Abstract
It was previously reported that the gag proteins of mammalian type C retroviruses are modified by the addition of myristate to the N-terminal glycine residue. We have performed oligonucleotide-directed mutagenesis to change this glycine codon in the Moloney murine leukemia virus genome to an alanine codon and also to specifically delete the glycine codon. Upon transfection into mammalian cells, these mutant genomes direct the synthesis of gag proteins, but these proteins are not myristylated. The mutants do not form virus particles or any recognizable virus-specific structures visible in thin sections with the electron microscope. Further, the mutant gag proteins appear to remain in the cytosol, whereas the wild type is found principally in particulate fractions of the cell. The results are consistent with the theory that myristate is required for the association of the gag protein with the plasma membrane and that this association is necessary for virus assembly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Phillips L. A., Kramer M. J., Haapala D. K., Peebles P. T., Nomura S., Fischinger P. J. Transformation of mouse 3T3 cells by murine sarcoma virus: release of virus-like particles in the absence of replicating murine leukemia helper virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. doi: 10.1128/jvi.32.3.1015-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Rein A., Levin J. G., Bassin R. H., Benjers B. M., Kashmiri S. V., Hopkins D., O'Neill B. J. Mutant of B-tropic murine leukemia virus synthesizing an altered polymerase molecule. J Virol. 1979 Sep;31(3):741–751. doi: 10.1128/jvi.31.3.741-751.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. H., Gilboa E. Expression of genes introduced into cells by retroviral infection is more efficient than that of genes introduced into cells by DNA transfection. J Virol. 1984 May;50(2):417–424. doi: 10.1128/jvi.50.2.417-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Garber E. A., Goldberg A. R. Differences in intracellular location of pp60src in rat and chicken cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4142–4146. doi: 10.1073/pnas.77.7.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Ozols J., Carr S. A., Strittmatter P. Identification of the NH2-terminal blocking group of NADH-cytochrome b5 reductase as myristic acid and the complete amino acid sequence of the membrane-binding domain. J Biol Chem. 1984 Nov 10;259(21):13349–13354. [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
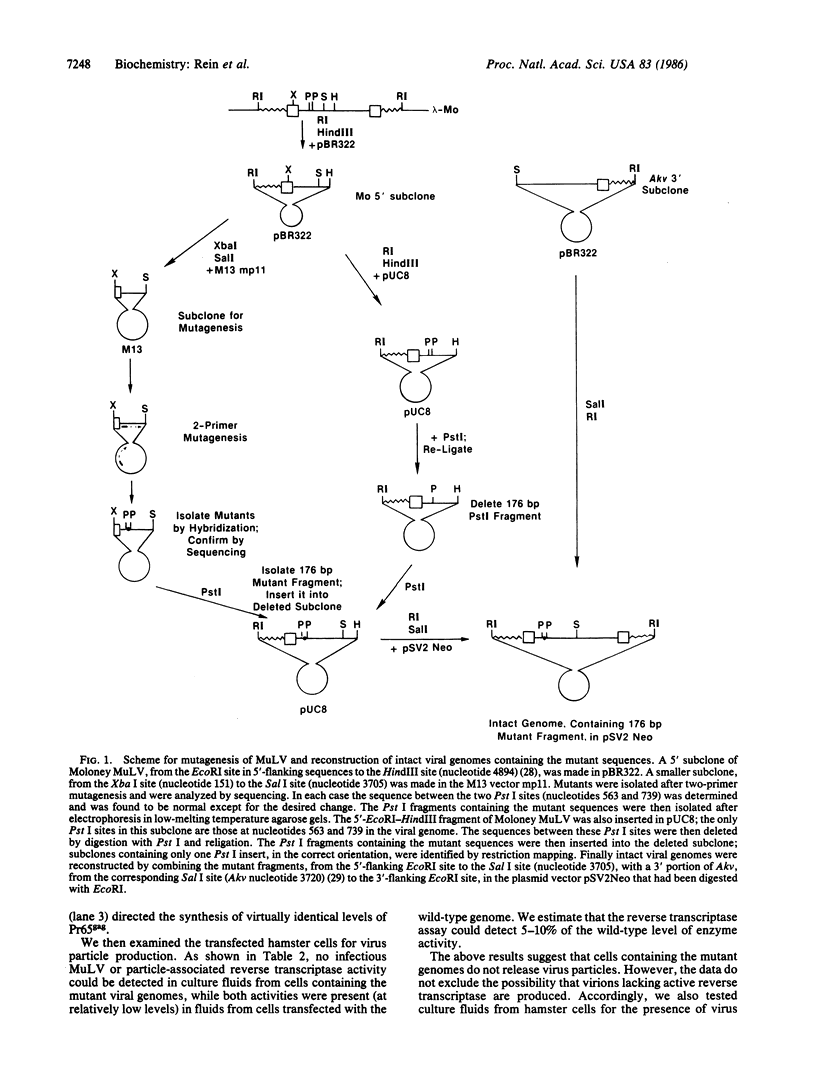
- Rein A., Benjers B. M., Gerwin B. I., Bassin R. H., Slocum D. R. Rescue and transmission of a replication-defective variant of moloney murine leukemia virus. J Virol. 1979 Feb;29(2):494–500. doi: 10.1128/jvi.29.2.494-500.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Lowy D. R., Gerwin B. I., Ruscetti S. K., Bassin R. H. Molecular properties of a gag- pol- env+ murine leukemia virus from cultured AKR lymphoma cells. J Virol. 1982 Feb;41(2):626–634. doi: 10.1128/jvi.41.2.626-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Luftig R. B. Microtubule-depolymerizing agents inhibit Moloney murine leukaemia virus production. J Gen Virol. 1982 Feb;58(Pt 2):339–349. doi: 10.1099/0022-1317-58-2-339. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Copeland T. D., Oroszlan S. The envelope proteins of bovine leukemia virus: purification and sequence analysis. Virology. 1984 Jun;135(2):417–427. doi: 10.1016/0042-6822(84)90197-1. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A., Oroszlan S. Myristylation of gag-onc fusion proteins in mammalian transforming retroviruses. Virology. 1984 Mar;133(2):431–437. doi: 10.1016/0042-6822(84)90409-4. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Versteegen R. J., Oroszlan S. Effect of chemical modification and fragmentation on antigenic determinants of internal protein p30 and surface glycoprotein gp70 of type C retroviruses. J Virol. 1980 Mar;33(3):983–992. doi: 10.1128/jvi.33.3.983-992.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. p65 of Gazdar murine sarcoma viruses contains antigenic determinants from all four of the murine leukemia virus (MuLV) gag polypeptides (p15, p12, p30, and p10) and can be cleaved in vitro by the MuLV proteolytic activity. Virology. 1982 Apr 30;118(2):380–388. doi: 10.1016/0042-6822(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]