procedures for large-scale production and purification of the connector have also been developed.17, 26-29 This protocol focuses on the cloning, expression and purification of the reengineered connector protein and its insertion into a lipid membrane setup, thereby creating a robust lipid bilayer-embedded nanopore platform that is capable of single-molecule detection of biopolymers and chemicals.
Comparison with other methods
Over the years, besides phi29 channels, several other biological pores have been extensively employed for nanopore-based sensing applications. These include α-hemolysin, Mycobacterium smegmatis porin A (MspA), and more recently, Ferric hydroxamate uptake component A (FhuA) channels. The crystal structures of these four channels are available, so that rational modifications by site-directed mutagenesis or chemical means can be carried out to reengineer mutant channels for desired applications.20, 30-34 Some fundamental differences exist between these channels with respect to structure and biophysical characteristics, especially with regard to nucleic acid translocation.
α-Hemolysin is a heptameric transmembrane pore that consists of an extramembranal cap connected to a transmembrane β-barrel. The narrowest cross-section of the hollow part of the pore is at the vestibule-transmembrane domain junction with a diameter of ~1.4 nm.35 To date, α-hemolysin has been the benchmark for sensing an extremely wide range of analytes, from metal ions, to organic molecules, to DNA, RNA, and peptides.36-41 Another nanopore is MspA, which is a octameric funnel-shaped channel with a diameter of ~1.2 nm at the narrowest point and a length of ~0.5 nm.30 α-hemolysin and MspA are both very robust pores and retain channel forming ability even at extreme pH (pH 2–12) and high temperatures (close to 100 °C)42-44. Given their small pore sizes at the narrowest constriction, the research applications of α-hemolysin and MspA are limited to the translocation of ssDNA, since dsDNA has a width of ~2 nm. In fact, although some evidence exists that some channels are capable of dsDNA transport,45-47 these nanopores have limited sensing applications due to their gating properties, variations in open-pore current due to conformational changes of protein as well as in response to changes in electric potential difference, and fluctuations of conductance (the reciprocal of resistance), defined as the ratio of current and voltage, under various experimental conditions. Over the past couple of years, a larger transmembrane β-barrel protein derived from the outer membrane of E. Coli, FhuA, has been inserted into a lipid bilayer to serve as a nanopore.33, 48 Compared with other channels, FhuA channels are monomeric in nature and are characterized by an elliptically shaped barrel (cross-sectional dimensions ~3.1 x 4.4 nm).33, 48 Heavily re-engineered FhuA channels remain active and structurally intact under a wide range of experimental conditions, so they can potentially be used for diverse purposes, such as examining the proteolytic activity of enzymes and studying protein–DNA kinetics33. However, the capability of FhuA channels to translocate dsDNA is yet to be demonstrated.
In order to achieve cross-membrane translocation of dsDNA, some researchers have switched to fabricating synthetic pores.49-53 The phi29 connector channel is the first viral protein channel that is neither a membrane protein nor an ion channel that has been reconstituted into a lipid bilayer.20 Bilayer-embedded connector channels exhibit biophysical and biochemical robustness under different experimental conditions, as revealed by single channel conductance measurements.20-25 The most significant advantage of the phi29 nanochannel with respect to the aforementioned systems is that it has a larger channel diameter, and hence it can easily translocate dsDNA as well as ssDNA. Furthermore, the large cavity size of this channel enables the relatively easy insertion of functional moieties with desirable characteristics via conjugation to amino acid residues lining the channel cavity or at the terminal ends for diverse applications.
Unlike the cases of α-hemolysin, MspA and FhuA, which can form protein channels in lipid membrane by simultaneous assembly and direct insertion, lipid membrane insertion of phi29 connector channels is a two-step process. The first step consists in the reconstitution of purified reengineered connectors into liposomes, and the second in the fusion of proteoliposomes with a planar lipid bilayer.20 The resulting system can then be studied by single-channel conduction assay using a conventional patch clamp setup, as used in other nanopore systems. Instrumentation and setup design protocols for synthetic and protein nanopore systems have been described previoulsy54. In general, the setup for electrophysiological assays requires: (1) a Faraday cage, to shield the assay setup from electromagnetic interference; (2) a vibration-isolating air table, to shield the assay setup from mechanical vibrations); (3) a high quality amplifier, to amplify and filter the electric current signal; (4) data acquisition hardware and software; (5) membrane support (such as bilayer cuvettes and chambers); (6) a source of illumination (for instance, a halogen lamp); (7) a stir plate; (8) a perfusion system, to exchange solutions; and (9) a thermo-cycler, to control temperature.
Potential Applications
The protocol described herein on the cloning, expression and purification of the re-engineered portal channel proteins, and their subsequent insertion into lipid membranes, can also be applied to portal channel proteins from other bacteriophages, such as SPP1, T3, T4, T5, T7, HSV-1, T7, ε15, λ, and P22, for desired nanotechnology and biotechnology applications.55
Nanopore-based sensing has a wide range of potential applications, for instance in the sensing of species as minute as single molecules of chemicals or as large as individual biopolymers.56-59 The single-molecule recognition approach that involves the use of nanopores is high-throughput and label-free, it requires very little sample volume and does not involve any sample preparations or modifications. Over the past decade, nanopore-based sensory techniques have been extensively used for diverse applications, including, but not limited to: sensing nucleic acid polymer structure and dynamics;20, 31, 36, 37, 60 potential sequencing of DNA;32, 34, 61 studying the mechanism of viral DNA packaging;22-24, 62 real-time sensing of chemicals via molecular adapters and non-covalent and/or covalent interactions;25, 63-65 detection of substrates that are relevant to medical diagnostics;66-70 studying single-molecule dynamics in RNA–protein, protein–protein or protein–ligand interactions;38, 71-74 generating biomimetics, such as substrate-responsive switches75 and biological sensors;76 investigating the possibility of sensing biopolymers like peptides and proteins64; and constructing bioreactors.77
Limitations
A significant challenge in the utilization of all biological pores is the mechanically fragile nature of the lipid matrix, which is kept together by weak intermolecular interactions and has a lifetime of several minutes to hours. This problem can in part be circumvented by using polymer membranes, solid supports, or droplet–interface bilayer.64, 78, 79 The employment of the mentioned approaches to decrease the fragility of the lipid matrix is particularly relevant when the objective is to integrate the biological pores in robust sensing platforms for high-throughput substrate detection.
MATERIALS
REAGENTS
1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) (Avanti Polar Lipids, cat. no. 850356C)
1,2- Dioleoyl-sn-Glycero-3-Phosphocholine (DOPC) (Avanti Polar Lipids, cat. no. 181PC-243)
N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (NBD-PE), (Invitrogen, cat. no. N 360)
Chloroform (Fisher, cat. no. C 298-500) ! CAUTION: Carcinogen; harmful by ingestion; skin and eye irritant. Wear gloves and goggles and dispense in a vented hood
n-Decane (Fisher, cat. no. O2128-500) ! CAUTION: Flammable liquid and vapor. Skin and eye irritant
Hexane (Fisher, cat. no. H 292-500) ! CAUTION: Flammable liquid and vapor. Skin and eye irritant
Tris (Fisher, cat. no. BP-152-5) ! CAUTION: Eye irritant
Hydrochloric acid (HCl) (Fisher, cat. no. A144-212) ! CAUTION: strong acid; very corrosive, and can cause severe burns. Wear gloves and goggles and dispense in a vented hood
Ethylenediaminetetraacetic acid (EDTA) (Fisher, cat. no. AC11843-0010) ! CAUTION: Skin, eye, and respiratory tract irritant
Ethanol, Absolute (200 Proof), Molecular Biology Grade (Fisher, cat. no. BP2818) ! CAUTION: Flammable liquid
Clorox® Regular-Bleach (VWR, cat. no. 37001-058). CAUTION: Corrosive, and can cause severe irritation or damage to eyes and skin. Harmful if swallowed
Restriction enzymes (New England BioLabs Inc.): SnaBI (cat. no. R0130S); ScaI (cat. no. R0122T), BstNI (cat. no. R0168S); EcoRI (cat. no. R0101T); SmaI (cat. no. R0141S); XhoI (cat. no. R0146S); NheI (cat. no. R0131S); BamHI (cat. no. R0136T); XbaI (cat. no. R0145T); NdeI (cat. no. R0111S); XhoI (cat. no. R0146S); EcoRV (cat. no. R0195L)
Ethidium bromide (Fisher, cat. no. P1302-10) ! CAUTION: Strong carcinogen; skin, eye, and respiratory tract irritant. Wear gloves and dispense in the hood
Amicon® Centricon® Centrifugal Filter Devices (YM-30) (Millipore, cat. no. 4210)
Qiaex II gel extraction kit (Qiagen, cat. no. 20051)
T4 DNA ligase (New England BioLabs, cat. no. M0202T)
Vector pET-21a(+) (Novagen) (Millipore, cat no. 69740-3)
Luria-Bertani (LB) medium (BD, cat. no. 244610)
Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Sigma, cat. no. I6758)
Ammonium persulfate (APS) (Sigma, cat. no. A3678). ! CAUTION: Eye irritant
N,N,N′,N′-Tetramethylethylenediamine (TEMED) (Sigma, cat. no. T9281). ! CAUTION: Skin, and respiratory tract irritant
DNA polymerase (Promega, Go Teq@, cat. no. M829)
Ampicillin (Fisher, cat. no. BP 1760-25) ! CAUTION: May be skin, eye, and respiratory tract irritant; May cause allergic reaction
Dithiothreitol (DTT) (Invitrogen, cat. no. P2325) ! CAUTION: Eye irritant
DNase I (RNase free) (Thermo Scientific, cat. no. EN0521)
RNase A (DNase and protease-free) (Thermo Scientific, cat. no. EN0531)
Acrylamide-bisacrylamide (37.5:1, wt/wt), 30% (Fisher, cat. no. BP1408-1)
Xylene cyanol (Sigma, cat. no. X-4126) ! CAUTION: Skin, and respiratory tract irritant.
Bromophenol blue (Sigma, cat. no. B-8026) ! CAUTION: Skin, and respiratory tract irritant
Poly-Prep Chromatography Columns (Bio-Rad Laboratories, Inc., cat. no. #731-1550)
Sodium Chloride (NaCl) (Fisher, cat. no. AC 11843-0010)
Adenosine Triphosphate (ATP) (Sigma, cat. no. A2383-25G)
Glycerol (Fisher, cat. no. G33-4)
Sodium Phosphate Tribasic (TSP) (Fisher, cat. no. S377-500)
Fluor Tag™ Fluorescein Isothiocyanate (FITC) conjugation kit (Sigma, cat. no. FITC1-1KT)
Cellulose acetate membrane- 0.45 µm (Life Science Products, Inc. cat. no. 10404026)
iQTM SYBR Green Supermix (Bio-Rad, cat. no. 170-8880)
Methylene chloride (CH2Cl2) (Fisher, cat. no. D37-1)
Stratagene Plasmid pBluescript (KS+) (Agilent Technologies, cat. no. #212207)
E. Coli DH5α™ Competent Cells (Invitrogen, cat. no. 18258-012)
PureYield™ Plasmid Maxiprep System (Promega, cat. no. A2393)
Novagen E.coli strain HMS174 (DE3) (EMD Millipore, cat. no. 69453)
His●bind resin (Novagen, EMD Millipore, cat. no. 69670)
His●bind buffer kit (Novagen, EMD Millipore, cat. no. 69755-3)
Strep-Tactin® Sepharose® (IBA, cat. no. #2-1201-500)
Open-top polyallomer centrifuge tubes (0.5 × 2 inch) (Seton Scientific, part no. 5022)
Dow Corning* High-Vacuum Grease (Fisher, cat. no. 146355)
EQUIPMENT
Freezer (–80 °C, and –20 °C) and 4 °C refrigerator
Access to UV spectrophotometer, ultracentrifuge, Milli-Q water purification system, optical and fluorescence microscopy, PCR machine (such as, Eppendorf master thermal cycler), real-time PCR detection system (such as, Roche LightCycler® 480 Real-Time PCR System), Nuclear Magnetic Resonance (NMR), Mass spectrometry (MS), Ultracentrifuge (such as, Beckman L-80 ultracentrifuge), and DNA Sequencing facilities
Vesicle Prep Pro system (Nanion Technologies, cat. no. 15-1001)
Gradient Master (Biocomp instruments, cat. no. 107-201M)
Optical microscope (Motic® company, cat.no.SFC-11)
Rotary evaporator (BÜCHI Labortechnik AG, cat. no. Rotavapor® R-210/R-215)
Vacuum Pump - oil free (BÜCHI Labortechnik AG, cat. no. V-700)
Vacuum Controller (BÜCHI Labortechnik AG, cat. no. V-850)
Mini-Extruder set (Avanti Polar Lipids, cat. no. 610000)
Gas-tight Syringe, 1000μL (Avanti Polar Lipids, cat. no. 610017)
Gas-tight Syringe, 250μL (Avanti Polar Lipids, cat. no. 610015)
100 nm polycarbonate membranes (Avanti Polar Lipids, cat. no. 610005)
400 nm polycarbonate membranes (Avanti Polar Lipids, cat. no. 610007)
Tabletop Ultrasonic Cleaner (Branson Ultrasonic Corporation Model 1510; cat. no. 610022)
French Press (SLM instrument INC, cat. no. FA078)
BLM Cells (horizontal chamber) (Eastern Scientific LLC. cat. no. BCH-1A)
Mini dish for BCH-1 (Eastern Scientific LLC, cat. no. MD-1)
Teflon Partition with 0.2 mm aperture (Eastern Scientific LLC, cat. no. TP-02)
BCH-13A (vertical chamber) (Warner Instruments LLC, cat. no. CD13A-200)
13-mm delrin cuvette with 200-μm aperture (Warner Instruments LLC, cat. no. 640409)
1-mm pin with 10-cm long, and 0.25-mm diameter bare silver wire package (Warner Instruments LLC, cat. no. 641327)
Bilayer thermocycler system (Warner Instruments LLC, cat. no. 640450)
Ag wire, 70 mm length, 10 pack (Warner Instruments LLC, cat. no. 64-1282 (AG25-10))
Ag/AgCl electrode, pellet, 1.5×3.0 mm (Warner Instruments LLC, cat. no. 64-1304 (E200))
1 mm pin, 10 pack (Warner Instruments LLC, cat. no. 64-1325(WC1-10))
Axon Axopatch 200B Capacitor Feedback Patch Clamp (Molecular Devices, LLC)
Axon Digidata 1440A Data Acuisition System (Molecular Devices, LLC)
PCLAMP 10 CNS software (Clampex and Clampfit) (Molecular Devices, LLC)
- Planar Lipid Bilayer workstation (Warner Instruments, LLC).
- ○ Bilayer clamp amplifier, resistive feedback (cat. no. 64-0432)
- ○ Faraday cage with active isolation table (cat. no. 64-0064)
- ○ Low pass 8-pole Bessel filter (cat. no. 64-0050)
- ○ SPIN-2,SUN-1,SUN Stir controller bundle (cat. no. 64-0076)
- ○ Bilayer perfusion system (cat. no. 64-0431)
- ○ Planar Lipid Bilayer Thermocycler (cat. no. 64-0450)
- ○ Headstage holder system (cat. no. 64-0435)
- ○ 14” tabletop instrument rack (cat. no. 64-0070)
- ○ Bilayer starter kit (cat. no. 64-0067)
REAGENT SETUP
▲CRITICAL STEP: Prepare all reagents and perform all experiments using pure water (Milli-Q, 18.2 MegaΩ/cm resistivity).
Tris-EDTA (TE) Buffer: 50 mM Tris-HCl (pH 7.8), 10 mM EDTA. This buffer can be stored at room temperature (RT, 23 °C) for 1 year.
Restriction and ligation enzyme buffers and reaction solutions: Use manufacturer (New England BioLabs, Inc: http://www.neb.com/nebecomm) supplied reagents for respective restriction enzymes. This buffer can be stored at –20°C till the manufacturer's expiration date.
1 × Tris-Borate EDTA (TBE) buffer: 89mM Tris, 200mM boric acid, 2mM EDTA. This buffer can be stored at RT for 1 year.
1 × Tris-Acetate EDTA (TAE) buffer: 40mM Tris-acetate and 1mM EDTA. This buffer can be stored at RT for 1 year.
0.8% (wt/vol) Agarose gel: Add 0.32 g agarose in 40 ml 1 × TBE or 1 × TAE buffer. Melt the agarose in the microwave and let it cool down to around RT. Add 1 μl of ethidium bromide and pour it in a gel caster. The gel needs to be prepared fresh. ! CAUTION: Ethidium bromide is a strong carcinogen; skin, eye, and respiratory tract irritant. Wear gloves and dispense in the hood.
12% Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE): 1.5M Tris-HCl (pH 8.8), 20% (wt/vol) sodium dodecyl sulfate (SDS), Acrylamide/Bis-acrylamide (30%/0.8% wt/vol), water, 10% (wt/vol) APS, TEMED; Stacking gel: 0.5M Tris-HCl (pH 6.8), 20% (wt/vol) SDS, Acrylamide/Bis-acrylamide (30%/0.8%, wt/vol), water, 10% (wt/vol) APS, TEMED. The gel needs to be prepared fresh.
2 × SDS-loading buffer: 100mM Tris-Cl (pH 6.8), 4% (wt/vol) SDS, 0.2% (wt/vol) bromophenol blue, 20% (vol/vol) glycerol, 200mM dithiothreitol (DTT). Store the SDS gel-loading buffer without DTT at RT. This buffer can be stored at 4 °C for 1 year. Add DTT from a 1M stock just before the buffer is used. 1M DTT can be stored at –20 °C for 1 year.
SDS running buffer: 25mM Tris-Cl (pH 8.3), 192mM glycine, 0.1% (wt/vol) SDS. This buffer can be stored at RT for 1 year.
10–15% (wt/vol) Urea denaturing PAGE: 10–15% (wt/vol) (37.5:1) acrylamide, 8M urea, 10% (wt/vol) APS, TEMED. The gel needs to be prepared fresh.
10–15% (wt/vol) native PAGE (TBM or TBE): 10–15% (37.5:1) acrylamide, 1× Tris-borate (TB) buffer (pH 7.8); 10mM MgCl2 (or 2mM EDTA); 10% (wt/vol) APS, TEMED. The gel needs to be prepared fresh.
Native PAGE loading buffer (1×final concentration): 50% (vol/vol) glycerol, 1×TBE buffer, 0.01% (wt/vol) bromophenol blue and 0.01% (wt/vol) xylene cyanol tracking dyes. This buffer can be stored at RT for 1 year.
Cell-washing buffer: 5% glycerol (vol/vol), 0.5M NaCl, 1mM EDTA, 10mM ATP, 100mM Tris-HCl, (pH 8.0). Replace 100mM Tris-HCl with 50mM Phosphate buffer (pH 7.0) for FITC labeling or 40mM MOPS (pH 8.0) for Analytical Ultracentrifugation. This buffer can be stored at 4 °C for 1 year.
Connector elution buffer: 5% glycerol (vol/vol), 0.5M NaCl, 1mM EDTA, 10mM ATP, 100mM Tris-HCl (pH 8.0), 2.5mM desthiobiotin for Strep tag connector. This buffer can be stored at 4 °C for 1 year.
Phosphate Buffer Saline (PBS): 137mM NaCl, 2.7mM KCl, and 10mM phosphate (pH 7.4). This buffer can be stored at RT for 1 year.
1 × TMS buffer: 50mM Tris (pH 8.0), 100mM NaCl, 10mM MgCl2. This buffer can be stored at RT for 1 year.
Proteoliposome rehydration buffer: 250mM sucrose, 1M NaCl, 5mM Tris (pH 7.6). This buffer can be stored at RT for 1 year.
BLM Cleaning Solution 1: Sodium Phosphate Tribasic (TSP): Place a tablespoon in a 500 ml wash bottle filled with Milli-Q water to prepare a dilute detergent solution. This buffer can be stored at RT for 1 year.
BLM Cleaning Solution 2: 0.1% (vol/vol) HCl. This buffer can be stored at RT for 1 year.
Conduction buffers: 5mM Tris (pH 7.9); or 5mM HEPES (pH 7.9), 1M NaCl or KCl (typically). Alter the salt concentration depending on desired application. This buffer can be stored at RT for 1 year.
Lipid solution A: DPhPC or DOPC lipids in hexane (concentration: 0.5 mg/ml). This buffer can be stored at –20 °C for 3 months.
Lipid solution B: DPhPC or DOPC lipids in n-Decane (concentration: 20–30 mg/ml). This buffer can be stored at –20 °C for 3 months.
PROCEDURE
Cloning of the gene coding for wild-type phi29 connector ●TIMING 1–2 wk
Extract the DNA of wild-type bacteriophage phi29 virion isolated from soil80 by heating at 70 °C for 15 min 5 μl of phi29 virion (1014 PFU/ml) in 100 μl TE buffer. This procedure will enable the isolation of 1–5 μl of pure DNA.
Digest the DNA obtained in step 1 sequentially with restriction enzymes SnaBI (cut site 11303), ScaI (cut site 5192), and BstNI (cut site 2742), following the guidelines of enzyme manufacturers. These digestions will result in the production of four DNA fragments of lengths 2,742, 2,450, 6,111, and 7,977 bp.5, 81-83
Purify the 6,111-bp fragment, which contains the gene for gp10, by electro-elution from a 0.8% (wt/vol) agarose gel as follows: Stain the agarose gel for 20 min in ethidium bromide staining solution at RT. Move the stained agarose gel under UV light. Excise out the front part of leading edge of the 6,111-bp DNA band. This excision will cause the formation of a well in front of the band. Try to keep the width of the well as narrow as possible (typically <0.5 mm). Insert a dialysis membrane into the excised well and push it under the agarose gel where the 6,111-bp DNA is positioned. Fill the well to the top of the gel with TAE buffer. Resume electrophoresis for ~15 min at 150 V until the DNA band has moved out of gel and into the well. Switch electrodes to apply reverse electrical field for 30 s at 150 V. Stop the electrophoresis and pipette out the DNA solution from the well carefully. Centrifuge the solution from the well for 5 min at 13,000 ×g force to cause the precipitation any agarose gel remnants, and collect the supernatant.
! CAUTION: Ethidium bromide is a strong carcinogen; skin, eye, and respiratory tract irritant. Wear gloves and dispense in the hood.
-
4
Concentrate the DNA fragment from step 3 by centrifugation using Centricon-YM-30 tubes (follow manufacturer's guidelines), and purify the DNA using Qiaex II gel extraction kit (follow manufacturer's guidelines).
-
5
Digest the resulting DNA fragment with EcoRI (cut site 9862) following the enzyme manufacturer's guidelines. This restriction will produce two fragments of 4,670 and 1,441 bp.
-
6
Restrict 1 μg of plasmid pBluescript (KS+) with EcoRI and SmaI following the enzyme manufacturer's instruction.
-
7
Purify the 1,441-bp EcoRI/SnaBI fragment from step 5, which contains parts of gene 10, from 0.8% agarose gel (see step 3). Insert the purified DNA fragment into the EcoRI/SmaI site of pBluescript (KS+), to generate plasmid pBlue10 using T4 DNA ligase (following enzyme manufacturer's instructions).
-
8
Restrict 1 μg of plasmid pBluescript (KS+) with EcoRI and XhoI following the enzyme manufacturer's guidelines.
-
9
The 4,670-bp DNA fragment, which contains part of gene 10, had an XhoI linker ligated to the ScaI blunt end. Ligate this fragment into the vector pBluescript (KS+) at the EcoRI/XhoI site using T4 DNA ligase (following manufacturer's instructions), thereby generating plasmid pBlueXhoI.
-
10
Restrict 1 μg plasmid pBlue10 with EcoRI/BamHI following the enzyme manufacturer's guidelines. Purify the 1,441-bp EcoRI/BamHI fragment from 0.8% agarose gel (see step 3).
-
11
Restrict 1 μg of plasmid pBlueXhoI with EcoRI/BamHI following the enzyme manufacturer's guidelines. Purify the 4,670-bp EcoRI/BamHI fragment from 0.8% agarose gel (see step 3).
-
12
Ligate the two purified DNA fragments (from step 10 and 11) into the EcoRI/BamHI sites of pBlueXhoI, to generate plasmid pBlue6K using T4 DNA ligase (following manufacturer's instructions). The plasmid pBlue6K will contain the two fragments, 1441-bp (excised from pBlue10) and 4670-bp (excised from pBlueXhoI).
-
13
Transform the plasmid into E. coli cells HMS17484.
-
14
Restrict 1 μg of plasmid pBlue6K with NheI/BamHI following the enzyme manufacturer's guidelines. Purify the 5,643-bp NheI/BamHI fragment from 0.8% agarose gel (see step 3).
-
15
Restrict 1 μg of plasmid pARgp75, 81-83 with NheI/BamHI following the enzyme manufacturer's guidelines. Ligate the 5,643-bp NheI/BamHI fragment from step 14 to plasmid pARgp7 at the NheI/BamHI sites using T4 DNA ligase (following enzyme manufacturer's instructions) to generate plasmid pARgp7-8-8.5-10.
-
16
Transform the plasmid into E. coli cells HMS17484.
-
17
Restrict 1 μg of plasmid pARgp7-8-8.5-10 with XbaI following the enzyme manufacturer's guidelines. Remove the XbaI fragment via 0.8% agarose gel. Self-ligate the plasmid using T4 DNA ligase to produce a plasmid pAR10.
-
18
Introduce a Strep-Tag (option A) or a His-Tag (Option B) at the terminal of the connector protein gp10.
Option A. Introduction of a terminal Strep-Tag.
Use the primer pair F1–R1 to amplify the GP10 gene in plasmid pAR10. Use ~1 μl of 1nM DNA template and 10 μl of 100μM each primer for 200 μL PCR reaction. The PCR protocol is as follows: 5 min at 95 °C and then 30 cycles of 1 min at 95 °C, 1 min at 55 °C, 1 min at 72 °C, with a final extension of 10 min at 72 °C.
Use the first PCR product as a template for a second step of PCR with primers F1, which contains the restriction site for NdeI, and R2, which contains affinity Strep-Tag as well as the restriction site for XhoI (Fig. 2). Use ~1 μl of 1nM DNA template and 10 μl of 100μM each primer for 200 μL PCR reaction and follow the same PCR protocol as in step 18A (i).
Figure 2. Example of plasmid construction for over-expression of connectors with C-terminal modification by two-step PCR.
(a) The linker was attached to the 3’-end of GP10 gene in the first PCR by a primer pair F1-R1. In a second PCR, amino acids were incorporated downstream using primer pair F1-R2, which contained NdeI and XhoI restriction sites, respectively. (b) The second PCR product was digested with both NdeI and XhoI, an ligated into the NdeI/XhoI sites of the vector pET-21a(+). (c) Sequences of primers. (d) Summary of the connector protein extension constructs. Underlined sequence represents TEV (Tobacco Etch Virus) protease recognition sequence; ↓ denotes the TEV cleavage site. Figures reproduced with permissions from: (a–c) Ref. 20, © Nature Publishing Group.
Option B. Introduction of a terminal His-Tag.
To introduce His-Tag at the terminal of the connector protein gp10, only one-step PCR is necessary. Use the primer pair F1–R3 to amplify the GP10 gene following the same PCR protocol as mentioned in step 28A (Fig. 2C).
-
19
Purify the PCR product by 0.8% agarose gel (see step 3), then digest the DNA product with NdeI and XhoI using the enzyme manufacturers’ directions.
-
20
Digest the vector pET-21a(+) with NdeI and XhoI employing the enzymes manufacturers’ instructions.
-
21
Ligate the PCR product from step 19 into the digested vector pET-21a(+) from step 20 to generate plasmid pETgp10-C-strep or pETgp10-C-his using T4 DNA ligase (following manufacturer's instructions).
-
22
Transform the ligated mixture into competent E. coli DH5α cells to amplify plasmid DNA following the cell manufacturers’ directions.
-
23
Purify the plasmid DNA extracted from transformed DH5α cells using Plasmid Maxiprep purification kit (following manufacturer's instructions).
-
24
Verify the recombinant DNA sequence by sequencing it (for example using Genwiz).
Expression and Purification of the reengineered phi29 connector ●TIMING: ~3 days
-
25
Transform E. coli strain HMS174 (DE3) following the cell manufacturers’ directions with plasmid pETgp10-C-his for His-tagged connector protein or pETgp10-C-strep for Strep-tagged connector protein. Incubate at 37 °C overnight.
-
26
Incubate a colony from the plate in 10 ml LB medium containing 50 μg/ml ampicillin at 37 °C with shaking at 250 r.p.m. until OD600 reaches 0.5–0.6 (~3 h).
-
27
Dilute the cell culture at least 100-fold. Induce expression of the connector protein by adding IPTG to a final concentration of 0.5mM and continue incubation for additional 3 h. At this stage the entire connector should assemble from its 12 subunits.
-
28
Test the induction of phi29 connector protein by 12% SDS-PAGE (wt/vol). To achieve this goal, take 100 μl samples from pre-induction culture (step 27, used as negative control) and post-induction culture (at end point of this step). Mix 10 μl of each pre-induction and post-induction samples with 10 μl 2× SDS-PAGE loading buffer and heat at 100 °C for 1 min before loading onto the gel. The molecular weight of one subunit of wild type phi29 connector is 35 kDa, whereas the molecular weight of the C-Strep-modified connector and CHis-modified connectors are 37.3 kDa, and 36.7 kDa, respectively.
-
29
Harvest the post-induction cells from the culture described in step 27 by centrifugation at 6,500 ×g for 30 min at 4 °C, and discard the supernatant.
▲PAUSE POINT: The cell pellets can be stored at –70 °C for several days.
-
30
Resuspend the cell pellet in 25 ml cell-washing buffer.
-
31
Lyse the resuspended cells in a French press at 12,000 psi (83 MPa). For complete lysis, pass through the French Press three consecutive times.
-
32
Remove genomic DNA and RNA from the cell suspension resulting from step 31 by adding DNase I to a final 5 μg/ml concentration and RNase A to a final concentration of 10 μg/ml. Keep the resulting mixture at 4 °C for 1 h.
-
33
Centrifuge lysed cells at 18,800 ×g for 30 min at 4 °C. Collect supernatant carefully without disturbing the pellet and store it at 4 °C.
▲CRITICAL STEP: Make sure that the supernatant is not cloudy, as, if it is, it will block the columns used in step 35 below.
-
34
(Optional) If the supernatant is cloudy, centrifuge one more time as detailed in step 33.
-
35
Choose a resin for the purification column according to the affinity tag introduced in the connector. For example, choose the Strep-tactin resin for a Strep-Tag, and His-bind resin for a His-Tag. Resuspend the appropriate resin gently by swirling. Add 2 ml of slurry to a Bio-Rad poly-prep column and wait for it to settle overnight at 4 °C.
-
36
Equilibrate the column at 4 °C by adding 10 ml of cell-washing buffer to it. While still keeping the system temperature at 4 °C, load the supernatant from step 33 and wash with 10 ml of cell-washing buffer.
-
37
Elute with 5 ml of elution buffer and collect 500 μl fractions of eluted solution, while keeping the system at 4 °C
▲CRITICAL STEP: Carry out washing and elution steps at 4 °C (in the cold room) to avoid protein degradation. ATP presence in the cell-washing buffer is essential in order to keep the protein soluble. A soluble connector is particularly important when preparing the liposome/connector complex (see below).
-
38
To test whether the connector has been eluted, and in which fractions, mix 10 μl samples of each eluted fraction with 10 μl 2× SDS-loading buffer and, after boiling the resulting solutions for 2 min, load them onto a 12% SDS-PAGE (wt/vol) gel. Run then an electrophoresis experiment.
▲PAUSE POINT: Aliquot the purified connector proteins and store it at –80 °C. It remains stable for several years.
▲ TROUBLESHOOTING
Fluorescence labeling of the reengineered phi29 connector ●TIMING: ~1 day
-
39
For small-scale FITC conjugation, dissolve around 1 mg of connector protein in elution buffer to obtain a 1 ml solution of connector.
-
40
Dialyze the solution obtained in step 39 against 1,000 ml PBS, pH 7.4 at 4 °C overnight.
-
41
Add 0.1 ml of 1M carbonate–bicarbonate buffer (provided in the FITC conjugation kit) to 0.9 ml of connector protein in PBS, so that the final concentration of carbonate–bicarbonate buffer is 0.1M, at pH 9.0.
-
42
Reconstitute 2 mg of lyophilized FITC (provided in the FITC conjugation kit) in 2 ml of 0.1M carbonate–bicarbonate buffer. Add 50 μl of the FITC solution thus obtained drop-wise (over 15 minutes) to the connector solution from step 41, while stirring. Incubate the solution at RT while gently stirring for 2 h. Shield the reaction vial from light using aluminum foil.
-
43
Remove free FITC using 3.5 ml G-25 sephadex columns (provided in the FITC conjugation kit). Equilibrate the column with PBS buffer, and elute the connector protein using 2.5 ml PBS. Collect fractions (0.25 ml each) and measure absorbance of each fraction at 280 nm using a UV/Vis spectrophotometer. Combine the fractions containing the connector.
-
44
Load 3–5 μg of FITC-labeled connector protein to a 12% SDS-PAGE (wt/vol), with SDS running buffer. Run then an electrophoresis experiment. Visualize FITC-labeled connector protein by gel imaging.
-
45
Determine the protein concentration (molarity) using the equation: , where A280 is the absorbance of the protein at 280 nm; Amax is the Absorbance of FITC dye at 495 nm wavelength; CF is the correction factor to account for FITC dye absorbance at 280 nm (0.300); ε is the molar extinction coefficient of the corrector (approximately 500,000 M−1cm−1).
-
46
Determine the degree of labeling using the equation: , where Amax is the Absorbance of FITC-protein complex at 495 nm wavelength and ε* is the molar extinction coefficient of FITC dye (68,000 M−1cm−1).
Preparation of giant unilamellar vesicles (GUVs) ●TIMING: 1–2 days.
-
47
Syringe 1 ml of 1 mg/ml DOPC or DPhPC in chloroform in a 2 ml glass vial or 25 ml round-bottom flask. Please note that both lipids are zwitterionic (carrying both negative and positive charges) and work equally well for this procedure. We have not tested the suitability of other lipids, with net negative or positive charges.
-
48
(Optional) If you wish to conduct fluorescence imaging experiments on the GUVs, add 1% molar ratio NBD-PE to the lipid solution from step 47.
-
49
Remove chloroform from the lipid solution according to the manual option A or to the automated option B. Please note that option B yields high quality GUVs and proteoliposomes.
Option A. Manual removal of chloroform from the lipid solution.
Apply a slow stream of nitrogen to the surface of the lipid solution until all the chloroform evaporates. Keep rotating the glass vial or round bottomed flask during the drying process to ensure the formation of a uniform thin film on the glass surface. Keep the glass vial under house vacuum for approximately 1 h to remove residual traces of solvent.
Option B. Automated removal of chloroform from the lipid solution.
Use a Rotary evaporator (such as, BÜCHI Labortechnik AG) to slowly evaporate the chloroform under vacuum over 3–4 h (Drop the pressure gradually from 700–800 mbar to 0 mbar over 3–4 hours; set rotation around 50–100 r.p.m.).
-
50
Rehydrate the lipid film adding to it 2 ml of filtered 200mM sucrose solution.
-
51
Prepare GUVs according to option A, which yields non-uniform sized GUVs that are however suitable to study biological membranes and their properties, or option B, which is an electro-swelling approach that gives a near-uniform size distribution of GUVs. Please note in addition that, by applying option B, the size of the GUVs can be controlled with relative ease for desired application by tuning the voltage amplitude and frequency.
Option A. GUV preparation by budding process.
After rehydration, store the solution overnight at RT.
Option B. GUV preparation by electro-swelling approach.
Implement Nanion Vesicle Prep Pro system materials and manufacturer's instructions to prepare GUVs.
-
52
To prepare GUVs without connector protein (control), rehydrate the lipid film with 2 ml of filtered 200mM sucrose solution. Then implement step 51A or 51B.
-
53
To prepare GUVs with connector protein, add 100 μl of 1 mg/ml FITC-labeled (for imaging) or unlabeled (for conduction assays) reengineered connectors in a 200 mM sucrose solution to the dried lipid with a final lipid:connector mole ratio of 75:1. Then implement step 51A or 51B.
-
54
Take an aliquot from the middle of each of the solutions from steps 52 and 53 and transfer each aliquot into a different Petri dish. Image the FITC-labeled proteoliposomes (or NBD-labeled giant vesicles, if step 48 had been implemented) by epi-fluorescence microscopy. The typical diameter of the vesicles is in the 20–50 μm range (Fig. 3a)
Figure 3. Images of fluorescently labeled liposomes containing the connector.
(a) Epifluorescence images of liposome: lipid labeled with NBD-PE without connector (left); a proteoliposomes reconstituted by FITC-labeled connectors (middle); FITC-connector mixed non-specifically with liposomes (right). (b) Separation of liposome/FITC-connector complexes by sucrose gradient sedimentation. Free connectors appeared in the top fractions while proteoliposomes remained in the lower fractions. Fractions 1–12 are not shown. Figures reproduced with permissions from Ref. 20, © Nature Publishing Group.
▲PAUSE POINT Store giant vesicles at 4 °C, as they remain stable at this temperature for up to 3 days.
Separation and detection of liposome/connector complex by sucrose gradient sedimentation ●TIMING 2–3 h
-
55
Perform a 5–20% linear sucrose gradient sedimentation in 1× TMS buffer to separate the liposome/connector complexes from the free connector. For this purpose, add 5% sucrose (2.5 ml) to open-top polyallomer centrifuge tubes. Place 2.5 ml of 20% sucrose in a syringe attached to a large (5-cm) needle. Place the needle at the bottom of the centrifuge tube and slowly inject the 20% sucrose solution without agitating the 5% sucrose layer. Place the tube (along with a balancing tube containing the same solution) inside a Biocomp Gradient Master to form the gradient. The protocol is as follows: time: 47 s; tilt angle, 86.5°; speed: 25 r.p.m.
-
56
Load 0.1 ml of liposome/connector complexes gently at the top of a 5-ml centrifuge tube. Centrifuge the sample in a Beckman L-80 ultracentrifuge (other ultracentrifuges with different rotors may also be used, but the experimental protocols need to be optimized according to the manufacturer instructions) at 27,000 r.p.m. for 30 min at 20 °C in a SW55 rotor. Collect 30 fractions (11 drops per fraction ~160 μl) from the bottom of the tube and analyze by 10% SDS-PAGE (wt/vol). Fraction 13–15 will contain connector-liposome complexes, as evidenced by epifluorescence microscopy experiments (Fig. 3b).
Preparation of small unilamellar vesicles (SUV) containing the reengineered phi29 connector ●TIMING ~5 h
-
57
Syringe 1 ml of 1 mg/ml DOPC or DPhPC lipids in chloroform into a 2 ml glass vial. Implement step 49A or 49B to remove the chloroform.
-
58
Rehydrate the lipid film with 1 ml proteoliposome rehydration buffer containing the reengineered connector protein (concentration 0.5 mg/ml) and vortex thoroughly.
-
59
Extrude the rehydrated protein–lipid complex with a mini-extruder (follow manufacturers’ instructions) through polycarbonate membranes (100 nm or 400 nm) to generate SUVs. Aliquot the extruded proteoliposomes (~50 μL in each tube).
▲PAUSE POINT: For long-term storage (up to several years) keep the SUVs at –80 °C. For short-term storage (up to a few weeks), place SUVs at 4 °C.
Setup for electrophysiological assays ●TIMING: ~1 h, but can take several days to setup the system for the first time.
-
60
Rinse a pair of Ag/AgCl electrodes with ethanol and water. Immerse the electrode pellets in Clorox bleach until a light gray color is observed on the electrode surface. Typically, 10–15 min will be sufficient.
▲CRITICAL STEP: Do not store the electrodes in contact with metals. Avoid exposing the electrode to light.
-
61
Connect the electrodes directly to the head-stage of a patch clamp current amplifier. Record the trace using an Axopatch 200B patch clamp amplifier coupled with the Axon DigiData 1322A or Axon DigiData 1440 analog-digital converter.
-
62
Acquire the data low-pass filtered at a frequency of 1–10 kHz and sampling frequency of 2–200 kHz, as needed. Use PClamp Clampex software (Axon Instruments) to collect the data, and the software Clampfit for data analysis.
▲CRITICAL STEP: Set the sampling frequency so that its value is at least 5 times higher than the filter cutoff frequency to avoid possible sampling artifacts.
Cleaning the BLM Chamber and Partition ●TIMING: ~30 min.
-
63
Pre-rinse the chambers with deionized water to remove any chemicals. Squirt BLM cleaning solution 1 (that contains TSP) with force to remove any solid residues. Rinse with BLM cleaning solution 2 to remove residual phosphates. Finally, wash with Milli-Q water to remove any residual HCl. Dry off everything with an air stream.
-
64
(Optional) For heavy duty cleaning, sonicate top chamber in ethanol for 10 min; rinse then with ethanol and profusely with Milli-Q water.
-
65
Incubate the Teflon partition in a test-tube with n-decane for 2 min at RT. Assemble the partition on the chamber using vacuum grease. Vortex to remove the grease completely. Rinse the partition twice with n-decane, twice with ethanol and twice with nanopure water. Let it dry to the air on a kimwipe.
▲CRITICAL STEP: For single-molecule analysis, it is important to ensure that the chambers and partitions are cleaned thoroughly to eliminate artifacts arising from contaminants.
Electrophysiological Chamber Assembly and experiment setup ●TIMING: ~2 h
-
66
Assemble horizontal chambers (Eastern Scientific-BCH-1A, option A) or vertical chambers (Warner instruments - BCH-13A, option B) to conduct electrophysiological assays (Fig. 4a). The two setups are essentially equivalent for the purposes of this protocol.
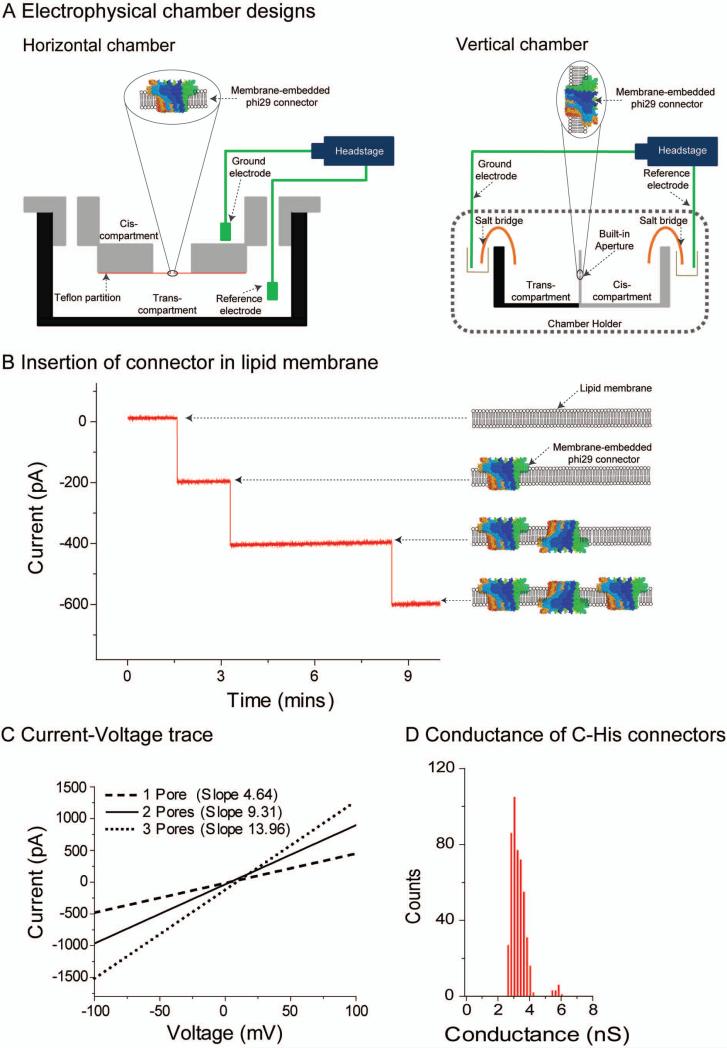
Figure 4. Insertion of connector channels in the planar lipid membrane.
(a) Schematic representation of electrophysiological assay setup: horizontal (left) and vertical (right) chambers. Note: figure not drawn to scale. (b) Insertion of the re-engineered phi29 connector into lipid membrane and demonstration of robust conductivity property. Discrete steps of current representing the insertion of one connector for each step (left) and illustration (right). Connector insertion into the bilayer only occurred when connector-reconstituted proteoliposomes were fused into the bilayer. Note that the orientation of the connector channel is random. (c) Data demonstrating robust properties of connector indicated by strong linear I–V relationship. Increased number of connectors is associated with larger slopes but identical conductance per channel. (d) Histogram showing uniform conductance of connector channels in the membrane. Figures reproduced with permissions from: (c–d) Ref. 21, © The Royal Society of Chemistry.
Option A. Horizontal chamber setup.
Apply grease to the top chamber and gently attach the Teflon partition, which has hole with a 200-μm diameter in it. Make sure you place the hole in the middle of the chamber.
Assemble the setup by placing the top chamber on top of the bottom chamber. The Teflon partition separates the cell into two compartments: the cis compartment (top) and the trans compartment (bottom).
Option B. Vertical chamber setup.
In the vertical chamber, the cuvette already has a 200 μm aperture drilled on the side. Simply assemble the cuvette in the holder with the screws provided. Make sure the aperture sits in the middle and that the screws are finger tight. Depending on applications, custom apertures can be ordered from the manufacturer (Warner instruments).
-
67
Pre-paint both sides of Teflon partition or cuvette with lipid solution A (typically less than 0.5 μl is sufficient). Wait 20–40 min for it to dry. Repeat this step one more time.
-
68
For the horizontal chamber setup, fill the cis and trans compartments with 0.25 ml and 2 ml of conduction buffer, respectively. For the vertical chamber setup, fill both compartments with 1 ml of conduction buffer. Place the head-stage Ag/AgCl electrode in the trans compartment and the ground Ag/AgCl electrode in the cis compartment.
-
69
(Optional) Connect the Ag/AgCl electrodes to the cis and trans compartments using salt bridges (Fig. 4a, right). This setup serves two purposes: it facilitates electrical coupling with the amplifier, and minimizes the transfer of ions or solute from the cis/trans compartment to electrode wells.
-
70
(Optional) Set up a bilayer perfusion system (follow manufacturer guidelines) to enable automated, efficient and rapid exchange of buffers in cis and trans compartments.
-
71
(Optional) Set up a bilayer thermocycler (follow manufacturer guidelines) to conduct single channel measurements at any temperature between 5 °C and 50 °C.
-
72
Place the chamber setup on a rubber mat. Place the whole assembly (Fig. 4a) inside a Faraday cage, so as to shield it from electrical and mechanical interference.
▲ CRITICAL STEP: Make sure that all the instruments are connected to a common ground, and the ground connection is isolated from all circuits.
▲ TROUBLESHOOTING
-
73
Familiarize yourself with the instrument control panel before use. Calibrate and set desired parameters (using guidelines from manufacturer).
Insertion of connector into planar bilayer lipid membrane ●TIMING: ~1–2 h
-
74
To paint a planar membrane, first locate the aperture in the Teflon partition (horizontal chamber) or cuvette (vertical chamber) with an optical microscope. Pipette then 1–2 μl of lipid solution B into the aperture by placing the tip of the pipette close to the aperture and gently squeezing lipids out. The membrane should form spontaneously within a few seconds.
-
75
Remove excess lipid from the aperture. To do so, place the pipette near the side of the aperture and squeeze out air bubbles from the pipette. Gently brush against the side of the hole. When bubbles touch the lipid solution, they absorb a part of it on a phase border and take the excess lipids out of the hole. Wait for a few minutes, the membrane will stabilize, and a straight line will be observed on the recording device with the noise level around ±2–3 pA.
▲CRITICAL STEP: Do not place the air bubble over the hole, as this will rupture the bilayer.
-
76
Calculate the thickness of the lipid membrane by measuring the capacitance (C) of the membrane under an applied voltage ramp. Use equation, to find the capacitance of the membrane, where I is the current measured, and is the applied voltage ramp. Determine the thickness of the lipid membrane from the equation , where ε0 and εs are permittivity of free space (8.854×10−12 F.m−1) and lipid ( ~ 3 ; no units, relative), respectively, and A is the cross-sectional area of the aperture (typical diameter of 200 um). For example, for I = 60×10−12 A and , then C = 60×10−12(0.4)−1 = 150×10−12F. Therefore, d = 5.56×10−9m
▲CRITICAL STEP: Make sure that the thickness of the membrane is around the optimal value for these experiments, 5 nm. If the membrane is too thin, in fact, the connector channel will be unstable; if the membrane is too thick, the proteoliposomes will not fuse with the planar membrane.
-
77
For single conductance measurements, dilute the stock SUV solutions from step 59 10–20-fold before use. Add 0.5–2 μl of the diluted liposome solution into the cis compartment on top of the aperture. Wait for a few minutes for the proteoliposomes to fuse with the planar bilayer, inserting thus the connector channel(s) in the planar membrane (Fig. 4b).
▲CRITICAL STEP: If too many channels are inserted in the membrane, they can negatively affect membrane stability. If membrane stability is compromised, repaint the membrane and wait for connector insertion.
-
78
After the insertion of connector channels in the membrane, observe discrete stepwise increases in current to determine the single-channel conductance of the connector. Measure the conductance under constant potential, option A, or under varying potential, option B. The data obtained using the approach detailed in option B are more accurate, since the slope of the curve reporting current versus electrostatic potential, which represents the conductance of the system, is determined using several data points. Furthermore, the influence of electrode and contact potentials is excluded from the calculation.
Option A. Conductance measurement at constant potential
Derive the value of the conductance of the system at specific, constant potentials, and either positive or negative transmembrane voltage (we typically use 75 mV) using the ratio of the measured current jump induced by a discrete step to the applied voltage. Acquire and statistically analyze data from multiple channel insertion events (from multiple experiments) to obtain reliable values for the single channel conductance of the connector. Plot a histogram (Y-axis: number of events vs. X-axis: conductance) to obtain the distribution for channel conductance (Fig. 4b, d).
Option B. Conductance measurement at varying (ramping) potential
Measure the conductance of the system by calculating the slope of the current–voltage trace. Do so by applying a ramping potential from –100 mV to +100 mV (Fig. 4c). As an illustration, traces from one, two and three connector insertions under a ramp voltage are shown in Fig. 4c. The corresponding slopes (that represent the conductance of the systems) are 4.64 nS, 9.31 nS, and 13.96 nS, respectively.
▲ TROUBLESHOOTING
DNA translocation experiments ●TIMING: ~2 days
-
79
Prepare DNA for translocation experiments according to option A, for short (less than 100-nt-long) DNA strands, or option B, for long (100 bp–10,000 kbp-long) DNA strands.
Option A. Preparation of short DNA samples
For short-length ssDNA, order the desired sequences. If necessary, purify the individual strands using 10–15% (wt/vol) denaturing PAGE.
To obtain dsDNA, anneal two complementary single strands by heating them at 95 °C for 5 min and then slowly cooling the sample to RT over a few hours.
Purify the dsDNA using 10–15% (wt/vol) native PAGE.
Option B. Preparation of long dsDNA samples
For long dsDNA, use restriction enzymes (such as EcoRV) to cut plasmid DNA into desired linear fragments with a blunt end, following manufacturer's guidelines and the buffers and reaction solutions provided with each enzyme.
Purify the DNA using 0.8% (wt/vol) agarose gel (see step 3) or elute with a Qiaex II gel extraction kit (follow manufacturer's guidelines).
-
80
Add purified DNA to the BLM chamber either by pre-mixing it in the conducting buffer before the connector-containing bilayer has been assembled in the chamber, or after the connector-containing bilayer has already been assembled. Choose the second approach specifically to observe the instantaneous effects of adding DNA in real-time.
▲CRITICAL STEP: Be aware that the orientation of the connector channel in the lipid membrane is random, because it relies on vesicle fusion of proteoliposomes with a planar bilayer to insert the channel in the membrane. The connector channel can, therefore, be oriented with either the C-terminal (wide) end or the N-terminal (narrow) end facing the trans compartment. The orientation of the connector is important, because DNA traffic across the connector protein is one-directional (with DNA entry occurring at the protein's N-terminal end, and DNA exit at the C-terminal end)22) (Fig. 5e–g).
Figure 5. Translocation of dsDNA through membrane-embedded connector channel.
(a) Illustration of dsDNA translocation in vitro; (b) Translocation of linear dsDNA induced numerous partial current blockages. Insert: Magnified image of a current blockage event caused by the translocation of a single DNA molecule. (c) Histogram of current blockage percentage induced by linear dsDNA (2 kbp) translocations with a total 3,264 events in the presence of 1M NaCl, pH 7.8 under (–)75 mV constant potential. (d) Comparison of dwell times for translocation of 38 bp and 5.5 kbp dsDNA. (e–g) DNA translocation events through a single connector channel observed under a ramping potential without DNA (e) and with dsDNA in both cis and trans compartments (f) and (g). Data prove the one-directional trafficking of dsDNA across the channel. Figures reproduced with permissions from ref. 22 (a,e-g); ref. 21 (c); ref. 20 (d).
▲ CRITICAL STEP: Exercise caution when adding DNA after the connector-containing membrane has been assembled, as the membrane can rupture: switch applied voltage of 0 mV and add DNA slowly.
▲ TROUBLESHOOTING
-
81
Record the current traces associated with DNA translocation at moderate-to-high sampling frequency (20–200 KHz). In the presence of DNA, a burst of transient (temporary) current blockage events will be observed, as DNA, a non-electrolyte, physically blocks ion current (Fig. 5b). Each of the events (magnified in the graph in Fig. 5b) represents the translocation of a single DNA molecule.
-
82
Characterize the individual translocation events using the parameters outlined: , where Ipore is the current when the connector channel is open (i.e. the step size of the current for one-connector insertion), and IDNA is the current observed during DNA translocation. In the presence of dsDNA, the channel blockage percentage will be centered at ~32%, which is consistent with the dimension of the channel (3.6 nm diameter at its narrowest end) and dsDNA (~2 nm in diameter) (Fig. 5c)
Dwell time = The time taken for the passage of DNA from one end of the channel to the other end (Fig. 5b). Dwell time distributions typically follow an exponential decay profile (Fig. 5d).
▲CRITICAL STEP: Acquire and statistically analyze data on a large number of translocation events to obtain reliable values for the mentioned parameters.
-
83
To verify that the observed current blockage events indeed result from dsDNA translocation through the connector channel, add dsDNA to the trans compartment to a final concentration of 25–100 nM, after addition of connector/liposome complexes. As a negative control, add the DNA without the addition of connector/liposome complexes.
-
84
Apply a potential of –95 mV and collect samples from the cis compartment at specific time intervals (such as 30 min).
-
85
Determine the DNA concentration using a UV/Vis spectrophotometer, and use this concentration to determine the copy number of DNA molecules (absolute quantification) in the samples collected.
-
86
Perform Q-PCR experiments using iQTM SYBR Green Supermix and LightCycler® 480 Real-Time PCR System. Use appropriate forward and reverse primers depending on the sequence for the dsDNA used in the experiment.
-
87
Construct a standard curve using dsDNA with 10-fold dilutions of known concentrations and assay each dilution in triplicates (Fig. 6a). In the graph reported in figure 6a, baseline-subtracted PCR curve-fits of relative fluorescence units (CF RFU) for different DNA copy numbers (reported in the graph itself) are plotted against PCR amplification cycle. The observed blockage events from the current trace should closely associate with the number of DNA molecules passing through the pores quantified by Q-PCR (Fig. 6e), which will validate the DNA translocation experiments.
Figure 6. Quantitative PCR (Q-PCR) analysis of DNA translocation events.
(a) Q-PCR amplification curves of the dilution series run in triplicate (top) using SYBR Green dye, which binds to dsDNA. The threshold level (set in the exponential amplification phase) is the detection point at which the PCR reaction reaches a fluorescent intensity above background. The cycle at which the DNA sample reaches this threshold level is called the Threshold Cycle (CT); A standard curve with the CT plotted against the log of the starting quantity of template for each dilution (bottom). (b) Quantitative analysis of the total number of DNA passing through one of the connectors in the lipid membrane from the trans compartment to the cis compartment (top). Negative controls (bottom) were carried out under the same condition but without connectors. The error bars represent standard deviations of the mean from nine independent experiments and four negative control experiments. (c) Plot of the total number of DNA passing through multiple connectors in the lipid membrane (noted on the right). (d) Plot of the total number of DNA molecules translocated under membrane leaking conditions over time without any connector channels in the membrane. (e) A comparison of copy number of translocated dsDNA measured by Q-PCR with the relevant blockage events counted from current trace recorded in two independent experiments (Note: Only the events with 29–34% current blockages were counted). The error bars represent the standard deviation of three independent Q-PCR measurements. Figures reproduced with permissions from: (a–d) Ref. 20, © Nature Publishing Group; (e) Ref. 21, © The Royal Society of Chemistry.
▲CRITICAL STEP: Generally speaking, as the number of connectors in the bilayer increases, so will DNA translocation events per unit time; however, given that DNA traffic across the connector is one-directional, some of the channels that are in the ‘wrong’ orientation will not translocate DNA (Fig. 6b, c). It is important to validate the inference that the increase of the DNA copy number in the cis compartment is due to DNA translocation through the channels, rather than membrane leakage. If the Teflon partition is leaking or the lipid bilayer ruptured during the course of the experiment, the copy number of DNA per μl of solution in the cis compartment will be approximately 104–105-fold higher than those experiments without leakage (Fig. 6d).
Capture and Fingerprinting of chemicals and macromolecules ●TIMING ~2 days
-
88
Insert chemical probes on the channel surface by implementing option A, if probes are to be attached to the channel wall cysteine residues, and/or option B, if probes are to be attached the N-terminus and/or C-terminus of the connector.
Option A. Inserting probes in the channel wall.
The native connector contains two cysteine residues (C76 and C275) per gp10 protein that are buried within the wall and therefore are not accessible for the conjugation of analytes or of recognition moieties. Introduce accessible cysteines by site-directed mutagenesis within the inner wall of the connector, for instance, to generate the K234C mutant. After assembly, the internal mutation generates a cysteine ring, composed of 12 evenly spaced residues in the same plane within the dodecameric connector channel (See steps 18–24).
Purify the reengineered connectors to homogeneity (See steps 25–38).
Characterize the conductance properties of the reengineered connectors from multiple experiments (See step 78). For reference, C-His K234C channels have a smaller cross-sectional area (conductance: 2.2 ± 0.17 nS) than the C-His connectors without the mutation (conductance: 3.2 ± 0.2 nS)
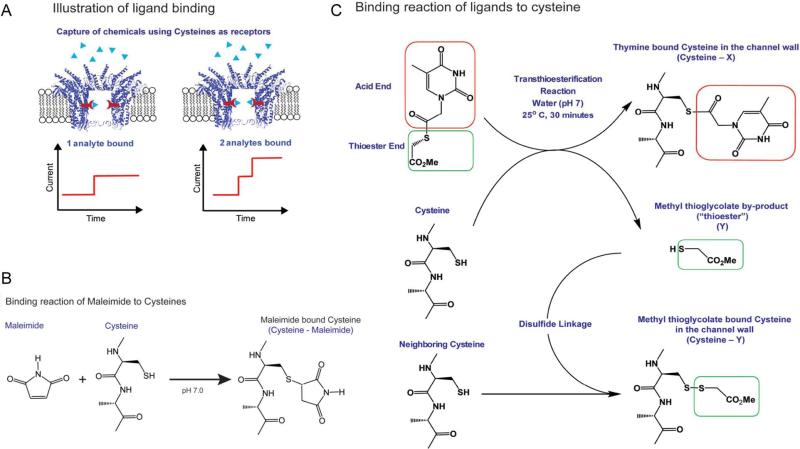
Utilize well-known methodologies to selectively conjugate chemicals to the sulfhydryl side chain of cysteine residues. Examples include chemicals containing maleimides and iodoacetamides that form C-S bounds; sulfhydryls that form disulfides under oxidizing conditions; and transthioesterification reactions linking synthesized thioesters to the channel cysteines85(Fig. 7, 8).
Monitor the binding of chemicals to the channel wall over time (See step 89).
Figure 7. Conjugation of chemical ligands to channel wall.
(a) Schematic of ligand binding to the inner wall of the connector pore which will result in the reduction of channel size as indicated by uniform stepwise blockage of channel current. (b) Reaction scheme of maleimidecysteine reaction. (c) Transthioesterification method, showing that cysteines are readily modified with nucleobases when exposed to thioesters, using thymine thioester as an example; a second reaction via disulfide linkage results in the binding of the methyl thioglycolate by-product. Figures reproduced with permissions from: Ref. 25, © American Chemical Society.
Figure 8. Capture and fingerprinting of chemicals in the channel lumen.
(a) Data showing discrete blocking steps due to the binding of thioesters groups containing ethane to accessible cysteine residues introduced by mutagenesis in the channel wall. Insert: Magnified current trace showing the transient and permanent current blockage events. (b) Analysis of current blockage events induced by the binding of thioesters to cysteine residues located in the channel wall. Histogram of permanent binding events for the binding of thioesters groups containing ethane, thymine, and benzene respectively. Figures reproduced with permissions from: Ref. 25, © American Chemical Society.
Option B. Inserting probes at the connector's terminal ends.
Re-engineer the connector at the N–terminal and C–terminal modifications with tags for added functionality, such as His, Strep, Biotin, TAT peptide, and poly-arginine peptide (See steps 18–24).
Purify the re-engineered connectors to homogeneity (See steps 25–38).
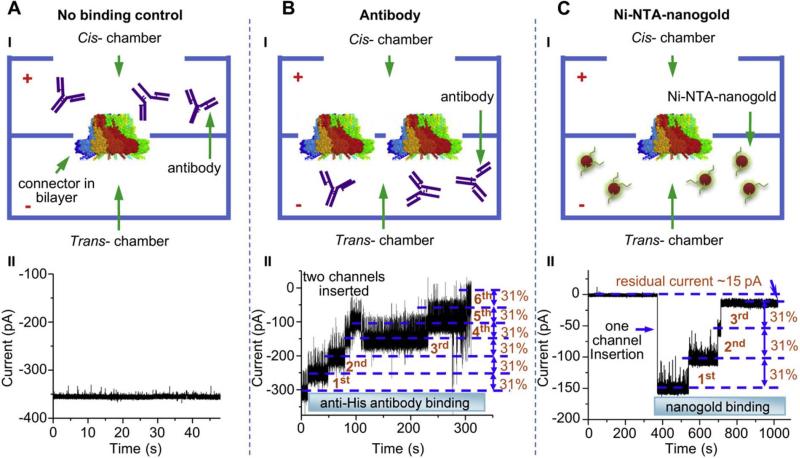
If, for instance, a C-His tag had been added, to confirm that it is present, add rabbit polyclonal antibodies specific against 6 His-tag to the trans compartment under asymmetric ionic conditions. Use 1M NaCl, 20mM Tris (pH 7.6) for the cis compartment. Use 150mM NaCl, 20mM Tris (pH 7.6) in the trans compartment to avoid the high salt effect that might interfere with the binding of nanogold to the His-tagged connector.Alternatively, add Ni-NTA nanogold (1.8 nm; Nanoprobes) to the trans compartment under asymmetric ionic conditions.
Monitor the binding of antibodies or nanogold particles to the connector termini over time (See step 89) (Fig. 9).
-
89
Characterize the binding events (from steps 88A and 88B) by monitoring the changes in the intensity of the current signal in a BLM setup (Fig. 8, 9). Use the following two parameters:
Figure 9. Capture and fingerprinting of antibodies or chemical ligand at the C-terminal end of the channel.
(a) Negative control. Since the antibody was placed in the cis compartment opposite to the C-terminal, no antibody binding occurs and no changes in current are observed. (b) Six discrete steps of changes for two C-His-tagged connector channels induced by anti-His tag antibodies. (c) Three discrete steps of changes for one C-His-tagged connector channels induced by Ni-NTA nanogold binding (1.8 nm). Figures reproduced with permissions from: Ref.24, © Elseiver.
Current blockage amplitude: Observe the step-wise decrease in conductance associated the binding of chemicals or macromolecules sequentially to each cysteine probe or C-terminal probe due to the physical hindrance of the channel (Fig. 7–9).
Current blockage signature: The characteristic signature of the captured analyte is given by the unique blockage amplitude and pattern of the signals. Count the number of molecules bound from the step-wise blocks and deduce the concentration from the number of binding events to a single pore per unit time.
▲ CRITICAL STEP: Acquire and statistically analyze data on a large number of single-molecule binding events.
TROUBLE SHOOTING
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 38 | Connector precipitation | Aggregation | Add 10mM ATP to the solution buffer |
| 38 | The purified connector complex contains free unassembled protein and assembled connectors. | The column procedure cannot separate the assembled and unassembled gp10 protein. Unassembled free gp10 protein will interfere with the subsequent membrane insertion work and all the subsequence quantitative and quantitative assays. | Carry out sucrose gradient sedimentation: 1.Load purified connectors on top of a linear 5–20% sucrose gradient in TMS. 2.Centrifuge in a Beckman L-80 ultracentrifuge at 35,000 r.p.m. for 1 h at 20 °C in a SW55 rotor. 3.Collect fractions from the bottom of the tube. |
| 72 | High noise in the current recordings | Poor shielding and grounding | Check grounding cables and connections to each electrical components Adjust the bandwidth (internal and external low-pass and high-pass filters) to provide optimal time resolution and signal-to-noise ratio |
| 74–78 | Membrane instability and signal fluctuations | Poor Teflon partition preparation and pre-painting | Thoroughly clean the Teflon partition and chambers Replace the used Teflon partition with a new one Adequately pre-treat the aperture with lipid A If necessary, prepare fresh lipid solutions A and B |
| Leaking between compartments | Broken seal between the partition and chamber | Seal the Teflon partition with chamber again carefully and uniformly If necessary, replace the used Teflon partition with a new one |
|
| Low channel insertion efficiency | Proteoliposomes are too diluted Poor quality of the lipid membrane |
Adjust the dilution to increase the number of channel insertion events Prepare fresh lipid solutions A and B. |
|
| Protein Degradation | Store proteoliposomes at 4 °C for a week or at –80 °C for long-term storage to preserve them. If there is any precipitate, discard the sample and take out a fresh aliquot stored at –80 °C | ||
| Noise spikes in current traces | Too many lipid vesicles without connectors can result in nonspecific strokes and collision Poor shielding and grounding Poor cleaning of partition and chambers |
Increase the connector-to-lipid ratio while preparing the proteoliposomes Check grounding cables and connections to eliminate noise spikes Thoroughly clean the Teflon partition and chambers |
|
| 80 | No DNA translocation observed after channel insertion | The connector inserted in the lipid bilayer is orientated opposite to what assumed | Apply a ramping potential from –100 mV to +100 mV. DNA translocation will be observed at either positive or negative voltages, depending upon the connector orientation in the membrane |
TIMING
Steps 1–24, Cloning of the gene coding for wild type phi29 connector: 1–2 wk.
Steps 25–38, Expression and Purification of the re-engineered phi29 connector: ~3 days.
Steps 39–46, Fluorescence labeling of the re-engineered phi29 connector: ~1 day.
Steps 47–54, Preparation of giant unilamellar vesicles (GUV): 1–2 days.
Steps 55–56, Separation and detection of liposome/connector complex by sucrose gradient sedimentation: 2–3 h.
Steps 57–59, Preparation of small unilamellar vesicles (SUV) containing the re-engineered phi29 connector: ~5 h.
Steps 60–62, Setup for electrophysiological assays: ~1 h, (but can take several days to setup the system for the first time).
Steps 63–65, Cleaning the BLM Chamber and Partitions: ~30 min.
Steps 66–73, Electrophysiological Chamber Assembly and experiment setup: ~2 h.
Steps 74–78, Insertion of connector into planar bilayer lipid membrane: 1–2 h.
Steps 79–87, Double-stranded DNA translocation experiments: ~2 days (Note: translocation experiments: 1–2 h; data processing can take several days).
Steps 88–89, Capture and fingerprinting of chemicals and macromolecules: ~2 days (Note: electrophysiological assays: 1–2 h; data processing can take several days)
ANTICIPATED RESULTS
In this protocol, we have provided an in-depth procedure for cloning, expressing and purifying the phi29 DNA packaging motor connector, and for inserting it into a lipid bilayer to serve as a model for a membrane-embedded nanopore. We have also provided general instructions on how to translocate DNA through the channel, append substrate-specific probes to the connector and to use conductance experiments to ascertain the presence of such substrates in solution. Explicit engineering of the phi29 connector is possible given that its crystal structure is available.28, 29 Anticipated results of this protocol are as follows:
The connector protein can be reengineered and inserted into liposomes (Fig. 3a) and lipid bilayers (Fig. 4b) efficiently.20
The conductance associated with the presence of each pore as measured in the BLM will be near-identical (Fig. 4d). The current-voltage trace will be perfectly linear demonstrating the robust nature of the connector nanochannel (Fig. 4c). 20, 21
Upon addition of dsDNA to the BLM chamber, numerous transient partial current blockage events, due to dsDNA translocation across the pore, will be observed in real time with a characteristic ~32% current blockage (Fig. 5b, c).20-22
The connector channel is expected to be stable under a wide range of experimental conditions, including high salt and extreme pH.21
A simple, reproducible approach can be applied to determine the number of pores embedded in the bilayer (Fig. 4c).21
DsDNA translocation across the phi29 connector channel will be one-directional (Fig. 5e–g).22
Insertion or conjugation of chemical groups or macromolecules can be achieved for single-molecule-sensing and diagnostic applications (Fig. 7–9).24, 25
This Protocol has important potential applications since artificial membrane architecture for DNA packaging motor would enable detailed investigations into discrete mechanisms of motor operation as well as opening future avenues for therapeutically relevant dsDNA packaging, sampling, and delivery.
ACKNOWLEDGEMENTS
Research was supported by NIH Grant EB012135 (P.G.). We thank Peng Jing for developing the procedure for the incorporation of the reengineered connectors into lipid membranes; Ying Cai and Feng Xiao for constructing the recombinant connectors; Dr. Brent Hallahan, Chad Schwartz, and Shaoying Wang for assisting in the preparation of the manuscript. P.G. is a co-founder of Kylin Therapeutics, Inc., and Biomotor and Nucleic Acid Nanotechnology Development Corp. Ltd.
Footnotes
AUTHOR CONTRIBUTIONS
P.G. conceived, and led the project in collaboration with C.M. in single pore measurements. F.H. and J.G. designed and conducted the experiments and co-wrote the manuscript with P.G.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests (see the HTML version of this article for details). P.G. is a co-founder of Kylin Therapeutics, Inc., and Biomotor and Nucleic Acid Nanotechnology Development Corp. Ltd.
REFERENCES
- 1.Guo P, Grimes S, Anderson D. A defined system for in vitro packaging of DNA-gp3 of the Bacillus subtilis bacteriophage phi29. Proc. Natl. Acad. Sci. USA. 1986;83:3505–3509. doi: 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DE, et al. The bacteriophage phi29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- 3.Rickgauer JP, et al. Portal motor velocity and internal force resisting viral DNA packaging in bacteriophage phi 29. Biophysical Journal. 2008;94:159–167. doi: 10.1529/biophysj.107.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo P, Erickson S, Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi29 DNA. Science. 1987;236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- 5.Lee CS, Guo P. In vitro assembly of infectious virions of ds-DNA phage f29 from cloned gene products and synthetic nucleic acids. J. Virol. 1995;69:5018–5023. doi: 10.1128/jvi.69.8.5018-5023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo P, Zhang C, Chen C, Trottier M, Garver K. Inter-RNA interaction of phage phi29 pRNA to form a hexameric complex for viral DNA transportation. Mol. Cell. 1998;2:149–155. doi: 10.1016/s1097-2765(00)80124-0. [DOI] [PubMed] [Google Scholar]
- 7.Xiao F, Moll D, Guo S, Guo P. Binding of pRNA to the N-terminal 14 amino acids of connector protein of bacterial phage phi29. Nucleic Acids Res. 2005;33:2640–2649. doi: 10.1093/nar/gki554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu D, Zhang H, Jin J, Guo P. Counting of six pRNAs of phi29 DNA-packaging motor with customized single molecule dual-view system. EMBO J. 2007;26:527–537. doi: 10.1038/sj.emboj.7601506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao F, Zhang H, Guo P. Novel mechanism of hexamer ring assembly in protein/RNA interactions revealed by single molecule imaging. Nucleic Acids Res. 2008;36(20):6620–6632. doi: 10.1093/nar/gkn669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu D, Moll WD, Deng Z, Mao C, Guo P. Bottom-up assembly of RNA arrays and superstructures as potential parts in nanotechnology. Nano Lett. 2004;4:1717–1723. doi: 10.1021/nl0494497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu D, Shu Y, Haque F, Abdelmawla S, Guo P. Thermodynamically stable RNA three-way junctions as platform for constructing multifuntional nanoparticles for delivery of therapeutics. Nature Nanotechnology. 2011;6:658–667. doi: 10.1038/nnano.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque F, et al. Ultrastable Synergistic Tetravalent RNA Nanoparticles For Targeting To Cancers. Nano Today. 2012;7:245–257. doi: 10.1016/j.nantod.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson AA, et al. Structure determination of the head-tail connector of bacteriophage phi29. Acta Cryst. 2001;D57:1260–1269. doi: 10.1107/s0907444901010435. [DOI] [PubMed] [Google Scholar]
- 15.Guasch A, et al. Detailed architecture of a DNA translocating machine: the high-resolution structure of the bacteriophage phi29 connector particle. J. Mol. Biol. 2002;315:663–676. doi: 10.1006/jmbi.2001.5278. [DOI] [PubMed] [Google Scholar]
- 16.Simpson AA, et al. Structure of the bacteriophage phi29 DNA packaging motor. Nature. 2000;408:745–750. doi: 10.1038/35047129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao F, et al. Fabrication of Massive Sheets of Single Layer Patterned Arrays Using Reengineered Phi29 Motor Dodecamer. ACS Nano. 2009;3:100–107. doi: 10.1021/nn800409a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao F, et al. Adjustable ellipsoid nanoparticles assembled from re-engineered connectors of the bacteriophage phi29 DNA packaging motor. ACS Nano. 2009;3:2163–2170. doi: 10.1021/nn900187k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F, Demeler B, Guo P. Assembly Mechanism of the Sixty-Subunit Nanoparticles via Interaction of RNA with the Reengineered Protein Connector of phi29 DNA-Packaging Motor. ACS Nano. 2010;4:3293–3301. doi: 10.1021/nn100158k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendell D, et al. Translocation of double-stranded DNA through membrane-adapted phi29 motor protein nanopores. Nature Nanotechnology. 2009;4:765–772. doi: 10.1038/nnano.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing P, Haque F, Vonderheide A, Montemagno C, Guo P. Robust Properties of Membrane-Embedded Connector Channel of Bacterial Virus Phi29 DNA Packaging Motor. Molecular BioSystems. 2010;6:1844–1852. doi: 10.1039/c003010d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing P, Haque F, Shu D, Montemagno C, Guo P. One-Way Traffic of a Viral Motor Channel for Double-Stranded DNA Translocation. Nano Lett. 2010;10(9):3620–3627. doi: 10.1021/nl101939e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang H, Jing P, Haque F, Guo P. Role of channel Lysines and “Push Through a Oneway Valve” Mechanism of Viral DNA packaging Motor. Biophysical Journal. 2012;102:127–135. doi: 10.1016/j.bpj.2011.11.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng J, Fang H, Haque F, Zhang L, Guo P. Three reversible and controllable discrete steps of channel gating of a viral DNA packaging motor. Biomaterials. 2011;32:8234–8242. doi: 10.1016/j.biomaterials.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haque F, Lunn J, Fang H, Smithrud D, Guo P. Real-Time Sensing and Discrimination of Single Chemicals Using the Channel of Phi29 DNA Packaging Nanomotor. ACS Nano. 2012;6:3251–3261. doi: 10.1021/nn3001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibanez C, Garcia JA, Carrascosa JL, Salas M. Overproduction and purification of the connector protein of Bacillus subtilis phage f29. Nucleic Acids Res. 1984;12:2351–2365. doi: 10.1093/nar/12.5.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MA, et al. Affinity of molecular interactions in the bacteriophage phi29 DNA packaging motor. Nucleic Acids Res. 2006;34:2698–2709. doi: 10.1093/nar/gkl318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Blocker F, Xiao F, Guo P. Construction and 3-D computer modeling of connector arrays with tetragonal to decagonal transition induced by pRNA of phi29 DNA-packaging motor. J. Nanosci. Nanotechnol. 2005;5:856–863. doi: 10.1166/jnn.2005.143. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Xiao F, Guo P. The effect of N- or C-terminal alterations of the connector of bacteriophage phi29 DNA packaging motor on procapsid assembly, pRNA binding, and DNA packaging. Nanomedicine. 2008;4:8–18. doi: 10.1016/j.nano.2007.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derrington IM, et al. Nanopore DNA sequencing with MspA. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16060–16065. doi: 10.1073/pnas.1001831107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH. Single-molecule DNA detection with an engineered MspA protein nanopore. Proceedings of the National Academy of Sciences. 2008;105:20647–20652. doi: 10.1073/pnas.0807514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manrao EA, et al. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat Biotechnol. 2012;30:349–353. doi: 10.1038/nbt.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammad MM, et al. Engineering a Rigid Protein Tunnel for Biomolecular Detection. J. Am. Chem. Soc. 2012 doi: 10.1021/ja3043646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherf GM, et al. Automated forward and reverse ratcheting of DNA in a nanopore at 5-A precision. Nat Biotechnol. 2012;30:344–348. doi: 10.1038/nbt.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song L, et al. Structure of Staphylococcal alpha -Hemolysin, a Heptameric Transmembrane Pore. Science. 1996;274:1859–1865. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 36.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akeson M, Branton D, Kasianowicz JJ, Brandin E, Deamer DW. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 1999;77:3227–3233. doi: 10.1016/S0006-3495(99)77153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheley S, Xie H, Bayley H. A genetically encoded pore for the stochastic detection of a protein kinase. Chembiochem. 2006;7:1923–1927. doi: 10.1002/cbic.200600274. [DOI] [PubMed] [Google Scholar]
- 39.Guan X, Gu LQ, Cheley S, Braha O, Bayley H. Stochastic sensing of TNT with a genetically engineered pore. Chembiochem. 2005;6:1875–1881. doi: 10.1002/cbic.200500064. [DOI] [PubMed] [Google Scholar]
- 40.Kang XF, Cheley S, Guan X, Bayley H. Stochastic detection of enantiomers. J. Am. Chem. Soc. 2006;128:10684–10685. doi: 10.1021/ja063485l. [DOI] [PubMed] [Google Scholar]
- 41.Meller A, Nivon L, Brandin E, Golovchenko J, Branton D. Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1079–1084. doi: 10.1073/pnas.97.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang XF, Gu LQ, Cheley S, Bayley H. Single protein pores containing molecular adapters at high temperatures. Angew. Chem. Int. Ed Engl. 2005;44:1495–1499. doi: 10.1002/anie.200461885. [DOI] [PubMed] [Google Scholar]
- 43.Franceschini L, Mikhailova E, Bayley H, Maglia G. Nucleobase recognition at alkaline pH and apparent pKa of single DNA bases immobilised within a biological nanopore. Chem. Commun. (Camb. ) 2012;48:1520–1522. doi: 10.1039/c1cc16124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20647–20652. doi: 10.1073/pnas.0807514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo I, Tombola F, Martinucci S, Zoratti M. DNA interacts with Bacillus subtilis mechano-sensitive channels in native membrane patches. Cell Physiol Biochem. 2002;12:127–134. doi: 10.1159/000063789. [DOI] [PubMed] [Google Scholar]
- 46.Mobasheri H, Lea EJ. Biophysics of gating phenomena in voltage-dependent OmpC mutant porin channels (R74C and R37C) of Escherichia coli outer membranes. Eur. Biophys. J. 2002;31:389–399. doi: 10.1007/s00249-002-0235-1. [DOI] [PubMed] [Google Scholar]
- 47.Carneiro CM, et al. Probing the volume changes during voltage gating of Porin 31BM channel with nonelectrolyte polymers. Biochim. Biophys. Acta. 2003;1612:144–153. doi: 10.1016/s0005-2736(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 48.Mohammad MM, Howard KR, Movileanu L. Redesign of a plugged beta-barrel membrane protein. J. Biol. Chem. 2011;286:8000–8013. doi: 10.1074/jbc.M110.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeets RM, et al. Salt dependence of ion transport and DNA translocation through solid-state nanopores. Nano Lett. 2006;6:89–95. doi: 10.1021/nl052107w. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Branton D. Nanopores with a spark for single-molecule detection. Nature Biotechnology. 2001;19:622–623. doi: 10.1038/90216. [DOI] [PubMed] [Google Scholar]
- 51.Venkatesan M, Yemenicioglu S, Dorvel B, Bashir R. Fabrication and characterization of low stress, mechanically robust Al2O3 nanopores for the electronic detection of biomolecules. Advanced Materials. 2009;21:1–6. [Google Scholar]
- 52.Iqbal SM, Akin D, Bashir R. Solid-state nanopore channels with DNA selectivity. Nature Nanotechnology. 2007;2:243–248. doi: 10.1038/nnano.2007.78. [DOI] [PubMed] [Google Scholar]
- 53.Chang H, et al. DNA counterion current and saturation examined by a MEMS-based solid state nanopore sensor. Biomed. Microdevices. 2006;8:263–269. doi: 10.1007/s10544-006-9144-x. [DOI] [PubMed] [Google Scholar]
- 54.Wanunu M, Meller A. In: Single-molecule analysis of nucleic acids and DNA-protein interactions using nanopores in Single-Molecule Techniques: A Laboratory Manual. Selvin PR, Ha T, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2008. pp. 395–420. [Google Scholar]
- 55.Cuervo A, Carrascosa JL. Viral connectors for DNA encapsulation. Curr. Opin. Biotechnol. 2011 doi: 10.1016/j.copbio.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesan BM, Bashir R. Nanopore sensors for nucleic acid analysis. Nature Nanotechnology. 2011;6:615–624. doi: 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- 57.Healy K. Nanopore-based single-molecule DNA analysis. Nanomedicine. 2007;2:459–481. doi: 10.2217/17435889.2.4.459. [DOI] [PubMed] [Google Scholar]
- 58.Majd S, et al. Applications of biological pores in nanomedicine, sensing, and nanoelectronics. Current Opinion in Biotechnology. 2010;21:439–476. doi: 10.1016/j.copbio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Branton D, et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008;26:1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meller A, Branton D. Single molecule measurements of DNA transport through a nanopore. Electrophoresis. 2002;23:2583–2591. doi: 10.1002/1522-2683(200208)23:16<2583::AID-ELPS2583>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 61.Huang S, et al. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010;5:868–873. doi: 10.1038/nnano.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Schwartz C, De Donatis GM, Guo P. Hexameric viral DNA packaging motor using a “Push through a one-way valve” mechanism. Adv. Virus Res. 2012;83:415–465. doi: 10.1016/B978-0-12-394438-2.00009-8. [DOI] [PubMed] [Google Scholar]
- 63.Wu HC, Astier Y, Maglia G, Mikhailova E, Bayley H. Protein nanopores with covalently attached molecular adapters. J. Am. Chem. Soc. 2007;129:16142–16148. doi: 10.1021/ja0761840. [DOI] [PubMed] [Google Scholar]
- 64.Wei R, Gatterdam V, Wieneke R, Tampe R, Rant U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 2012;7:257–263. doi: 10.1038/nnano.2012.24. [DOI] [PubMed] [Google Scholar]
- 65.Gu LQ, Braha O, Conlan S, Cheley S, Bayley H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature. 1999;398:686–690. doi: 10.1038/19491. [DOI] [PubMed] [Google Scholar]
- 66.Wanunu M, et al. Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat Nanotechnol. 2010;5:807–814. doi: 10.1038/nnano.2010.202. [DOI] [PubMed] [Google Scholar]
- 67.Mirsaidov U, et al. Nanoelectromechanics of Methylated DNA in a Synthetic Nanopore. Biophysical Journal. 2009;96:L32–L34. doi: 10.1016/j.bpj.2008.12.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Q, et al. Detecting SNPs using a synthetic nanopore. Nano Letters. 2007;7:1680–1685. doi: 10.1021/nl070668c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer A, et al. Nanopore based sequence specific detection of duplex DNA for genomic profiling. Nano Lett. 2010;10:738–742. doi: 10.1021/nl100058y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zheng D, Tan Q, Wang MX, Gu LQ. Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat Nanotechnol. 2011;6:668–674. doi: 10.1038/nnano.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie H, Braha O, Gu LQ, Cheley S, Bayley H. Single-molecule observation of the catalytic subunit of cAMP-dependent protein kinase binding to an inhibitor peptide. Chem. Biol. 2005;12:109–120. doi: 10.1016/j.chembiol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Rotem D, Jayasinghe L, Salichou M, Bayley H. Protein detection by nanopores equipped with aptamers. J. Am. Chem. Soc. 2012;134:2781–2787. doi: 10.1021/ja2105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hornblower B, et al. Single-molecule analysis of DNA-protein complexes using nanopores. Nat Methods. 2007;4:315–317. doi: 10.1038/nmeth1021. [DOI] [PubMed] [Google Scholar]
- 74.Cockroft SL, Chu J, Amorin M, Ghadiri MR. A single-molecule nanopore device detects DNA polymerase activity with single-nucleotide resolution. J. Am. Chem. Soc. 2008;130:818–820. doi: 10.1021/ja077082c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia F, et al. Gating of single synthetic nanopores by proton-driven DNA molecular motors. J. Am. Chem. Soc. 2008;130:8345–8350. doi: 10.1021/ja800266p. [DOI] [PubMed] [Google Scholar]
- 76.Han C, et al. Enantioselective recognition in biomimetic single artificial nanochannels. J. Am. Chem. Soc. 2011;133:7644–7647. doi: 10.1021/ja2004939. [DOI] [PubMed] [Google Scholar]
- 77.Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahl PL, Price R, Smuda J, Gaber BP, Singh A. Insertion of bacteriorhodopsin into polymerized diacetylenic phosphatidylcholine bilayers. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1990;1028:141–153. doi: 10.1016/0005-2736(90)90148-h. [DOI] [PubMed] [Google Scholar]
- 79.Knoll W, et al. Functional tethered lipid bilayers. J. Biotechnol. 2000;74:137–158. doi: 10.1016/s1389-0352(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 80.Reilly BE, Spizizen J. Bacteriophage Deoxyribonucleate Infection of Competent Bacillus subtilis. J Bact. 1965;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee CS, Guo P. Sequential interactions of structural proteins in phage phi29 procapsid assembly. J. Virol. 1995;69:5024–5032. doi: 10.1128/jvi.69.8.5024-5032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo P, Rajogopal B, Anderson D, Erickson S, Lee C-S. sRNA of bacteriophage phi29 of B.subtilis mediates DNA packaging of phi29 proheads assembled in E. coli. Virology. 1991;185:395–400. doi: 10.1016/0042-6822(91)90787-c. [DOI] [PubMed] [Google Scholar]
- 83.Guo P, et al. Regulation of the phage f29 prohead shape and size by the portal vertex. Virology. 1991;183:366–373. doi: 10.1016/0042-6822(91)90149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg AH, et al. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56:125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 85.Ura Y, Beierle JM, Leman LJ, Orgel LE, Ghadiri MR. Self-assembling sequence-adaptive peptide nucleic acids. Science. 2009;325:73–77. doi: 10.1126/science.1174577. [DOI] [PubMed] [Google Scholar]