Abstract
Despite numerous revisions and reformulations, dopamine (DA) hypothesis of schizophrenia remains a pivotal neurochemical hypothesis of this illness. The aim of this review is to expose and discuss findings from positron emission tomography (PET) or single-photon-emission computed tomography (SPECT) studies investigating DA function in the striatum of medicated, drug-naïve or drug-free patients with schizophrenia and in individuals at risk compared with healthy volunteers.
DA function was studied at several levels: i) at a presynaptic level where neuroimaging studies investigating DOPA uptake capacity clearly show an increase of DA synthesis in patients with schizophrenia; ii) at a synaptic level where neuroimaging studies investigating dopamine transporter availability (DAT) does not bring any evidence of dysfunction; iii) and finally, neuroimaging studies investigating DA receptor density show a mild increase of D2 receptor density in basic condition and, an hyperreactivity of DA system in dynamic condition.
These results are discussed regarding laterality, sub-regions of striatum and implications for the at-risk population. Striatal DA abnormalities are now clearly demonstrated in patients with schizophrenia and at risk population and could constitute an endophenotype of schizophrenia. Subtle sub-clinical striatal DA abnormalities in at risk population could be a biomarker of transition from a vulnerability state to the expression of frank psychosis.
Keywords: Dopamine, striatum, schizophrenia, PET; imaging.
1. INTRODUCTION
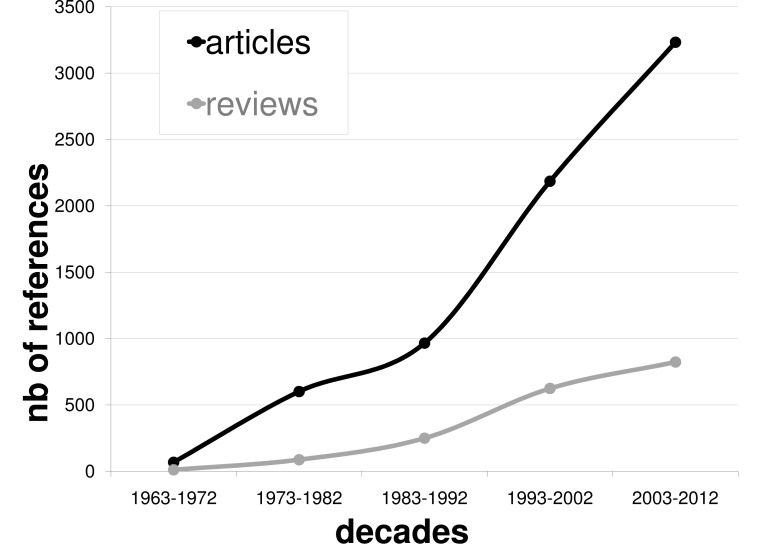
Although undergoing revisions and reformulations, the original dopamine (DA) hyperactivity hypothesis of schizophrenia, even over about 40 years ago [1] remains a pivotal neurochemical hypothesis increasingly studied in literature (see Fig. (1)). This hypothesis is based on two main observations: 1) it is possible to induce a psychotic episode in healthy subjects with pharmacological DA agonist and 2) all effective antipsychotic drug provide at least some degree of D2-type DA receptor blockade. The advent in the eighties of neuro-imaging techniques based on positron emission tomography (PET) or single-photon-emission computed tomography (SPECT) opened the possibility of direct investigation of theses hypotheses. A hyperactivity of DA systems could be already present in the early stage of the illness, before the expression of frank psychosis experience and may constitute a biomarker of transition from a vulnerability state to full blown episode of schizophrenia.
Fig. (1).
Number of original studies and reviews concerning dopamine and schizophrenia per decades (NIH Pubmed search with dopamine and schizophrenia as key words, until 2012, July).
Multiple components of dopaminergic neurotransmission may cause dopaminergic overactivity, including increased DA synthesis, release, receptor number and/or affinity, and DA-mediated postsynaptic effector mechanisms, and decreased inactivation.
Thanks to methodological advances, certain of these processes can now be quantified in vivo in patients, allowing a more comprehensive test of the DA overactivity hypothesis than was previously available and can test to confirm or refute the hyperDA hypothesis in patients with schizophrenia as well as in individuals at risk to develop the illness. After giving a brief overview of DA system, this paper reviews findings from PET and SPECT studies investigating dopaminergic impairments in patients with schizophrenia and in individuals at risk at the presynaptic and synaptic levels, in phasic and basal conditions in the striatum.
2. OVERVIEW OF THE DOPAMINERGIC SYSTEM
2.1. Anatomy of the DA System
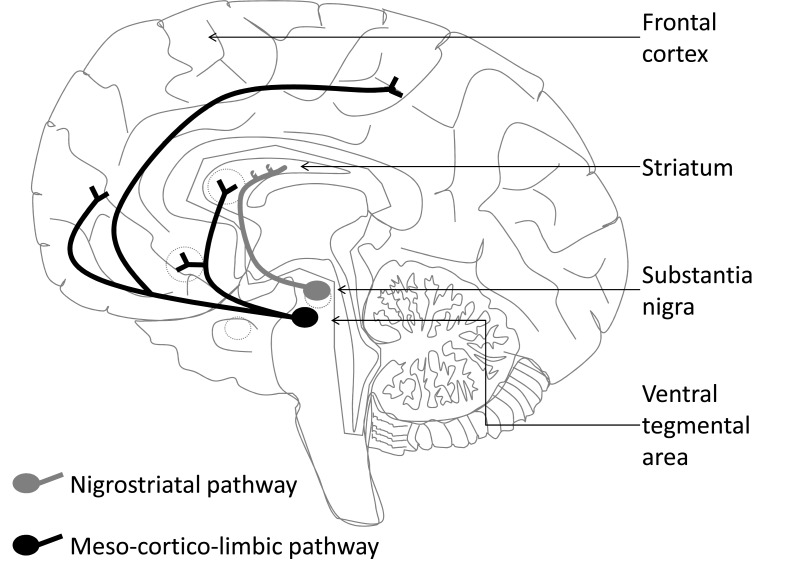
DA neurons are located in the mesencephalon and project into the forebrain along three major pathways forming a complex modu-latory network (see Fig. (2)). The nigrostriatal system is composed of DA neurons located in the substantia nigra compacta that project into the dorsal striatum. This system is mainly involved in motor control. The mesolimbic DA system is composed of neurons located in the ventral tegmental area (VTA) that project to limbic structures including the nucleus accumbens located into the ventral part of the striatum (VS), the basolateral amygdala and the hippocampus. Mesolimbic system involving the VS is thought crucial for motivation and reward seeking while the DA system involving basolateral amygdala and hippocampus is implicated in long-term memory and emotion. Finally, the DA mesocortical system is composed of neurons located into the VTA that project to cortical and cerebellar areas including the prefrontal cortex (PFC), an area that plays a pivotal role in mediating cognitive functions (such as short term memory), attention, and executive functions (such as abstract reasoning and planning).
Fig. (2).
Schematic drawing of dopaminergic pathways
2.2. DA Transmission
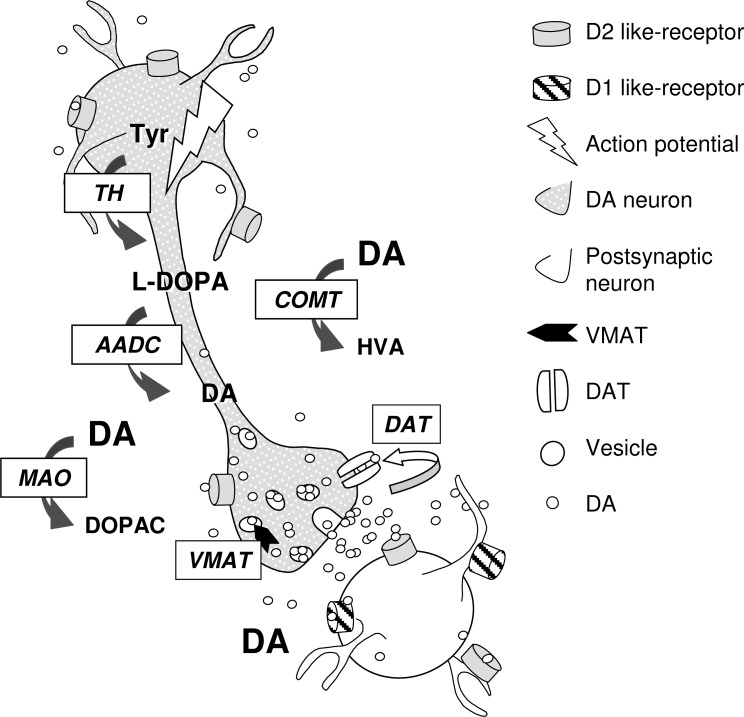
Thanks to animal studies it is now well admitted that DA transmission is governed by two signaling modes, a single neuron being able to switch from one mode to the other. The tonic mode corresponds to a discharge of DA neurons in a low regular spike mode (mean firing rate about (4-6 Hz) [2, 3] establishing a steady state level of DA efflux responsible for a low basal DA level. Change in tonic DA efflux can be too weak to change postsynaptic stimulation but sufficient to be involved in homeostatic processes such as autoregulation [4]. The phasic signaling corresponds to a discharge in a high frequency bursting pattern [3, 5, 6] eliciting transient large DA effluxes on top of DA tone [7, 8]. Usually, bursts consist of two to six spikes at high frequency (15 Hz-100 Hz) [5, 9]. Physiological stimulations such as sensory and appetitive stimuli favours bursting activity suggesting that phasic activity convey relevant information. The relationship between discharge activity and DA release is dependent on various mechanisms including DA synthesis and storage, reuptake, metabolism and autoregulation [10, 11, 12]. DA synthesis occurs within DA neurons from tyrosine hydroxylated to L-3.4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase (TH). Upon synthesis, DA arising from L-DOPA decarboxylation by aromatic amino acid decarboxylase (AADC) is transported into synaptic vesicles by the vesicular monoamine transporter (VMAT). In the striatum, re-uptake into DA terminals, achieved by DA transporter (DAT), is the main mechanism responsible for the clearance of the released DA [12].
In contrast, in the prefrontal cortex DA released is primarily metabolized by catechol-O-methyltransferase (COMT) or cleared by reuptake into noradrenergic terminals. DA action is mediated via five distinct G-protein-coupled receptor subtypes (DA1-DA5). D1-like receptors (D1, D5) and D2-like receptors (D2, D3 and D4) are positively and negatively coupled to adenylate cyclise, respectively. D1 receptors are found in DA projection areas while D2 receptors are found in DA projection and cell body areas. D2 autoreceptors located on DA axonal terminals modulate DA synthesis and release (see Fig. 3).
Fig. (3).
Schematic drawing of mechanism participating in DA transmission. AADC: aromatic amino acid decarboxylase; COMT: catechol-Omethyltransferase; DA: dopamine; DAT: Dopamine transporter; DOPAC: 3,4-Dihydroxyphenylacetic acid ; HVA: Homovanillic; L-DOPA: L-3.4- dihydroxyphenylalanine; MAO: Monoamine Oxydase; TH: tyrosine hydroxylase; Tyr: tyrosine; VMAT: vesicular monoamine transporter
In a physiopathological context, altogether these results underline the importance to consider, beyond the changes in basal extracellular level, the changes in tonic versus phasic DA signaling mode [13].
In this way, Grace proposed in schizophrenia an imbalance between tonic and phasic DA transmission within the ventral striatum resulting in a functional impairment in the integration of the information convergent from limbic and cortical areas [14]. In schizophrenia, most of the processes involved in DA transmission has been used as target in imaging studies.
3. RESULTS: DOPAMINEGIC IMPAIRMENTS IN SCHIZOPHRENIA
3.1. Presynaptic Dopamine Function
3.1.1. Imaging Measuring DOPA Decarboxylase Activity Indicative of Dopamine Synthesis
Although it is not possible to measure DA synthesis directly in humans, recent neuroimaging provides indirect indices of DA synthesis. A method of imaging presynaptic dopaminergic function under baseline conditions measures the formation and storage of DA in presynaptic terminals with l-[β-11C]DOPA (11C-DOPA) or 6-[18F]fluoro-L-DOPA (18F-DOPA), 2 radioactive analogues of L-DOPA, the precursor of DA. The radioactive analogue of DOPA, peripherally injected under the scanner, is taken up by presynaptic monoaminergic neurons and metabolized by decarboxylase AADC to radioactive tagged DA which will be trapped and stored within vesicles in the nerve terminals. The uptake of radioactive L-DOPA, quantified as the influx constant Ki, measures AADC activity and vesicular storage capacity [15] and thus is indicative of DA synthesis capacity. High values for 18F-DOPA Ki are observed in areas of dense DA nerve terminal innervation (e.g., the striatum), and 18F-DOPA uptake correlates with surviving nigrostriatal cell numbers in both monkey and human studies [16, 17]. In this way, 18F-DOPA was extensively used to probe the structural and functional integrity of striatal dopaminergic neurons, particularly in Parkinson disease and other movement disorders (for review see [18]).
To date, compared to healthy subjects, elevations of 18F-DOPA or 11C-DOPA in the striatum of both antipsychotic-naive and antipsychotic-free patients were observed in the majority of PET studies [19-25]. Two studies reported different results. One study reported no difference [26] and another one reported reduced 18F-DOPA striatal uptake in patients as compared to controls [27]. Both were conducted in chronic, remitted patients. Finally, a recent meta-analysis (including 11 different studies from 1994 to 2011), comparing 113 patients with schizophrenia and 131 healthy controls reported a relative elevation of on average 14% of dopamine synthesis capacity measured by L-DOPA (11C and 18F) uptake in patients in both caudate and putamen [28]. The authors have reported no effect of exposure to antipsychotic medication, illness duration, age or intensity of psychotic symptoms.
Interestingly, 18F-DOPA uptake was also investigated in individuals at-risk to develop schizophrenia, without experiencing frank psychotic symptoms. This target population included unaffected first degree relatives of patients with schizophrenia (FDR), individuals with an At-Risk Mental State (ARMS) for psychosis or twin pairs discordant for schizophrenia [29, 30].
The studying of first degree relatives of patients with schizophrenia [30] revealed that 18F-DOPA uptake was higher in the FDR group than in the control group suggesting that the changes of striatal presynaptic DA synthesis seen in previous studies in antipsychotic-naive and antipsychotic-free patients with schizophrenia are also present in FDRs. These findings have implications for the early detection of psychosis as well as for pharmacological interventions in individuals at risk for psychosis. This finding was corroborated in a study of ARMS showing higher 18F-DOPA uptake in the associative part of the striatum (AST) of ARMS subjects compared to controls [29]. However, another study of ARMS subjects [31] and a study of twin pairs discordant for schizophrenia [32] did not confirmed these promising observations and reported no difference in 18F-DOPA uptake between at-risk subjects and controls. The wide variability between at-risk individuals due to the lack of specificity in the features used to characterize the populations targeted in these studies could explain the discrepancies. For example, post-hoc examination of the data corresponding to the subjects enrolled in the study of Allen and coworkers who have developed psychosis (3 of 20 subjects) [31], indicated that 18F-DOPA uptake was higher in these 3 ARMS subjects than in the rest of the sample.
Altogether, these results are consistent with a 18F-DOPA uptake increase in the striatum of patients with schizophrenia as well as of subjects with an high risk to develop the pathology. Moreover 18F-DOPA uptake correlated significantly with severity of prodromal symptom [24].
Totally, in schizophrenia and at-risk subjects, imaging data suggest an abnormal striatal DA transmission at the presynaptic level which can be viewed as an increased DA synthesis capacity. From these studies it is not clear if and how the increase in presynaptic DA could account for DA output changes, because measurements depend on AADC activity in a non rate-limitating step of DA synthesis. Moreover, because the operating levels of processes involved in intracellular DA storage, such as vesicular transport, are missing in schizophrenia, an increase in storage cannot be ruled out.
3.1.2. Imaging Striatal Dopamine Transporter (DAT)
The striatal DA transporters (DAT) are located on the presynaptic membrane of DA neuron terminals. DAT regulate DA transmission by removing released DA from the synapse through reuptake. The measurement of DAT is usually considered as an index of the density of DA terminals or innervation into the striatum. Using either PET or SPECT, a lot of studies have examined striatal DAT density in antipsychotic-naive and in antipsychotic-free patients with schizophrenia. The majority of studies failed to report any significant differences between patients and controls [33-37]. A recent meta-analysis [38] comparing 202 subjects with schizophrenia with 147 matched healthy controls (from 13 independent studies) did not report evidence indicating altered density of striatal dopamine terminals in schizophrenia independently of illness duration or antipsychotic medication intake. These results suggest that the increased presynaptic striatal DA activity in schizophrenia indicated by other imaging parameters is not secondary to dysfunctional DAT or an elevation in the density of DA terminals.
However, some alterations in DAT availability were reported in schizophrenia, particularly using SPECT technique. On the one hand, DAT availability was found significantly larger (up-regulation) in chronic schizophrenic patients [39] and in a subgroup of patients with predominantly positive symptoms than in controls [40]. On the other hand, some studies clearly showed a significant decrease of DAT binding in patients with schizophrenia, in young antipsychotic-free patients [41] as well as in chronic medicated patients [42]. Using recent [123I] FP-CIT tracer, in two independent studies, Mateos and co-workers [43, 44] reported and confirmed that DAT binding was lower in antipsychotic-naive first episode patients than in healthy subjects, at baseline and after a 4-week-treatment period. Interestingly, in a 4-year follow up study, Mané and co-workers [45] reported a specific relation between striatal DAT number and negative symptoms suggesting that there is a close relationship between DAT number and clinical features of patients. Finally, supporting abnormal dopamine transmission in schizophrenia, using a dual-isotope SPECT technique, a positive association between DAT and D2 receptor availability has been found in first-episode, antipsychotic-naive patients whereas this relation does not exist in controls [35, 37].
Altogether, results show many discrepancies. Discrepancies can be explained by the heterogeneity in the age range and illness duration of the samples, the type and length of treatments, the radiotracers used ([99mTc]TRODAT-1, [18F]CFT or [123I] FP-CIT) , and the different methods of quantification applied. Thus, pending further replication, most of the evidence at this point does not shows if DAT is affected or unaffected in schizophrenia.
3.2. Synaptic Dopamine Function
3.2.1. Striatal D2 Receptor Density
Another approach to measure DA activity in the human striatum consists in measuring DA receptor density through the receptor occupancy by a radioligand evaluated by its binding potential. Because reversibly radioligand competes with endogenous DA for receptor binding, it is assumed that radiotracer binding potential is indicative of DA level. This measurement is achieved either in basal or in dynamic conditions. In dynamic conditions, DA release is evoked either by the administration of a DA-releasing drug or by subjecting individuals to DA-releasing activity such as stress. In these conditions, reduction in radiotracer binding potential is thought indicative of DA system reactivity by assessing evoked DA release. It is undoubtedly because post-mortem studies of D2 receptors in schizophrenia were crucial to the genesis of the DA hypothesis that D2 receptor density has been extensively study in schizophrenia. Dopamine D2-receptor imaging, combined with pharmacological manipulation of DA release enables a more direct evaluation of DA presynaptic activity than 18 F-DOPA study.
In the study of schizophrenia, two imaging techniques are usually associated with various D2 receptor radiotracers. Among radiotracers available, iodobenzamide (IBZM) and raclopride are the most widely used. IBZM is a DA antagonist which is currently used as a radioactive tracer for SPECT where the radioactive isotope is iodine-123. [123I]IBZM is considered to measure striatal D2 DA receptor density. Raclopride is a synthetic compound that acts as an antagonist on D2 DA receptors. It can be radiolabelled with the carbon-11 radioisotope and used in PET scanning to assess the degree of DA binding to the D2/D3 receptors.
3.2.1.1. Imaging Striatal D2 Receptor Density in Basal Conditions
Many years ago, increased striatal D2 receptor density was reported [46, 47], but these first findings were questioned on the basis that the data were obtained in antipsychotic-treated patients. Indeed, classical antipsychotic therapy could, in itself, cause D2 receptor upregulation [48, 49]. Imaging studies using PET and SPECT could control for this confound by studying never-treated people with schizophrenia. Since these first studies, striatal D2 receptor density in schizophrenia has been extensively studied. In a meta-analysis in 2001, Weinberger & Laruelle [50] have identified 17 imaging studies using various radiotracers comparing D2 receptors in 245 patients with schizophrenia (112 antipsychotic naive, 133 antipsychotic free) and matched 231 healthy controls for age and sex. Despite only 2 studies (on 17 selected studies) reporting a significant elevation of D2 receptor density in patients, the metaanalysis revealed a small but significant global elevation of striatal D2 receptor (12%). The more recent radioligand [18F]fallyprid confirmed abnormal D2 receptor density in the striatum of patients with schizophrenia [51, 52].
However, before any conclusion, it is important to note that these findings may be confounded by physiological and methodological factors. D2 imaging results from several parameters including the number of D2 receptors, the ability of the radioligand in displacing DA and the level of DA in the vicinity of receptors. Moreover discrepancies may result from the radioelement used probably because of wide variations in their specific activity and thus of competition with endogenous DA.
Efforts have been made to overcome the problem of a competition between the radioligand and endogenous DA [53] by developing D2 imaging in acute DA depletion condition in humans [54, 55]. Such depletion is achieved by blocking the rate limiting step of DA synthesis via orally administering the tyrosine hydroxylase enzyme inhibitor, AMPT (Alpha-methyl-p-tyrosine) over 48 h. It is assumed that, compared to baseline, the D2 receptor density measured in the depleted state is extended to D2 receptors occupied by endogenous DA at baseline. The unmasked D2 receptor density is then considered as an index of DA level at baseline [55]. Using this approach with SPECT and the D2 radiotracer [123I]IBZM, Abi-Dargham and colleagues [56] reported that the increase in D2 receptor availability after acute DA depletion was significantly larger in patients with schizophrenia (during an acute psychotic episode) than in controls. Assuming normal affinity of D2 receptor for DA, this result was interpreted as an increased occupancy of striatal D2 receptors by DA at baseline in schizophrenia, and thus as consistent with higher DA synaptic levels in patients. It was further noted that AMPT exposure led to a significant reduction in severity of positive symptoms among patients; the higher occupancy of striatal D2 receptors by DA the greater was reduction in positive symptoms after administration of antipsychotic treatment [56].
D2 receptors are highly heterogeneous in various aspects leading to heterogeneity in their function and response to drug [57]; such heterogeneity hampers the interpretation of D2 imaging data. Data are interpreted from the hypothesis that D2 affinities are similar between patients and controls. This hypothesis is questioned by the demonstration of the existence of two D2 affinity states for agonist binding, DA competing first at high-affinity D2 site coupled to G-protein while low affinity D2 site is uncoupled. In vitro studies in animal model for psychosis demonstrated an increase in high-affinity D2 state suggesting that this increase may be responsible of DA hyperactivity in schizophrenia [58]. In humans, a specific imaging of each D2 affinity state is still under discussion [59]. The wide D2 distribution across constituting neural elements in the striatum makes also difficult the functional interpretation of D2 density measurements. D2 receptors, existing as long and short variants, are distributed across dendritic spines of efferent neurons and interneurons, as well as DA terminals and other terminals such as glutamatergic ones. Moreover, the accessibility of D2 receptors depends on their localization, synaptically or extrasynaptically, internalized or addressed to the membranes. Imaging data do not bring information about which D2 receptor population is affected. Finally, in schizophrenia alterations in processes regulating D2 receptors such as an impaired D2 trafficking [60] cannot be ruled out. Totally, to date, altered D2 receptor binding found in schizophrenia would not simply indicative of DA level change.
3.2.1.2. Imaging Striatal D2 Receptor Density in Dynamic Conditions
Using a Pharmacological Challenge
The acute DA releasing effect of amphetamine has been demonstrated in schizophrenia by measuring changes in D2 receptor availability combined with SPECT and PET [61-63]. The degree of amphetamine-induced DA release has been shown dependent on patient status in terms of phase of illness and intensity of symptoms. When comparing different populations of patients, Laruelle and co-workers have observed that, after amphetamine, the subgroup of schizophrenia patients in the acute phase of illness displayed the strongest reduction in striatal [123I]IBZM binding potential. The DA releasing effects of amphetamine were similarly large in antipsychotic naïve and previously treated patients experiencing exacerbation of positive symptoms whereas the response evoked in patients in remission was found lower and not different from that of control group [64]. The findings suggest that excessive evoked DA release at striatal D2 receptors is involved in the experiencing of psychotic symptoms, and highlight a role for a dysregulated, hyperresponsive subcortical DA system that fluctuates in its degree of dysregulation during the different phases of the illness.
More recently, it has been shown that following amphetamine, patients with schizotypal personality disorder (SPD) present a larger reduction in striatal [123I]IBZM binding potential compared to controls that remained, however, lower than that seen in acutely ill patients [65]. This result has been replicated in subjects at risk to psychosis using other D2 radioligands. An abnormal supra striatal DA response has been found after a metabolic stress challenge with no direct DA impact in unaffected siblings of patients with schizophrenia [66], after psychosocial stress [67, 68] and also after amphetamine challenge in psychometric schizotypal subjects [69]. Interestingly, according to the relationship between DA transmission and stress (for a comprehensive review see [70]), it has been shown that the over-DA response evoked by psychosocial stress was correlated with salivary cortisol response to stress [68].
Taken together, these results suggest that a modest over-increase in DA release (or a hyperreactivity) may characterize individuals at risk for psychosis including subjects with spectrum disorders or unaffected relatives and patients between episodes. A high degree of DA dysregulation can characterize those reaching the threshold of active psychosis including the first episode of the illness. The hypothesis of an increase DA release in schizophrenia and individuals at risk of psychosis is consistent with the data suggesting an increase in DA synthesis (see above).
The gradation in DA release dysregulation between patients with schizophrenia and their unaffected at-risk siblings compared to healthy controls has been also confirmed by indirect measures of DA activity after a metabolic stress challenge using plasma level measurements of homovanillic acid (HVA), a breakdown product of DA [71]. Altogether data suggest that DA dysregulation is involved in the process of transition across the stages of illness. However, according to the modulation of DA activity by other neurotransmitters, activity changes, such as hyperactivity in serotonergic systems could moderate DA release and thus constitute a protective factor against DA hyperactivity in high risk subjects [72].
By Acting on Frontal Activity
Imaging data support the concept of fronto-striatal dysfunction in schizophrenia. It has been reported a relationship between dorsolateral prefrontal cortex hypoactivity and abnormal striatal 18-F DOPA uptake in patients with schizophrenia [19]. Moreover, Kegeles and co-workers [73] suggest that elevated subcortical dopamine function might adversely affect performance of the dorsolateral prefrontal cortex in schizophrenia. In the same way, in ARMS individuals, an abnormal relation has been highlighted between striatal L-DOPA uptake and medial temporal lobe [31] and frontal activations [29], providing additional support for the relation between prefrontal dysfunction and potentiated striatal DA activity in the illness and its early phase. In this way, schizophrenia could be associated to a failure in the inhibitory brake provided by the frontal activity on striatal DA system [74].
According to the relation between frontal cortex activity and ventral striatal DA transmission, striatal DA transmission has been investigated using [11C] raclopride displacement following external frontal activation in patients with schizophrenia. In this way, it has been reported in a young drug naive patient with refractory negative symptoms that repetitive transcranial magnetic excitatory stimulation (rTMS) applied over the left DLPFC induced a DA release in the ventral striatum (especially on the right ventral striatum; [75]), as seen in healthy volunteers [76].
Before any conclusion, it is important to note that some parameters which could be confounding at baseline could be also confounding in the interpretation of data obtained in dynamic conditions by measuring D2 DA receptor density through the binding potential of a radiolabelled antagonist. An increase in D2 affinity for DA in schizophrenia cannot be excluded. The development of radiolabeled agonist of D2 receptor could settle this issue.
3.2.2. Striatal D1 Receptor Density
Compared to studies investigating D2 availability in schizophrenia, fewer studies aimed to investigate D1 receptor density.
In post-mortem studies only one study has reported lower dopamine D1 receptors in the striatum in patients with schizophrenia compared to healthy [77] while no significant change has been reported in numerous other studies [78,79].
PET studies using different radiotracers have reported contradictory results showing an unaltered binding potential of D1 receptor over the brain of patients with schizophrenia compared with control subjects [80]. When structures were specifically analyzed, either a decrease or an increase in D1 receptor density was found in the prefrontal and/or striatal regions [81-83].
Further studies are needed in order to elucidate the role of striatal D1 receptors in the pathophysiology of schizophrenia.
3.3. Anatomical and Functional Features
3.3.1. Striatal Subregions
The advent of high-resolution PET scanners and the development of methods permitting examination of DA receptor parameters in the various subregions of the striatum allow to probe aspects of DA transmission separately for the functional subdivisions of the striatum [84, 85]. Classically, abnormalities in DA system were reported in the ventral striatum. Combined with PET and [11C]raclopride, studies with high resolution PET have demonstrated that patients with schizophrenia displayed a significantly larger increase in D2 receptor availability after DA depletion compared to controls in the associative striatum (AST), but not in the limbic or sensorimotor striatal regions [73]. This result was supported by measuring DA in response to psychological stress in at risk population and in patients [68].
Abnormalities in the AST have also been reported using F-DOPA uptake in ARMS (29; for a negative result see also [31]),
These findings have challenged the notion that in schizophrenia, alterations in subcortical DA transmission are most prominently localized to the ventral part and highlighted the potential role of the AST in the illness. This striatal region receives prominent input from the dorso lateral PFC, and is thought to play an important role in regulating the circuitry from various cortical regions.
3.3.2. Lateralisation of Dopamine Abnormalities
In schizophrenia, dopamine is known to be implicated in functional neuropsychophysiological asymmetry (for a review see [86]). McGowan and colleagues [20] as well as Hietala and co-workers
[21] reported that abnormal presynaptic dopamine density (measured by 6-[18F]-fluorodopa uptake) was lateralised to the left in patients with schizophrenia. Other studies have reported a dopaminergic asymmetry also in unaffected siblings [30], while no such asymmetries were observed in healthy subjects [87]. Strikingly, while the majority of PET studies did not report any D2 asymmetry in patients, Hirvonen and colleagues [88] noted more pronounced D2 increase in left vs. right head of the striatum. The finding that unmedicated patients with schizophrenia displayed a left-sided spontaneous turning preference (orientating spatial behaviour) that was associated with the severity of psychotic symptoms [89] is in favor an asymmetrical DA system dysregulation in this population, characterized by a more active DA system in the left hemisphere which could conceivably be corrected by antipsychotic medication [90]. This asymmetry has also been found in dynamic condition. In individuals at risk, a stress challenge evokes more DA release in the left part of the ventral striatum than in the right part [66]. This excessive DA release in the left part of the ST was correlated with the intensity of positive symptoms. All together, these results suggest a pre-existence of abnormal lateralization of DA transmission in the early stage of the illness with an excessive left DA transmission linked to an excessive DA synthesis.
In a case report [75], Brunelin and colleagues reported that after 20 sessions of rTMS applied to the left dorso lateral PFC, the patient had achieved remission of schizophrenic symptoms. The clinical improvement was accompanied with an induced asymmetrisation of D2 receptor binding potential in the striatum (Right>Left) while no asymmetry was observed in baseline condition in this drug-naïve patient. The slight binding potential increase in the right ventral striatum measured after TMS regimen suggests an up-regulation of dopaminergic D2 receptors evoking a change in basal dopaminergic transmission. This adaptive mechanism could contribute to a right subcortical dopamine signalization enhancement in dynamic/phasic conditions. Further studies are needed to confirm this abnormal lateralization of DA systems in schizophrenia.
CONCLUSION
In summary, recent studies and meta-analyses have highlighted that hyperDA hypothesis in schizophrenia and in at-risk individual seem to be a robust result, in terms of presynaptic DA function and of evoked DA release by pharmacological-drug or by stress in the striatum, probably more pronounced in the left AST.
DA dysregulation may not be a primary dysfunction in schizophrenia, but rather may represent a final common pathway. The aetiology of the striatal dopaminergic dysregulation in schizophrenia is still unknown, and evidence support this dysregulation is related to numerous alterations in other areas functionally linked, such as the frontal cortex and in other neurotransmitter systems that regulate DA transmission.
In addition, as the striatum represents an essential integrative node, it seems that DA dysregulation itself may play a role in other pathogenic effects. Indeed, the striatum receives inputs from the hippocampus and the cortex, two areas involved in the pathology of schizophrenia and projects directly and/or indirectly back to the cortex and DA midbrain neurons. Striatal dopaminergic dysfunction can be also reciprocally linked to a cortical dopaminergic dysfunction. Acting on the PFC using non invasive brain stimulation techniques permit a subcortical DA release and open the possibility to act on abnormal DA transmission in schizophrenia and probably from the earliest stages (see [91]).
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
LIST OF ABBREVIATIONS
- AADC
= Aromatic amino acid decarboxylase
- ARMS
= At-Risk Mental State for psychosis
- AST
= Associative striatum
- COMT
= Catechol-O-methyltransferase
- DA
= Dopamine
- DAT
= Dopamine transporter
- HVA
= Homovanillic Acid
- IBZM
= Iodobenzamide
- L-DOPA
= L-3.4-dihydroxyphenylalanine
- PET
= Positron Emission Tomography
- PFC
= Prefrontal cortex
- rTMS
= Repetitive transcranial magnetic stimulation
- SPECT
= Single-photon-emission computed tomography
- TH
= Tyrosine hydroxylase
- VMAT
= Vesicular monoamine transporter
- VS
= Ventral striatum
- VTA
= Ventral tegmental area
REFERENCES
- 1.Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. (Copenh.) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 2.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons single spike firing. J. Neurosci. 1984;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanghera MK, Trulson ME, German DC. Electrophysiological proper-ties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984;12:793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- 4.Dugast C, Brun P, Sotty F, Renaud B, Suaud-Chagny MF. On the involvement of a tonic dopamine D2-autoinhibition in the regulation of pulse-to-pulse-evoked dopamine release in the rat striatum in vivo. Naunyn Schmiedeberg Arch. Pharmacol. 1997;355:716–719. doi: 10.1007/pl00005004. [DOI] [PubMed] [Google Scholar]
- 5.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman AS, Bunney BS. Chronic neuroleptic effects on dopamine neuron activity: a model for predicting therapeutic efficacy and side effects?. Psychopharmacol Ser. 1987;3:225–235. doi: 10.1007/978-3-642-71288-3_26. [DOI] [PubMed] [Google Scholar]
- 7.Dugast C, Suaud-Chagny MF, Gonon F. Continuous in vivo monitoring of evoked dopamine releasein the rat nucleus accumbens by amperometry. Neuroscience. 1994;62:647–654. doi: 10.1016/0306-4522(94)90466-9. [DOI] [PubMed] [Google Scholar]
- 8.Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 9.Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85(4):1285–1309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- 10.Suaud-Chagny MF, Buda M, Gonon F. Pharmacology of electrically evoked DA release studied in the rat olfactory tubercle by in vivo electro-chemistry. Eur. J. Pharmacol. 1989;164:273–283. doi: 10.1016/0014-2999(89)90468-8. [DOI] [PubMed] [Google Scholar]
- 11.Suaud-Chagny MF, Ponec J, Gonon F. Presynaptic autoinhibition of the electrically evoked dopamine release studied in the rat olfactory tubercle by in vivo electrochemistry. Neuroscience. 1991;45:641–652. doi: 10.1016/0306-4522(91)90277-u. [DOI] [PubMed] [Google Scholar]
- 12.Suaud-Chagny MF, Dugast C, Chergui K, Msghina M, Gonon F. Uptake of dopamine released by impulse flow in the rat mesolimbic and stri-atal systems in vivo. J. Neurochem. 1995;65:2603–2611. doi: 10.1046/j.1471-4159.1995.65062603.x. [DOI] [PubMed] [Google Scholar]
- 13.Suaud-Chagny MF. In vivo monitoring of dopamine overflow in the central nervous system by amperometric techniques combined with carbon fibre electrodes. Methods. 2004;33:322–329. doi: 10.1016/j.ymeth.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Grace AA. Phasic versus tonis dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schiz-ophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 15.Brown WD, Taylor MD, Roberts AD, Oakes TR, Schueller MJ, Holden JE, Malischke LM, DeJesus OT, Nickles RJ. FluoroDOPA PET shows the nondopaminergic as well as dopaminergic destinations of levodopa. Neurology. 1999;53:1212–1218. doi: 10.1212/wnl.53.6.1212. [DOI] [PubMed] [Google Scholar]
- 16.Pate BD, Kawamata T, Yamada T, McGeer EG, Hewitt KA, Snow BJ, Ruth TJ, Calne DB. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann. Neurol. 1993;34:331–338. doi: 10.1002/ana.410340306. [DOI] [PubMed] [Google Scholar]
- 17.Snow BJ, Tooyama I, McGeer EG, Yamada T, Calne DB, Takahashi H, Kimura H. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann. Neurol. 1993;34:324–330. doi: 10.1002/ana.410340304. [DOI] [PubMed] [Google Scholar]
- 18.Fischman AJ. Role of [18F]-dopa-PET imaging in assessing movement disorders. Radiol. Clin. North Am. 2005;43(1):93–106. doi: 10.1016/j.rcl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 20.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch. Gen. Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 21.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 22.Hietala J, Syvalahti E, Vilkman H, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr. Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 23.Howes OD, Montgomery AJ, Asselin M, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br. J. Psychiatry. 2007;51:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howes OD, Montgomery AJ, Asselin M, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 25.Lindstrom LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol. Psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 26.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Attar-Levy D, Remy P, Crouzel C, Artiges E, Feline A, Syrota A, Martinot JL. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr. Res. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 27.Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-(18)F-DOPA PET study in patients with schizophrenia: positron emission tomography. Psychiatry Res. 2000;100:1–11. doi: 10.1016/s0925-4927(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 28.Fusar-Poli P, Meyer-Lindenberg A. Striatal Presynaptic Dopamine in Schizophrenia, Part II: Meta-Analysis of [18F/11C]-DOPA PET Studies. Schizophr. Jan 26 Bull. 2012 doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusar-Poli P, Howes OD, Allen P, Broome M, Valli I, Asselin MC, Montgomery AJ, Grasby PM, McGuire P. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol. Psychiatry. 2011;16(1):67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 30.Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S, Solin O, Ilonen T, Korkeila J, Ristkari T, McGlashan T, Salokangas RK, Hietala J. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol. Psychiatry. 2008;63(1):114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, Valli I, Day F, McGuire PK. Abnormal Relationship Between Medial Temporal Lobe and Subcortical Dopamine Function in People With an Ultra High Risk for Psychosis. Schizophr. May 2 Bull. 2011 doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shotbolt P, Stokes PR, Owens SF, Toulopoulou T, Picchioni MM, Bose SK, Murray RM, Howes OD. Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol. Med. 2011;41(11):2331–2338. doi: 10.1017/S0033291711000341. [DOI] [PubMed] [Google Scholar]
- 33.Lavalaye J, Linszen DH, Booij J, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr. Res. 2001;47:59–67. doi: 10.1016/s0920-9964(00)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao MC, Lin KJ, Liu CY, Tzen KY, Yen TC. Dopamine trans-porter change in drug-naive schizophrenia: An imaging study with 99mTc-TRODAT-1. Schizophr. Res. 2003;65:39–46. doi: 10.1016/s0920-9964(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 35.Yang YK, Yu L, Yeh TL, Chiu NT, Chen PS, Lee IH. Associated alterations of striatal dopamine D2/D3 receptor and transporter binding in drug-naive patients with schizophrenia: A dual-isotope SPECT study. Am. J. Psychiatry. 2004;161:1496–1498. doi: 10.1176/appi.ajp.161.8.1496. [DOI] [PubMed] [Google Scholar]
- 36.Laakso A, Vilkman H, Alakare B, et al. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am. J. Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt GJ, Frodl T, Dresel S, et al. Striatal dopamine transporter availability is associated with the productive psychotic state in first episode drug-naive schizophrenic patients. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:115–121. doi: 10.1007/s00406-005-0618-2. [DOI] [PubMed] [Google Scholar]
- 38.Fusar-Poli P, Meyer-Lindenberg A. Striatal Presynaptic Dopamine in Schizophrenia, Part I: Meta-analysis of Dopamine Active Transporter (DAT) Density. Schizophr Jan 26. Bull. 2012 doi: 10.1093/schbul/sbr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjoholm H, Bratlid T, Sundsfjord J. 123I b-CIT SPECT demonstrates increased presynaptic dopamine transporter binding sites in basal ganglia in vivo in schizophrenia. Psychopharmacology. 2004;173:27–31. doi: 10.1007/s00213-003-1700-y. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt GJ, la Fougere C, Dresel S, et al. Dual-isotope SPECT imaging of striatal dopamine: First episode, drug naive schizophrenic patients. Schizophr. Res. 2008;101:133–141. doi: 10.1016/j.schres.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Tatsch K, Scherer J, Linke R, Kerner M, Hahn K. Decrease of dopamine transporter binding in neuroleptic-free schizophrenic patients as-sessed with IPT-SPECT (abstr. . J. Nucl. Med. 1999;40:31. [Google Scholar]
- 42.Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Sivälahti E, Hietala J. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr. Res. 2001;52:115–120. doi: 10.1016/s0920-9964(00)00095-5. [DOI] [PubMed] [Google Scholar]
- 43.Mateos JJ, Lomeña F, Parellada E, Font M, Fernandez E, Pavia J, Prats A, Pons F, Bernardo M. Decreased striatal dopamine transporter binding assessed with [123I] FP-CIT in first episode schizophrenic patients with and without short-term antipsychotic-induced parkinsonism. Psy-chopharmacology. 2005;181:401–406. doi: 10.1007/s00213-005-2250-2. [DOI] [PubMed] [Google Scholar]
- 44.Mateos JJ, Lomeña F, Parellada E, Mireia F, Fernandez-Egea E, Pavia J, Prats A, Pons F, Bernardo M. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacol (Berl.) 2007;191(3):805–811. doi: 10.1007/s00213-006-0570-5. [DOI] [PubMed] [Google Scholar]
- 45.Mané A, Gallego J, Lomeña F, Mateos JJ, Fernandez-Egea E, Horga G, Cot A, Pavia J, Bernardo M, Parellada E. A 4-year dopamine transporter (DAT) imaging study in neuroleptic-naive first episode schizophrenia patients. Psychiatry Res. 2011;194(1):79–84. doi: 10.1016/j.pscychresns.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Lee T, Seeman P. Elevation of brain neuroleptic/ dopamine receptors in schizophrenia. Am. J. Psychiatry. 1980;137:191–197. doi: 10.1176/ajp.137.2.191. [DOI] [PubMed] [Google Scholar]
- 47.Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, Creese I, Synder SH. Increased brain dopamine and dopamine receptors in schizophrenia. Arch. Gen. Psychiatry. 1982;39:991–997. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- 48.Clow A, Theodorou A, Jenner P, Marsden CD. Changes in rat striatal dopamine turnover and receptor activity during one year's neuroleptic admin-istration. Eur. J. Pharmacol. 1980;63:135–144. doi: 10.1016/0014-2999(80)90437-9. [DOI] [PubMed] [Google Scholar]
- 49.Clow A, Theodorou A, Jenner P, Marsden CD. Cerebral dopamine function in rats following withdrawal from one year of continuous neurolep-tic administration. Eur. J. Pharmacol. 1980;63:145–157. doi: 10.1016/0014-2999(80)90438-0. [DOI] [PubMed] [Google Scholar]
- 50.Weinberger D, Laruelle M, Davis KL, Charney DS, Coyle J, et al. In: Neurochemical and neuropharmachological imaging in schizophrenia In Neuropsychopharmacology-The Fifth Generation of Progress. Lippincott Williams Wilkins. 2001 [Google Scholar]
- 51.Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, Dawant B, Zald D, Meltzer HY. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra limbic regions and cortex in schizophrenic subjects. Biol. Psychiatry. 2009;65(12):1024–1031. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kegeles LS, Slifstein M, Xu X, Urban N, Thompson JL, Moadel T, Harkavy-Friedman JM, Gil R, Laruelle M, Abi-Dargham A. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol. Psychiatry. 2010;68(7):634–641. doi: 10.1016/j.biopsych.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laruelle M, Martinez D, Talbot PS, Adi-Dargham A, Valk P, Bailey DL, Townsend DW, Maisey MN, Valk P, Bailey DL, Townsend DW, Maisey MN. In Molecular Imaging in psychiatric disorder In Positon Emission Tomography. Basic science and clinical practice Edited by Springer. 2003 [Google Scholar]
- 54. Fujita M, Verhoeff NPLG, Varrone A, Zoghbi SS, Baldwin RM, Jatlow PA, Anderson GM, Seibyl JP, Innis RB. Imaging extrastriatal dopamine D2 receptor occupancy by endogenous dopamine in healthy humans. Eur. J. Pharmacol. 2000;387:179–?188. doi: 10.1016/s0014-2999(99)00817-1. [DOI] [PubMed] [Google Scholar]
- 55.Laruelle M, D'Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162?–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 56.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, VanHeertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U S A. 2000;97:8104?–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlsson A, Carlsson ML. Adaptive properties and heterogeneity of dopamine D(2) receptors -pharmacological implications. Brain Res. Rev. 2008;58:374–378. doi: 10.1016/j.brainresrev.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Seeman P, et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. U S A. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinbjerg M, Sibley DR, Javitch JA, Abi-Dargham A. Imaging the high-affinity state of the dopamine D2 receptor in vivo: fact or fiction? . Biochem.pharmacol. 2012;83:193–198. doi: 10.1016/j.bcp.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and sig-naling but not D1 internalization. J. Neurosci. 2007;27:12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am. J. Psy-chiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 64.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: Relationship to illness phases. Biol. Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 65.Abi-Dargham A, Kegeles L, Zea-Ponce Y, et al. Amphetamine-induced dopamine release in patients with schizotypal personality disorders studied by SPECT and [123I]IBZM. Biol. Psychiatry. 2004;55:1001–1006. doi: 10.1016/j.biopsych.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Brunelin J, d'Amato T, Van Os J, Costes N, Suaud-Chagny MF, Saoud M. Increased left striatal dopamine transmission in unaffected siblings of schizophrenia patients in response to acute metabolic stress. Psychiatry Res. 2010;181:130–135. doi: 10.1016/j.pscychresns.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Soliman A, O'Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology. 2008;33(8):2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- 68.Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. Increased Stress-Induced Dopamine Release in Psychosis. Biol. Psychiatry. 2012;71(6):561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Woodward ND, Cowan RL, Park S, Ansari MS, Baldwin RM, Li R, Doop M, Kessler RM, Zald DH. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am. J. Psychiatry. 2011;168(4):418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lodge DJ, Grace AA. Developmental pathology, dopamine, stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29(3):207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunelin J, d'Amato T, van Os J, Cochet A, Suaud-Chagny MF, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr. Res. 2008;100(1-3):206–211. doi: 10.1016/j.schres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Brunelin J, d'Amato T, van Os J, Dalery J, Suaud-Chagny MF, Saoud M. Serotonergic response to stress: a protective factor against abnormal dopaminergic reactivity in schizophrenia? Eur. Psychiatry. 2007;22(6):362–364. doi: 10.1016/j.eurpsy.2007.01.1219. [DOI] [PubMed] [Google Scholar]
- 73.Kegeles L, Abi-Dargham A, Frankle W, et al. Increased synaptic dopamine in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 74.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizo-phrenia : new evidence. Annu. Rev. pharmacol. toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 75.Brunelin J, Szekely D, Costes N, Mondino M, Bougerol T, Saoud M, Suaud-Chagny MF, Poulet E, Polosan M. Theta burst stimulation in the negative symptoms of schizophrenia and striatal dopamine release.An iTBS-[11C]raclopride PET case study. Schizophr. Res. 2011;131(1-3):264–265. doi: 10.1016/j.schres.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 76.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine re-lease in the caudate nucleus. J. Neurosci. 2001;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hess EJ, Bracha HS, Kleinman JE, Creese I. Dopamine receptor subtype imbalance in schizophrenia. Life Sci. 1987;40:1487–1497. doi: 10.1016/0024-3205(87)90381-x. [DOI] [PubMed] [Google Scholar]
- 78.Seeman P, Bzowej NH, Guan HC, et al. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer's, Parkinson's, and Huntington's diseases. Neuropsychopharmacology. 1987;1(1):5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- 79.Knable MB, Hyde TM, Herman MM, Carter JM, Bigelow L, Kleinman JE. Quantitative autoradiography of dopamine-D1 receptors, D2 receptors, and dopamine uptake sites in postmortem striatal specimens from schizophrenic patients. Biol. Psychiatry. 1994;36 (12):827–835. doi: 10.1016/0006-3223(94)90593-2. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D1 dopamine receptor binding in neuroleptic-naïve patients with schizophrenia. Am. J. Psychiatry. 2002;159(5):761–767. doi: 10.1176/appi.ajp.159.5.761. [DOI] [PubMed] [Google Scholar]
- 81.Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 82.Kosaka J, Takahashi H, Ito H, et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 2010;86(21-22):814–8. doi: 10.1016/j.lfs.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22 (9):3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez D, Slifstein M, Broft A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography.Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 85.Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I.Accuracy and recision of D2 receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Gruzelier JH. Functional Neuropsychophysiological Asymmetry in Schizo-phrenia: A Review and Reorientation. Schizophr. Bull. 1999;25:91–120. doi: 10.1093/oxfordjournals.schbul.a033370. [DOI] [PubMed] [Google Scholar]
- 87.Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT Modulation of Dopamine Release in Basal Ganglia in Psilocybin-Induced Psychosis in Man—A PET Study with [11C]raclopride. Neuropsychopharmacology. 1999;20:424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 88.Hirvonen J, van Erp TG, Huttunen J, Aalto S, Någren K, Huttunen M, Lönnqvist J, Kaprio J, Hietala J, Cannon TD. Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch. Gen. Psychiatry. 2005;62:371–378. doi: 10.1001/archpsyc.62.4.371. [DOI] [PubMed] [Google Scholar]
- 89.Bracha HS, Livingston RL, Clothier J, Linington BB, Karson CN. Correlation of severity of psychiatric patients’ delusions with right hemispatial inattention (left-turning behavior). Am. J. Psychiatry. 1993;150:330–332. doi: 10.1176/ajp.150.2.330. [DOI] [PubMed] [Google Scholar]
- 90.Levine J, Martine T, Feraro R, Kimhi R, Bracha HS. Medicated chronic schizophrenic patients do not demonstrate left turning asymmetry. Neuropsychobiology. 1997;36:22–24. doi: 10.1159/000119355. [DOI] [PubMed] [Google Scholar]
- 91.Brunelin J, Pouet E, Bor J, Rivet A, Eche J, d’amato T, Saoud M. Transcranial magnetic stimulation (rTMS) and negative symptoms of schizo-phrenia. Ann. Med. Psychol. (Paris) 2010;168:422–427. [Google Scholar]