Abstract
Background
Our prior studies of lung cancer suggested that a novel biomarker (pro-surfactant protein B or pro-SFTPB) might serve as a predictive marker for this disease. We aimed to determine the potential utility of pro-SFTPB for distinguishing lung cancer cases from matched controls as a risk marker.
Methods
Study subjects were drawn from the longitudinal Physicians’ Health Study (PHS). Cases (n = 188) included individuals who were cancer-free at study enrollment but developed lung cancer during follow-up. Controls (n = 337) were subjects who did not develop lung cancer. Cases and controls were matched on date of study enrollment, age at enrollment, and smoking status and amount. Baseline plasma samples drawn at enrollment were analyzed for pro-SFTPB using ELISA to detect differences in protein expression levels for cases and controls.
Results
Pro-SFTPB-non-detectable status was significantly associated with lung cancer risk (OR = 5.88, 95% CI 1.24, 27.48). Among subjects with detectable levels of the protein, increasing plasma concentration of pro-SFTPB was associated with higher lung cancer risk (OR = 1.41 per unit increase in log pro-SFTPB, 95% CI 1.08, 1.84).
Conclusion
These results suggest a non-linear, J-shaped association between plasma pro-SFTPB levels and lung cancer risk, with both non-detectable and higher levels of the marker being associated with lung cancer.
Impact
These results show promise of a risk marker that could contribute to predicting risk for lung cancer development and to narrowing the high risk population for low-dose computed tomography (LDCT) screening.
Keywords: Proteomics, cancer risk, biomarker, lung cancer
INTRODUCTION
Lung cancer is the leading cause of cancer death among men, and the second leading cause among women worldwide (1). Despite efforts to improve lung cancer detection and treatment, the prognosis of lung cancer patients remains poor, with overall five-year survival rates in the United States of approximately 15%. However, when diagnosed at an early stage, five-year survival rates for lung cancer approach 50% (2). Recently, the National Lung Screening Trial (NLST) reported that low-dose computed tomography (LDCT) screening reduced lung cancer mortality by 20% in adults who were at high risk of lung cancer (3). Although LDCT screening is a promising approach for early detection, high rates of false positives, cost, and risk from radiation exposure are important limiting factors (4). Determining individual lung cancer risk based on a biomarker profile and known risk factors such as smoking could allow more efficient lung cancer screening. Circulating biomarkers that have been associated with greater risk of developing lung cancer include increased levels of interleukin 8 and surfactant protein D (SP-D) (5, 6). Increased levels of Krebs von Lungren-6 (KL-6) have also been associated with greater risk of interstitial lung disease and subsequent development of lung cancer (5). We previously demonstrated that levels of circulating mature SFTPB were increased among subjects with lung cancer both at the time of diagnosis and in a pre-diagnostic setting, compared to matched controls (7). We further examined SFTPB peptide sequences in mouse models of lung adenocarcinoma and in conditioned media from human lung adenocarcinoma cell lines. Mass spectrometry analysis indicated predominant release of an N-terminal pro-peptide containing form of SFTPB in both cell lines and mouse models, we therefore developed a sandwich ELISA assay that detects N-terminal SFTPB pro-peptide. Analysis of samples collected at the time of diagnosis indicated that pro-SFTPB yielded better discrimination of cases vs controls than mature SFTPB. In this study, we intended to determine whether pro-SFTPB levels were associated with risk of lung cancer in a nested case-control study from the Physicians’ Health Cohort (PHS).
METHODS
Study populations
The PHS cohort comprises two groups: PHS I and II. PHS I began in 1982 as a randomized trial of aspirin and beta-carotene for the primary prevention of heart disease and cancer among 22,071 male, Caucasian physicians initially aged 40–84 years. Men were excluded from the study if they had a history of cardiovascular disease (CVD), cancer (except non-melanoma skin cancer), and contraindications to aspirin use or were users of aspirin, or took platelet-active medications or vitamin A supplements. The aspirin and beta-carotene components of the PHS I trial have previously been reported (8, 9). The PHS II was a randomized trial that began in 1997 to evaluate the impact of beta-carotene, vitamin C, vitamin E, and a daily multivitamin on the prevention of cancer, CVD, age-related eye disease, and decline in cognitive function. The PHS II included 14,641 men, with 7,641 participants from the PHS I plus 7,000 new physicians, bringing the total number of PHS participants to 29,071. In PHS II, neither vitamin C nor vitamin E had an effect on CVD (10) or cancer (11). Follow-up of all PHS participants for major morbidity and mortality continues through annual questionnaires and endpoint follow-up. Written informed consent was obtained from each participant and the study was approved by the Human Research Committee at Brigham and Women’s Hospital. At baseline for PHS I and II, participants were sent blood kits and asked to have their blood drawn, fractionated by centrifugation, and packed on dry ice for return within 24 hours by overnight courier. Pre-randomization blood specimens were obtained from 14,916 (67.6%) of 22,071 PHS I participants, and 11,133 (76.0%) of 14,641 PHS II participants. Upon receipt in the central laboratory, blood components were immediately aliquoted, labeled, frozen, and stored at −82°C for PHS I samples and in liquid nitrogen at −170°C for PHS II samples. Eligible cases for the pro-SFTPB assays were subjects free of baseline cancer who developed lung cancer during follow-up and had plasma samples collected at baseline and available for laboratory analyses. Up to two controls who remained free of cancer were randomly selected and matched to cases based on date of recruitment into the cohort (+/− 24 months), age at recruitment (+/− 36 months), PHS I or II group, smoking status (never, former, current), and among current smokers, categories of cigarettes smoked per day (1–19, 20–39, 40 or more).
Pro-SFTPB assay
Samples were blinded and analyzed using anti-pro-SFTPB mouse monoclonal antibodies (#515 and #464) developed against the N-terminal pro-peptide of human SFTPB. Ninety-six well polystyrene plates (Corning, Canton, NY, USA) were coated with 1µg/ml of anti-pro-SFTPB mouse monoclonal antibody (#515) and blocked with 3% BSA blocking buffer. Plasma samples with 1:100 dilution and various amounts of N-terminal pro-peptide of SFTPB as standards were added to the wells. Anti-pro-SFTPB mouse monoclonal antibody (#464) was biotinylated with EZ-Link® Sulfo-NHS-LC-Biotin (Thermo Scientific) and used for incubation at 0.5µg/ml. After washing, each well was incubated with Streptavidin-HRP followed by incubation of color reagents and adding stop solution (R&D Systems). The absorbance was measured at 450nm with a SpectraMax M5 microplate reader (Molecular Devices).
Statistical Analyses
Descriptive statistics on age, duration of follow-up, pro-SFTPB detection status (detectable or non-detectable), and smoking status as well as the number of cigarettes smoked per day were compared for cases and controls. Cancer histology (adenocarcinoma or non-adenocarcinoma) and metastatic status (metastatic or non-metastatic) was also assessed for lung cancer cases. Blood samples from 53 of the PHS subjects (10.1%) were found to have levels of pro-SFTPB below the detection limit of the ELISA. These samples were assigned a value of 1.56 ng/ml, which corresponds to one-half of the detection limit (12). Pro-SFTPB levels were natural log-transformed to produce a more normal distribution of values. Using data from the controls with detectable pro-SFTPB levels, multivariable generalized estimating equations (GEE) (13) assessed associations between smoking status and age at enrollment and pro-SFTPB levels. GEE analyses account for non-independence of measures between pairs of controls matched to the same case. Conditional logistic regression analyses estimated the odds ratios (ORs, equivalent here to relative risk or RR) of lung cancer incidence in relation to baseline biomarker levels. The regression model included a variable for the natural log-transformed pro-SFTPB concentration levels and a dichotomous variable indicating samples that were above versus below the detection limit. Thus the regression model estimated an OR for the risk of lung cancer associated with having non-detectable pro-SFTPB levels and an OR associated with the per unit increase in log-transformed pro-SFTPB concentration. Analyses were performed among all subjects, then by strata of baseline smoking status (never, former, current), age (at median age of 65 years), and median follow-up (94.6 months) among cases. Polychotomous analyses were also conducted comparing adenocarcinoma cases to their matched controls and non-adenocarcinoma cases to their matched controls. Similarly, polychotomous analyses were completed comparing cases with metastatic disease to their matched controls and cases with non-metastatic disease to their matched controls.
RESULTS
To assess the potential of pro-SFTPB as a risk marker for lung cancer, we examined pro-SFTPB levels in plasma samples drawn from the prospective PHS set. Table 1 presents the characteristics of the PHS cases and controls. The average follow-up period between the baseline collection of blood samples and diagnosis/matching was 117.6 months (range 4.8 months to 304.8 months). A total of 188 cases and 337 matched controls were included from the PHS cohort, as 39 cases had one matched control and 149 cases had two matched controls (Table 1). Among controls, in multivariable GEE analyses, plasma levels of pro-SFTPB were higher among current smokers (+142%, p < 0.001) and former smokers (+21%, p = 0.09) than never smokers and increased with age (+18.1 % per 10 year difference in age, p = 0.02) (see Figure 1).
Table 1.
Descriptive statistics for cases of lung cancer and controls in the Physicians’ Health. Study.
| N (%) | Cases (N=188) Mean (SD) |
Controls (N=337) Mean (SD) N (%) |
|---|---|---|
| Age | 64.5 (10.0) | 64.7 (10.1) |
| Follow-up in months (Median, 25th, 75th percentile) | 94.6 (62.7, 148.9) | 111.2 (74.0, 163.3) |
| Blood pro-SFTPB (ng/ml) | 325.6 (313.9) | 260.8 (289.5) |
| Detectable pro-SFTPB | ||
| Yes | 170 (90) | 304 (90) |
| No | 18 (10) | 35 (10) |
| Smoking status | ||
| Never smoker | 41 (22) | 80(24) |
| Former smoker | 96 (51) | 185 (55) |
| Current smoker | 51 (27) | 72 (21) |
| Cigarettes per day | ||
| 1–19 | 12 (23) | 13 (18) |
| 20–39 | 28 (55) | 42 (58) |
| 40 or more | 11 (22) | 17 (24) |
| Histology | ||
| Adenocarcinoma | 78 (42) | NA |
| Non-adenocarcinoma | 110 (58) | NA |
| Metastatic Disease | ||
| Yes | 84 (45) | NA |
| No | 104 (55) | NA |
Means and percentages for matching variables are not equal between the case and control series because some matched sets have two controls for each case and some have one control per case.
Figure 1.
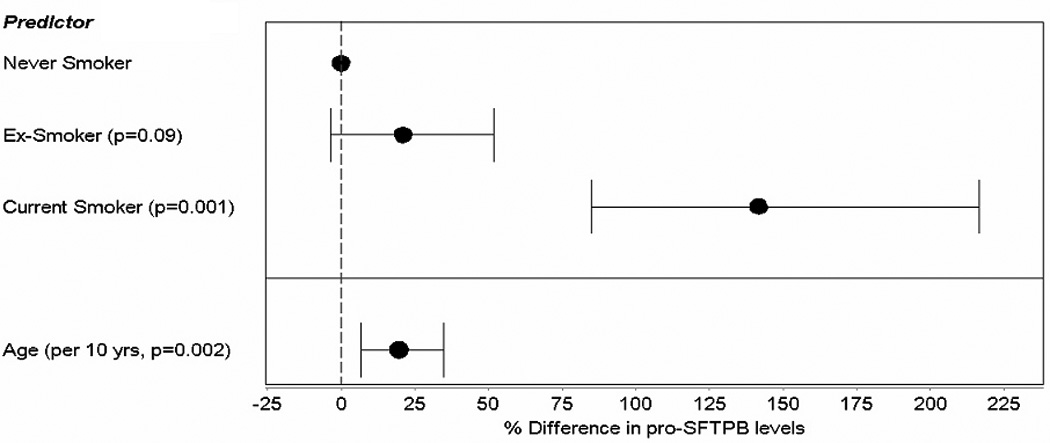
Associations between pro-SFTPB and smoking status and age.
Overall, conditional logistic regression analyses that accounted for matching factors found that pro-SFTPB-non-detectable status was significantly associated with lung cancer risk (OR = 5.88, 95% CI 1.24, 27.84). Increasing concentration of plasma pro-SFTPB was also associated with higher lung cancer risk (OR = 1.41 per unit difference in log pro-SFTPB, 95% CI 1.08, 1.84) (Table 2). These results suggest a non-linear, J-shaped association between plasma pro-SFTPB levels and lung cancer risk, with both non-detectable and higher levels of the marker being associated with lung cancer.
Table 2.
Unstratified analysis of lung cancer risk.
| Lung Cancer Risk OR (95% CI), P-value (N=188 case-control sets) |
|
|---|---|
| Detectable | 1 |
| Non-detectable | 5.88 (1.24, 27.48) p=0.03 |
| Ln concentration | 1.41 (1.08, 1.84) p=0.01 |
OR, odds ratio, OR for non-detectable status and ln of concentration are mutually adjusted and further adjusted for matching variables.
CI, confidence interval
Ln concentration, log-transformed concentration.
Table 3 portrays the results stratified by smoking status, age at enrollment, and duration of follow-up. In terms of smoking status, OR for pro-SFTPB-non-detectable status was higher in never smokers (OR = 9.25, 95% CI 0.56, 154.09), while OR for log-transformed pro-SFTPB concentration was higher in current smokers (OR = 2.08 per unit difference in log pro-SFTPB, 95% CI 0.97, 4.49). ORs for both pro-SFTPB-non-detectable status and log-transformed pro-SFTPB concentration were numerically larger among those with a longer follow-up and who were of younger age at enrollment. However, tests for interaction in these analyses did not reach statistical significance.
Table 3.
Stratified analyses of lung cancer risk.
| Stratification by smoking status | |||
| Never smokers* (N=41 case-control sets) | Former smokers* (N=96 case-control sets) | Current smokers (N=51 case-control sets) | |
| Detectable | 1 | 1 | 1 |
| Non-detectable | 9.25 (0.56, 154.09) p=0.12 | 2.53 (0.33, 19.65) p=0.38 | NA1 |
| Ln concentration | 1.42 (0.85, 2.40) p=0.18 | 1.26 (0.89, 1.76) p=0.19 | 2.08 (0.97, 4.49) p=0.06 |
| Stratification by age at enrollment | |||
| <=65 years† (N=94 case-control sets) | >65 years† (N=94 case-control sets) | ||
| Detectable | 1 | 1 | |
| Non-detectable | 14.79 (1.34, 163.36) p=0.03 | 5.11 (0.58, 44.96) p=0.14 | |
| Ln concentration | 1.77 (1.15, 2.72) p=0.01 | 1.30 (0.91, 1.86) p=0.15 | |
| Stratification by duration of follow-up | |||
| <94.64 months§ (N=94 case-control sets) | >=94.64 months§ (N=94 case-control sets) | ||
| Detectable | 1 | 1 | |
| Non-detectable | 2.81 (0.37, 21.24) p=0.32 | 23.49 (1.80, 307.18) p=0.02 | |
| Ln concentration | 1.13 (0.80, 1.59) p=0.48 | 1.93 (1.23, 3.03) p=0.04 | |
Odds ratios (ORs) and 95% confidence intervals (CI) reported as OR (95% CI), OR for non-detectable status and ln of concentration are mutually adjusted and further adjusted for matching variables.
Ln concentration, log-transformed concentration.
Among smokers, only one subject has non-detectable levels.
P for interaction between never/former smoking status and non-detectable levels = 0.46, P for interaction between never/ever smoking status and non-detectable levels = 0.62
P for interaction between never/former smoking status and ln of blood concentrations = 0.69,
P for interaction between never/ever smoking status and ln of blood concentrations = 0.92.
P for interaction between age group and non-detectable levels = 0.46
P for interaction between age group and ln of blood concentrations = 0.99
P for interaction between follow-up duration and non-detectable levels = 0.57
P for interaction between follow-up duration and ln of blood concentrations = 0.06
Table 4 presents the results of the polychotomous analyses in which cases were sub-grouped by histology and metastatic status, and compared to their respective controls. While the association between pro-SFTPB-non-detectable status and lung cancer was numerically larger for non-adenocarcinomas and for cases with metastatic disease, the confidence intervals were wide and overlapped, providing insufficient evidence for etiological heterogeneity (Table 3) due to the constraint of sample size limitations. Therefore, one cannot conclude that risk associated with pro-SFTPB varies by lung cancer risk factors or tumor characteristics.
Table 4.
Polychotomous analyses of lung cancer risk.
| Segregating cases by histology | ||
| Cases with adeno histology versus their controls (N=78 case-control sets) | Cases with non-adeno histology versus their controls (N=110 case-control sets) | |
| Detectable | 1 | 1 |
| Non-detectable | 3.30 (0.29, 37.41) p=0.34 | 11.78 (1.47, 94.58) p=0.02 |
| Ln concentration | 1.40 (0.89, 2.23) p=0.15 | 1.48 (1.05, 2.07) p=0.02 |
| Segregating cases by the presence of metastases | ||
| Cases with metastatic disease versus their controls (N=84 case-control sets) | Cases with non-metastatic disease versus their controls (N=104 case-control sets) | |
| Detectable | 1 | 1 |
| Non-detectable | 11.03 (0.87, 139.28) p=0.06 | 3.84 (0.54, 27.34) p=0.18 |
| Ln concentration | 1.48 (0.95, 2.30) p=0.09 | 1.36 (0.98, 1.90) p=0.07 |
Odds ratios (ORs) and 95% confidence intervals (CI) reported as OR (95% CI), OR for non-detectable status and ln of concentration are mutually adjusted and further adjusted for matching variables.
Ln concentration, log-transformed concentration.
DISCUSSION
We have validated pro-SFTPB as a promising new biomarker of lung cancer risk. In this study, initial plasma pro-SFTPB levels were associated with smoking status, age, and higher risk of lung cancer in men with up to 23.7 years follow-up. This work was preceded by studies in the samples collected at the time of diagnosis and two independent pre-diagnostic lung cancer cohorts using a newly developed ELISA against the N-terminal pro-peptide of SFTPB (submitted), based on mass spectrometric findings in lung adenocarcinoma mouse models and human lung adenocarcinoma cell lines (7).
Increased pro-SFTPB levels were associated with smoking status and age in control subjects, and the risk of lung cancer, concordant with the previous studies in the general population (14, 15), as well as our previous studies in pre-diagnostic cohorts (submitted). It was surprising that pro-SFTPB-non-detectable status was also significantly associated with lung cancer risk, predominantly in never smokers. The mechanism behind increased risk of lung cancer and decreased circulating pro-SFTPB levels needs to be elucidated. However, decreased concentrations of surfactant protein B in bronchoalveolar lavage fluid are associated with acute respiratory distress syndrome (16), lung injury induced by endotoxin (17), and late asthmatic response (18). Interestingly, lung sftpb gene expression levels were decreased in mice by exposure to nickel (19), one of the occupational carcinogens for lung cancer (20). These findings suggest that decreased plasma pro-SFTPB levels may reflect some pathological conditions that are associated with an increased risk of lung cancer, especially in never smokers. Currently no biomarker or prediction model has enough potential to identify a high risk group for lung cancer among never smokers. Thus it is critical to develop a risk prediction model for never smoker lung cancer in larger cohorts, integrating circulating pro-SFTPB levels and known risk factors of never smoker lung cancer (21).
Interestingly, both pro-SFTPB-non-detectable status and log-transformed pro-SFTPB concentration were more strongly associated with the risk of non-adenocarcinoma than adenocarcinoma, although confidence intervals overlap with each other. Increased pro-SFTPB levels may also reflect pathological conditions of the lung, as well as decreased pro-SFTPB levels as we mentioned above. While a transcription factor thyroid transcription factor 1 (TITF1)/NK2 homeobox 1 (NKX2-1), which regulates surfactant gene expression, decreases in sites of acute epithelial injury, TITF1 is markedly increased in regions of lung parenchyma undergoing regeneration and repair (22). Thus circulating proSFTPB levels might be altered under different pathological lung conditions caused by smoking, genetic, hormonal, and viral factors, which would result in the occurrence of different histological subtypes of lung cancer (21).
Based on the results in the NLST study (3), the American Cancer Society (ACS) recently published lung cancer screening guidelines (23). In the guidelines, the ACS recommends that clinicians should initiate a discussion about lung cancer screening with subjects who meet the NLST criteria (e.g. aged 55 years to 74 years, ≥ 30-pack-year smoking history and < 15 years since quitting). In this study, plasma pro-SFTPB levels are associated with an increased risk of lung cancer in middle-aged and older men. Thus pro-SFTPB might improve selection criteria for lung cancer screening or even might provide an opportunity to propose a personalized screening program, such as intensity of follow up. Further investigations are needed to clarify the relationship of circulating pro-SFTPB levels and lung function and other lung disease, such as chronic obstructive pulmonary disease (COPD). In addition, further studies of pro-SFTPB in pre-symptomatic subjects or in screening subjects combined with LDCT are of particular interest in improving lung cancer survival.
Acknowledgments
GRANT SUPPORT
This work was supported by the Canary Foundation; the Lungevity Foundation; the National Cancer Institute Early Detection Research Network; The Department of Defense W81XWH-10-1-0632; the National Institutes of Health (CA127532, CA097193, CA34944, CA40360, HL26490, HL34595); and the American Recovery and Reinvestment Act Supplement (CA 127532-02S1).
Footnotes
Conflict of interest: None
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiels MS, Chaturvedi AK, Katki HA, Gochuico BR, Caporaso NE, Engels EA. Circulating markers of interstitial lung disease and subsequent risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2262–2272. doi: 10.1158/1055-9965.EPI-11-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer Institute. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taguchi A, Politi K, Pitteri SJ, Lockwood WW, Faca VM, Kelly-Spratt K, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer Cell. 2011;20:289–299. doi: 10.1016/j.ccr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Final report on the aspirin component of the ongoing Physicians' Health Study. Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 9.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 10.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990;5:46–51. [Google Scholar]
- 13.Rundle AG, Vineis P, Ahsan H. Design options for molecular epidemiology research within cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1899–1907. doi: 10.1158/1055-9965.EPI-04-0860. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen AB, Rohatgi A, Garcia CK, Ayers CR, Das SR, Lakoski SG, et al. Interactions between smoking, pulmonary surfactant protein B, and atherosclerosis in the general population: the Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:2136–2143. doi: 10.1161/ATVBAHA.111.228692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robin M, Dong P, Hermans C, Bernard A, Bersten AD, Doyle IR. Serum levels of CC16, SP-A and SP-B reflect tobacco-smoke exposure in asymptomatic subjects. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2002;20:1152–1161. doi: 10.1183/09031936.02.02042001. [DOI] [PubMed] [Google Scholar]
- 16.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. American journal of respiratory and critical care medicine. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 17.Epaud R, Ikegami M, Whitsett JA, Jobe AH, Weaver TE, Akinbi HT. Surfactant protein B inhibits endotoxin-induced lung inflammation. American journal of respiratory cell and molecular biology. 2003;28:373–378. doi: 10.1165/rcmb.2002-0071OC. [DOI] [PubMed] [Google Scholar]
- 18.Haczku A, Atochina EN, Tomer Y, Cao Y, Campbell C, Scanlon ST, et al. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. American journal of physiology Lung cellular and molecular physiology. 2002;283:L755–L765. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- 19.McDowell SA, Gammon K, Bachurski CJ, Wiest JS, Leikauf JE, Prows DR, et al. Differential gene expression in the initiation and progression of nickel-induced acute lung injury. American journal of respiratory cell and molecular biology. 2000;23:466–474. doi: 10.1165/ajrcmb.23.4.4087. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, et al. The global burden of disease due to occupational carcinogens. American journal of industrial medicine. 2005;48:419–431. doi: 10.1002/ajim.20209. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nature reviews Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 22.Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1(TTF-1) in fetal and neonatal human lung. J Histochem Cytochem. 1996;44:673–678. doi: 10.1177/44.7.8675988. [DOI] [PubMed] [Google Scholar]
- 23.Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, et al. American Cancer Society lung cancer screening guidelines. CA: a cancer journal for clinicians. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]


