Abstract
Developing an effective vaccine against lymphatic filariasis will complement the WHO's effort to eradicate the infection from endemic areas. Currently 83 different countries are endemic for this infection and over 1 billion people are at risk. An effective vaccine coupled with mass drug administration will reduce the morbidity and social stigma associated with this gruesome disease. Several potential vaccine candidates that can confer partial protection in experimental animals have been reported from different laboratories. However, no licensed vaccines are currently available for this disease. Among the several vaccine antigens identified from our laboratory, three most promising antigens; rBmHSPαc (α crystalline domain and c-terminal extension of Heat Shock Protein 12.6), rBmALT-2 (Abundant larval transcript) and rBmTSP LEL (Tetraspanin large extracellular loop) was further developed as a recombinant fusion protein vaccine (rBmHATαc). In a mouse model this fusion protein vaccine gave close to 68% protection following a challenge infection. To improve the vaccine efficiency of rBmHATαc, in this study we evaluated various preparations of alum (AL007, AL019, Alhydrogel and Imject® Alum) as adjuvants. Our results show that mice immunized with rBmHATαc formulated in AL007 (alum from IDRI) and/or AL019 (alum plus TLR-4 agonist from IDRI) gave the highest IgG antibody titer compared to other groups. Subsequent in vivo challenge experiments confirmed that >95% protection can be achieved when AL007 or AL019 was used as the adjuvant. However, when Imject® Alum or alhydrogel was used as the adjuvant only 76% and 72% protection respectively could be achieved. These results show that AL007 or AL019 (IDRI) is an excellent choice of adjuvant for the rBmHATαc vaccine against B. malayi L3 in mice.
Keywords: TLR-4 agonist, vaccine, Adjuvant, lymphatic filariasis, Alum, ADCC, Brugia malayi
1. Introduction
Lymphatic filariasis caused by the nematodes Wuchereria bancrofti and Brugia malayi affects more than 120 million people in 72 countries. The World Health Organization (WHO) identified filariasis as a second leading cause of permanent and long term disability [1]. Developing an effective vaccine against this infection will complement the effort towards eliminating lymphatic filariasis from the endemic areas. Our laboratory and others have identified several potential vaccine antigens that can confer significant protection in experimental animal models [2-5]. By screening a phage display cDNA expression library of B. malayi third stage infective larvae (L3) with sera from putatively immune endemic normal individuals (EN) we identified several potential vaccine candidates [6]. After evaluating the vaccine potential of each antigen in animal models, the three most promising vaccine candidates [Abundant Larval transcript (rBmALT-2) [6], Tetraspanin Large extracellular loop (rBmTSP LEL) [7] and small Heat Shock Protein 12.6 (rBmHSP12.6) [2] were down selected to prepare a single multivalent fusion protein vaccine, rBmHAT [BmHsp12.6+BmALT-2+BmTSPLEL] [8]. One of our recent studies showed that BmHSP12.6 can bind to human IL-10 receptor-α (hIL-10R) and may exhibit IL-10 like function [2], which is potentially not advantageous for developing BmHSP12.6 as a vaccine [9,10]. Subsequently, we analyzed the BmHSP12.6 sequence and identified the presence of hIL-10R binding sequences within Val5 to Glu42 in the N-terminus region of BmHSP12.6 [2]. We then deleted the hIL-10R binding sequence from BmHSP12.6 and created a mutant, HSPαc (α crystalline domain and c-terminal extension of HSP12.6). Another recent study demonstrated that deleting hIL-10R binding region did not significantly affect the immunogenicity of rBmHSP12.6 [2]. Therefore, in this study we cloned BmHSPαc along with other two promising vaccine candidates (rBmALT-2 and rBmTSP LEL) to construct the multivalent vaccine protein (rBmHATαc).
Adjuvants are known to boost the potency and duration of specific immune responses to antigen [11]. Among these, alum is the most commonly used adjuvant both in the human and veterinary vaccines [12,13] and induces strong humoral immunity [14]. Toll-like receptors (TLRs), constitutes a receptor family that recognizes a wide variety of conserved microbial molecular patterns and plays an important role in activating the memory B cells and enhancing the antibodies titer [15,16]. Recent studies by Baldwin et al [17] and Fox et al [18] showed that inclusion of a TLR4 agonist along with alum emulsion could promote both humoral and cellular responses. Our initial vaccination trials with rBmHATαc plus Imject® Alum gave only 72% protection [unpublished data]. This is approximately 23% less protection compared to the parent rBmHAT vaccine. Thus, the mutation induced in BmHSP12.6 appears to reduce the vaccine efficacy. Therefore, in this study we have attempted to evaluate and compare various preparations of alum and alum plus TLR4 for their ability to improve the vaccine efficacy of rBmHATαc in a mouse model.
2. Materials and Methods
2.1. Parasites
Brugia malayi infective third stage larvae (L3) were obtained from Filariasis Research Reagent Resource Center (FR3) at the University of Georgia, Athens, GA under NIAID supply contract AI#30022.
2.2. Cloning, Expression and purification of rBmHATαc
Bmhsp12.6αc+Bmalt-2+Bmtsp lel (rBmHATαc) gene construct was amplified from pRBmHAT plasmid (cloned for our previous study, [8]) using the forward primer 5’-CGGATTCCATGGTCATTCACTGCAGACATG-3’ with BamHI restriction sites designed to amplify only α crystalline domain and c-terminal extension of Bmhsp12.6 (Bmhsp12.6αc) and reverse primer to amplify Bmtsp lel 5’-CGGAATTCTCAATCTTTTTGAGATGAAT-3’ with EcoRI restriction sites. PCR conditions were denaturation at 95°C, annealing at 50°C, and extension at 75°C. Amplified products were cloned into pRSETA vector. After confirming the DNA sequence, recombinant BmHATαc was expressed in BL21 (DE3) PLysS E.coli and purified using IMAC column. Purity was confirmed by SDS PAGE and immunoblot analysis with anti-his antibodies. Endotoxin in the prep was removed by passing through a High Capacity Endotoxin removal resin column (ThermoFisher Scientific, Rockford, IL) and the level of endotoxin in the final prep was found to be <4EU/ml.
2.3 Adjuvants
Only alum based adjuvants were used in this study since our previous vaccination trials with rBmHAT showed that alum adjuvant promoted better immunogenicity and protection compared to several other adjuvants. The following adjuvants were used in this study; (1) alum (AL007) obtained from Infectious Diseases Research Institute (IDRI, Seattle, WA), (2) alum plus TLR4 agonist (AL019) obtained from IDRI (3) Imject® Alum (ThermoFisher Scientific) and (4) Alhydrogel (Brenntag Biosector, Denmark, Europe).
2.4. Immunization and blood collection
Six weeks old male Balb/c mice purchased from Charles River laboratory (Wilmington, MA) were divided into six groups with five mice in each group. Use of animals in this study was reviewed and approved by the Animal Care Committee of the University of Illinois Rockford. Mice were immunized four times subcutaneously with 15μg of rBmHATαc plus 100μg of adjuvant formulation given at 2 weeks interval. The groups were 1) rBmHATαc plus AL007, 2) rBmHATαc plus AL019. 3) rBmHATαc plus Imject® Alum , 4) rBmHATαc plus Alhydrogel, 5) rBmHATαc alone with no adjuvants, 6) control animals that received only AL007 and 6) control animals that received only AL019. Blood was collected from the retro-orbital space of each mouse before immunization and after the final dose of immunization. The immunization experiment was repeated three times.
2.5. Evaluation of the correlates of vaccine-induced protection in mice
Levels of anti-BmHATαc, anti-BmHSP12.6αc, anti-BmALT-2 and anti-BmTSP LEL IgG antibodies and IgG isotypes were determined in the sera of immunized mice by an indirect ELISA as described previously [8]. We also performed an in vitro antibody-dependent cell-mediated cytotoxicity assay (ADCC) to determine the protective ability of anti-BmHATαc [8]. Briefly, 2×105 peritoneal exudates cells were incubated with 50μl of sera from immunized mice and 10 B. malayi L3 for 48hrs at 37°C in 5% CO2. Larval viability was determined as described previously [4]. Percentage larval death was calculated using the formula: number of dead parasites/number of recovered parasites×100. We also performed an in vivo micropore chamber challenge study as described previously [8,19]. Twenty B. malayi L3 were placed in the peritoneal cavity of immunized mouse in a micropore chamber and 48 h later chambers were removed and examined microscopically for larval death. Percent larval viability was calculated as mentioned above.
2.6. Evaluation of vaccine-induced immunity
Spleens were collected and single cell suspension was prepared. Presence of antigen-responding cells were determined by a proliferation assay described previously [8]. We also measured the secreted levels of cytokines (IL-4, IL-5, IL-10, IL-12, IFN-γ and TNF-α) in the culture supernatants using a multianalyte ELISA array kit (SA biosciences, Valencia, CA). Each sample was analyzed in triplicate wells and the experiment was repeated three times. Values from cells incubated with media alone were used as the background reading.
2.7. Statistical analysis
GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA) was used to analyze the data. One way ANOVA with Tukey-kramers or Dunnets post test or Student's t test was applied where appropriate. P value of <0.05 was considered statistically significant.
3. Results
3.1. rBmHATαc generated antibodies against each antigen
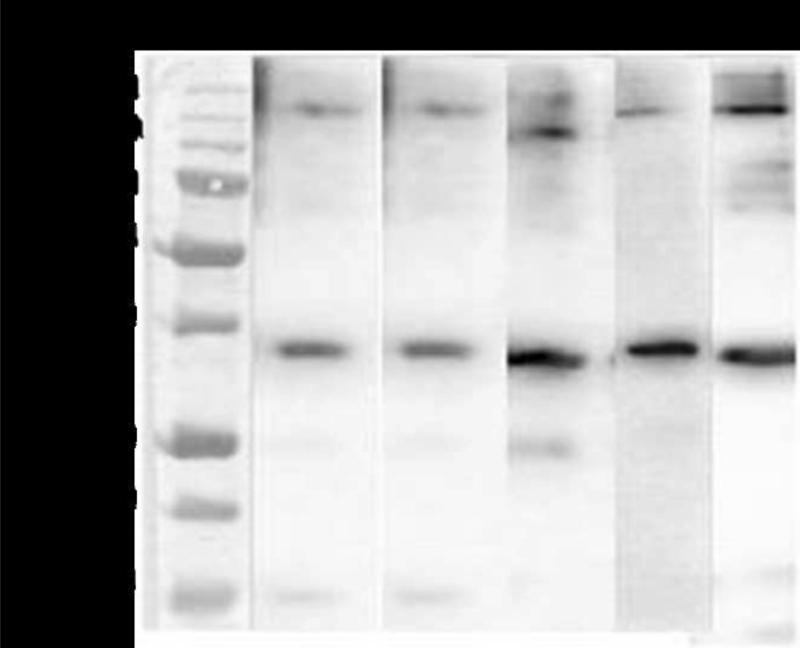
Immunoblot analysis on purified rBmHATαc confirmed that antibodies against rBmHSP12.6, rBmALT-2 and rBmTSP LEL recognized a single band at 35 kDa (fig.1) suggesting that all three component antigens in rBmHATαc are immunogenic.
Figure 1.
Purified rBmHATαc was separated on a 12% SDS-PAGE, transblotted onto nitrocellulose sheet and probed with polyclonal anti-rBmHSP12.6 (lane 1), anti-rBmALT-2 (lane 2), anti-rBmTSP LEL (lane 3), anti rBmHATαc (lane 4) and anti-penta- his antibodies (lane 5). Results show that all the antibodies reacted with a major single band around 35 kDa. Few multimeric species were also evident around 140 kDa.
3.2. Mice immunized with rBmHATαc plus AL019 or AL007 as adjuvant developed the highest titer of antigen-specific IgG antibodies
Significant titers of anti-rBmHSP12.6, anti-rBmALT-2 and anti-rBmTSP LEL IgG were present in the sera of rBmHATαc immunized mice (Table-1). Among the adjuvants, AL007 and AL019 were more efficient in promoting the highest titer of IgG antibodies (P<0.001) compared to Alhydrogel and Imject® Alum (P<0.05) (Table 1). Control animals had no anti-BmHATαc antibodies (Table-1).
Table 1.
| Animal groups immunized with | IgG antibody titer against | |||
|---|---|---|---|---|
| rBmHSP12.6 | rBmALT−2 | rBmTSP LEL | rBmHATαC | |
| rBmHATαc plus AL007 | 24000** | 40000** | 24000** | 24000** |
| rBmHATαc plus AL019 | 24000** | 40000** | 24000** | 24000** |
| rBmHATαc plus Imject alum | 12800* | 24000** | 24000** | 12800* |
| rBmHATαc plus Alhydrogel | 12800* | 24000** | 24000** | 12800* |
| rBmHATαc (No Adjuvants) | 6400* | 24000** | 6400* | 12800* |
| Control | 100 | 100 | 100 | 100 |
P<0.001 **P<0.05 Statistically significant IgG antibody titer compare to all other groups of mice (One way ANOVA along with Tukey-Kramer post statistics test was used).
3.3. Sera from mice immunized with rBmHATαc plus AL019 or rBmHATαc plus AL007 participated in significant killing of B. malayi L3
In vitro ADCC assay showed that sera from mice immunized with rBmHATαc plus Imject® Alum participated in the killing of 75±6.9% of L3. However, when AL007, AL019 or alhydrogel was used as an adjuvant, there was 91±5.13%, 90±7.3% and 79±5.06% killing of L3 respectively (Table-2). When sera from mice immunized with rBmHATαc with no adjuvant were used there was 73±8.8% killing compared to 0% death when sera from alum control groups were used. Thus, inclusion of alhydrogel or Imject® Alum as adjuvants did not significantly improve the killing ability of the rBmHATαc vaccinated sera against B. malayi L3.
Table 2.
| Animal groups immunized with | % Larval death (Mean± S.D) | |
|---|---|---|
| In vitro ADCC | In vivo micropore chamber | |
| rBmHATαc plus AL007 | 91±5.13** | 94.98±6.895** |
| rBmHATαc plus AL019 | 90±7.320** | 88.89±8.115** |
| rBmHATαc plus Imject alum | 75±6.9* | 72± 6.74* |
| rBmHATαc plus Alhydrogel | 79±5.062* | 76.36±5.403* |
| rBmHATαc (No Adjuvants) | 73.33±8.867* | 68.88±5.357* |
| Control | 0±0 | 5.82±4.337 |
(P<0.05)
(P<0.001)Statistically significant protection compare to all other groups of mice analyzed by one way ANOVA followed by Dunnett's post ANOVA test.
3.4. Mice immunized with or rBmHATαc plus AL007 or rBmHATαc plus AL019 showed the highest level of protection against a challenge dose of L3
In vivo challenge experiments also showed that mice immunized with rBmHATαc plus AL007 or rBmHATαc plus AL019 gave the highest percentage of larval death (Table-2), 94±6.8% and 88±8.1% respectively compared to adjuvant controls (5.8±4.3%). When Alhydrogel or Imject® Alum was used as the adjuvant, larval killing was only 76±5.4% and 72± 6.74% respectively. Vaccination with rBmHATαc with no adjuvants resulted in 68±5.3% larval death (Table 2) suggesting that the AL007 or AL019 are better adjuvants for rBmHATαc vaccination in mice.
3.5. Immune correlates of protection after immunization with rBmHATαc
3.5.1. Both IgG1 and IgG2 isotypes are elevated
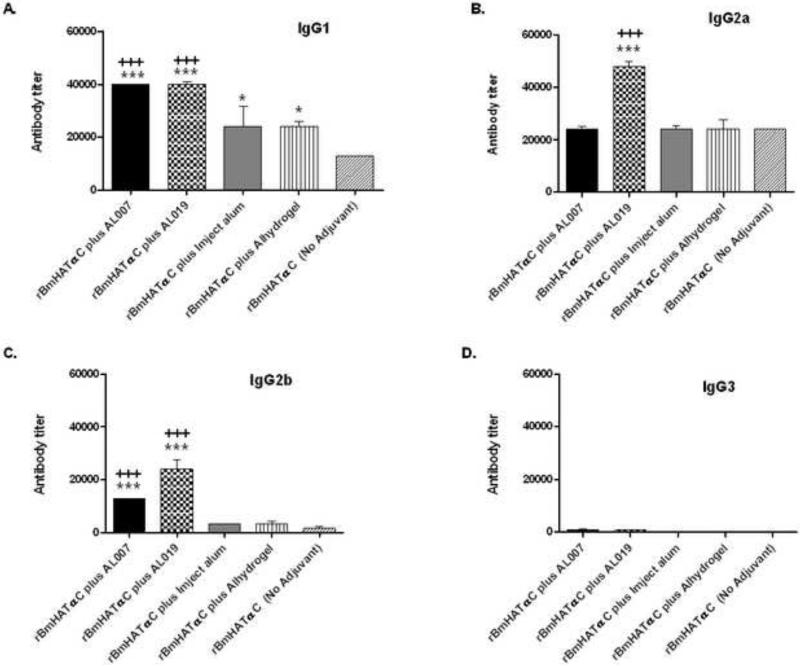
Levels of antigen-specific IgG1 antibodies were significantly elevated in the sera of all the vaccinated animals including rBmHATαc with no adjuvant group compared to controls (Fig. 2). This suggested that the vaccine antigens by itself can stimulate significant levels of IgG1 antibodies without the need for an adjuvant. However, inclusion of AL007 or AL019 as an adjuvant resulted in a 4 fold increase in anti-BmHATαc IgG1 antibodies compared to the no adjuvant group. Vaccination with Imject® Alum or alhydrogel adjuvant increased the anti-BmHATαc specific IgG1 antibodies to 2 fold. AL019 was more efficient in promoting the high anti-BmHATαc IgG2a antibody responses. No significant differences were observed in the levels of IgG2a and IgG3 antibodies in the sera of AL007, Imject® Alum or alhydrogel group compared non-adjuvanated group.
Figure 2. Titer of IgG Isotype of anti-rBmHATαc antibodies in the sera of mice.
Levels of (A) IgG1, (B) IgG2a, (C) IgG2b and (D) IgG3 were measured in the sera of mice using an indirect ELISA. Each bar represents titer of Mean ± SD of sera samples from 5 animals. Significant ***(P<0.001) *(P<0.05) levels compared to “no adjuvant” groups. Significant +++ (P<0.05) levels compared between the different adjuvant groups (One way ANOVA along with Tukey-Kramer post statistics test was used).
3.5.2. Spleen cells from vaccinated animals showed antigen specific recall response
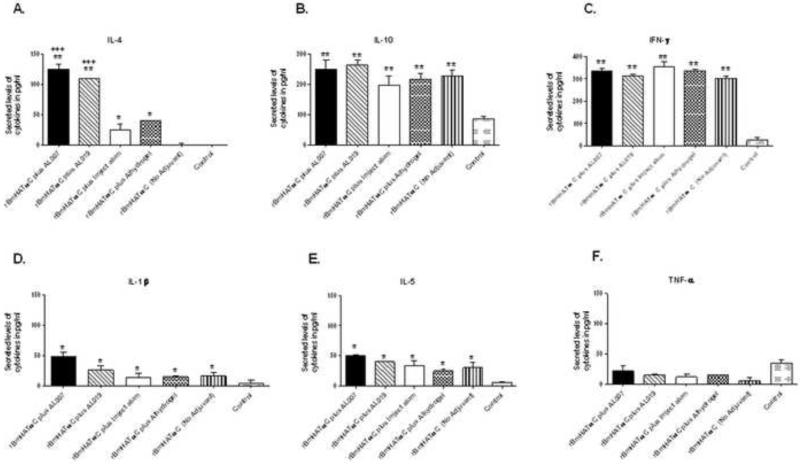
Spleen cells from all rBmHATαc vaccinated mice, irrespective of whether adjuvant was used or not, proliferated significantly (P<0.001) in response to rBmHATαc stimulation compared to control groups. The stimulation index (SI) were 3.412±0.426 (rBmHATαc plus AL007), 3.285±0.625 (rBmHATαc plus AL019), 3.193±1.017 (rBmHATαc plus alhydrogel), 2.53±0.913 (rBmHATαc no adjuvants) compared to the alum control group (1.04±0.0012). SI after Con A stimulation was 4.5-4.8 for all the groups. Culture supernatants of the spleen cells from vaccinated animals stimulated with rBmHATαc secreted significantly high levels of IL-4, IL-5, IL-10, IFN-γ (P<0.001) and IL-1β (P<0.05), (Fig. 3) compared to the adjuvant controls. Overall the cytokine responses were comparable in the vaccinated animals, except that when we used AL007 or AL019 as the adjuvant there was a 6 fold increase in the secreted levels of IL-4 (P<0.001).
Figure 3. Secreted levels of cytokines in the culture supernatants of spleen cells stimulated with rBmHATαc.
Cytokine levels (pg/ml) of (A) IL-4, (B) IL-10, (C) IFN-γ, (D) IL-1β, (E) IL-5 and (F) TNF-α were measured in the culture supernatants using an ELISA. Results show that significant level of IL-4, IL-10, IFN-γ, IL-1β and IL-5 were present in the culture supernatants of all vaccinated. Levels of TNF-α showed no significant differences between the groups. When AL007 or AL019 was used as the adjuvant the secreted levels of IL-4 was significantly higher compared to the other adjuvant groups. Data shown are OD normalized with the unstimulated controls. Each bar represents the mean ±SD levels of cytokines secreted by spleen cells. Significant levels of cytokines **(P<0.001) *(P<0.05) compared to control groups. Significant levels of cytokines +++ (P<0.05) compared to “no adjuvant” and also between the different adjuvant groups (One way ANOVA along with Tukey-Kramer post statistics test was used). Data is from one of two similar experiments showing comparable results.
4. Discussion
A multivalent fusion protein vaccine, rBmHAT developed in our laboratory conferred close to 95% protection against challenge infections in mice [8]. rBmHAT is a fusion of three proteins; rBmHSP12.6, rBmALT-2 and rBmTSP LEL. Recently we showed that the N-terminal region of BmHsp12.6 has an epitope that can bind to hIL-10R and potentially induce IL-10-like effects in vitro [2]. Subsequently, we mapped and identified the hIL-10R binding sequences of BmHSP12.6 and created a deletion mutant, BmHSP12.6αc that retained all the immunogenic potential of BmHSP12.6 [2]. In this study we reconstructed the BmHAT fusion protein by replacing BmHSP12.6 with the HSP deletion mutant (BmHSP12.6αc). Vaccination trials with the newly constructed rBmHATαc multivalent fusion protein showed that substitution with the deletion mutant significantly reduced the level of vaccine-induced protection. Therefore, to improve the vaccine efficacy of rBmHATαc we compared 4 different adjuvant formulations for their ability to improve the BmHSP12.6αc vaccine efficacy. Results presented in this study show that inclusion of AL007 (a GMP manufactured alum) or AL019 (a GMP manufactured alum plus a TLR-4 agonist) as adjuvant substantially improved the vaccine efficacy of rBmHATαc.
Our previous vaccination trials with rBmHAT plus Imject® Alum in the mouse model showed that high anti-rBmHAT IgG antibody titer correlated with higher (95%) protection [8]. When we compared similar responses in our present studies, we found that only moderate levels of anti-rBmHATαc IgG antibodies were developed in mice vaccinated with rBmHSP12.6αc plus Imject® Alum. This finding correlated with low levels of vaccine-induced protection (72%). Thus, under similar conditions rBmHATαc appears to be not as effective as its parent fusion protein (rBmHAT) mainly because of lowered immunogenicity. Imject® Alum contains aluminum hydroxide (40 mg/mL) and magnesium hydroxide (40 mg/mL) and inactive stabilizers [20]. We felt that the adjuvanating effects of Imject® Alum may be insufficient for rBmHATαc, therefore, we tested other alum preparations to see if that would improve the vaccine efficacy. We decided to stay with alum-based adjuvant because our earlier studies showed that alum plus rBmHAT was sufficient to induce the desired protective immune responses against B. malayi [8].
Alhydrogel is a positively charged aluminium hydroxide wet gel suspension that is used in several human vaccines. In comparative studies, alhydrogel was shown to be a better adjuvant than Imject® Alum [21,22]. Although the mechanism is still unknown adsorption of the antigen onto the alum is believed to be a critical step, whereby the antigen is presented in particulate form to the phagocytic cells. In our studies rBmHATαc adsorbed well to all of the alum formulations as no protein was left in the supernatant after incubation.
Alum in general induces a pronounced Th2 biased response and can directly modulate B cells to secrete predominantly IgG1 antibodies and to certain extend IgG2b antibodies [23]. In our studies, inclusion of Imject® Alum or alhydrogel as adjuvants resulted in comparable titers of anti-BmHATαc IgG1, IgG2a and IgG2b antibodies despite the differences in the composition of the two alum adjuvants [20] However, when AL007 and AL019 were used as the adjuvant we observed a stronger IgG antibody response with specific increases in IgG1 and IgG2b isotypes. As expected, inclusion of AL019 resulted in an increase in IgG2a antibody levels. Thus, there was a clear difference in the type and strength of antibody responses elicited following use of AL007 and AL019 as adjuvants.
The extent of the antibody responses elicited by alum adjuvants is shown to be proportional to the local inflammatory reaction [24]. Unfortunately, we did not measure the degree of inflammatory responses at the site of injection. Therefore, we do not know if differences in the degree of local inflammation may account for the better responses associated with AL007 and AL019 adjuvants. Another possible reason may be a difference in the level of host chromatin deposited around the alum-adjuvant complex [25]. Host DNA associated with the alum were shown to deliver the antigen to the cytosol of APCs leading to activation of antigen-specific IL-4 secreting CD4+ T cells. In general alum promotes an IL-1β, IL-4 and IL-5 responses [24]. In our studies also antigen-specific secretion of IL-4, IL-1β and IL-5 were observed in all vaccinated mice compared to controls. The IL-4 levels were significantly high in AL007 and AL019 group. The increase in IL-4 corresponded with the high IgG1 antibody titer and the protection in vitro (ADCC) and in vivo (micropore challenge). Although disputed [26], kinetics of release of antigens from the alum complex at the site of injection can determine the robustness of the immune responses. At this time we do not know why AL007 and AL019 gave stronger immune responses and better protection than other alum preparations.
Toll-like receptors (TLRs) are a family of type 1 trans-membrane glycoproteins that can function as pattern-recognition receptors and are expressed on the surface of a variety of immune cells [16]. Studies by Fox et al [18] show that monophosphoryl lipid A (MPL®) is an excellent TLR-4 agonist that can mimic the function of pattern associated molecular patterns and can be used as a safe adjuvant to stimulate Th1 responses. Previous studies showed that in human both Th1 (IFN-γ) and Th2 (IL-4) biased responses are important for protection against B. malayi. Since alum largely bias the response to a Th2 pattern, we wanted to test if including a TLR-4 agonist along with alum (AL019) can elicit a better protective responses in our vaccination regimen. MPL® formulated with alum is known to trigger high levels of antibody responses and cytotoxic T lymphocyte (CTL) responses [27,28]. In fact, several formulations of MPL® plus alum were successfully developed and commercially used in human vaccines [reviewed in 18]. In our studies inclusion of AL019 as the adjuvant induced a higher IgG2a response. However, the IFN-γ response was not significantly different from the no-adjuvant group. Surprisingly, spleen cells from all our vaccinated animals secreted high levels of IL-10 in response to the vaccine antigen. We believe that the increased levels of IL-10 may have influenced the secretion of IFN-γ in the cultures. rHSP12.6 trigger IFN-γ secretion from sensitized T cells [4,8]. This might explain the high levels of IFN-γ present in the culture supernatants of cells collected from no adjuvant group.
Vaccination trials using several potential vaccine candidates showed that a balanced Th1/Th2 immune response is associated with protection in mice [4,8,29-31]. A similar cytokine pattern was evident when AL007 or AL019 was used as an adjuvant along with rBmHATαc immunization. Presence of IL-10 secreting cells in vaccinated animals suggests that these cells may be important for preventing the development of highly polarized Th1 responses [32]. This may also be responsible for the reduced IFN-γ response observed following use of AL019 as an adjuvant. The cytokine responses correlated well with the IgG1 and IgG2a antibody response suggesting a balanced Th1 and Th2 responses.
Previous studies demonstrated that antibodies are critical in the killing B. malayi L3 through an ADCC mechanism [2]. In this study also we confirm that the antibodies, generated following vaccination with rBmHATαc, can participate in the killing of B. malayi L3 in an ADCC fashion. Inclusion of AL007 or AL019 as adjuvants for rBmHATαc vaccination substantially increased the ability of serum antibodies to kill B. malayi L3 resulting in 91% and 90% respectively compared to 73% protection with non-adjuvanated rBmHATαc. However, inclusion of Imject® Alum or alhydrogel as adjuvant for rBmHATαc resulted in only 72% and 76% protection respectively. This suggested that both AL007 and AL019 are equally effective as adjuvants for rBmHATαc in mice. The study also showed that there is a direct correlation between the titer of antibodies and protection. This was further confirmed in our in vivo B. malayi L3 challenge experiment. Similar to our ADCC finding, AL007 appeared to be a better adjuvant than Imject® Alum or alhydrogel. Since similar results were obtained with AL007 and AL019 adjuvants, we believe that the AL007, which is also present in AL019, may be a critical adjuvant for rBmHATαc amplifying the protective immune responses. At this time we do not know the exact reasons for the difference in the protection conferred by different alum preparations. It is well established that the immunogenicity of antigens largely depends on the degree of antigen adsorption to adjuvant and the dose of adjuvant [33]. Adjuvant molecules must be appropriately formulated for both maximum effect and stability. Thus manufacturing practices and physicochemical properties of the final alum preparation can significantly influence its adjuvant effect [34,35]. We believe that the AL007 alum prepared by IDRI offered the optimum physicochemical properties that favored the immunogenicity of rBmHATαc. Our findings thus confirm that AL007 or AL019 can be used as an excellent adjuvant for rBmHATαc vaccination in mice.
In conclusion, deletion of N-terminal sequences from rBmHSP12.6 diminished the vaccine efficiency of the multivalent vaccine construct, rBmHATαc. Inclusion of a GMP manufactured alum (AL007) or alum plus a TLR4 agonist (AL019) as adjuvant was shown to increase the antibody titer and its vaccine efficiency to the levels (95%) close to the parent vaccine. These studies thus confirm that the protective immune responses against B. malayi is predominantly driven by a strong Th1 and Th2 type antibody responses and an effective vaccine against lymphatic filariasis should include adjuvants that can drive a balanced Th1/Th2 responses against the antigen.
Highlights.
rBmHAT is an excellent multivalent vaccine candidate for lymphatic filariasis.
However, rBmHSP12.6, a component of rBmHAT was shown to bind to human IL-10R.
Deletion mutant of BmHSP12.6 was created to remove the IL-10R binding region.
Multivalent vaccine formulated with the BmHSPαc deletion mutant was 40% less effective.
Including alum or alum plus TLR-4 agonist adjuvant regained the vaccine efficacy of rBmHATαc to 95%.
Acknowledgment
B. malayi third stage infective larvae (L3) were obtained from NIH/NIAID Filariasis research reagent resource center, College of Veterinary Medicine, University of Georgia, Athens, GA under NIAID supply contract AI#30022. This study was supported by the NIH grant AI064745. AL007 and AL019 were provided by Dr. Steven. G. Reed, Founder, president & Chief Scientific officer of Infectious Diseases Research Institute, Seattle, WA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Report. Bridging the gap. Report of the Director General. World Health Organization; Geneva-3: 1995. [Google Scholar]
- 2.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Shinde GB, Nutman T, Reddy MV, et al. Biochemical Characterization and Evaluation of a Brugia malayi Small Heat Shock Protein as a Vaccine against Lymphatic Filariasis. PLoS One. 2012;7(3):e34077. doi: 10.1371/journal.pone.0034077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhumathi J, Prince PR, Anugraha G, Kiran P, Rao DN, Reddy MV, et al. Identification and characterization of nematode specific protective epitopes of Brugia malayi TRX towards development of synthetic vaccine construct for lymphatic filariasis. Vaccine. 2010;28(31):5038–48. doi: 10.1016/j.vaccine.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Kalyanasundaram R, Balumuri P. Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Res Rep Trop Med. 2011;2011(2):45–56. doi: 10.2147/RRTM.S13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Gnanasekar M, Nandakumar K, et al. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol. 2007;116(4):483–91. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnanasekar M, Rao KV, He YX, Mishra PK, Nutman TB, Kaliraj P, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004;72(8):4707–15. doi: 10.1128/IAI.72.8.4707-4715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dakshinamoorthy G, Munirathinam G, Stoicescu K, Reddy MV, Kalyanasundaram R. Large Extracellular Loop of Tetraspanin as a potential vaccine candidate for filariasis. PLoS One. 2013 Oct 11; doi: 10.1371/journal.pone.0077394. DOI: 10.1371/journal.pone.0077394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett BL, Tyring SK. Safety and efficacy of vaccines. Dermatol Ther. 2009;22(2):97–103. doi: 10.1111/j.1529-8019.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 10.Curlin G, Landry S, Bernstein J, Gorman RL, Mulach B, Hackett CJ, et al. Integrating safety and efficacy evaluation throughout vaccine research and development. Pediatrics. 2011;127(Suppl 1):S9–15. doi: 10.1542/peds.2010-1722C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wack A, Rappuoli R. Vaccinology at the beginning of the 21st century. Curr Opin Immunol. 2005;17(4):411–8. doi: 10.1016/j.coi.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–72. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 13.Sayers S, Ulysse G, Xiang Z, He Y. Vaxjo: a web-based vaccine adjuvant database and its application for analysis of vaccine adjuvants and their uses in vaccine development. J Biomed Biotechnol. 2012:831486. doi: 10.1155/2012/831486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2(10):947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 16.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27(43):5956–63. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 18.Fox CB, Friede M, Reed SG, Ireton GC. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. Subcell Biochem. 2010;53:303–21. doi: 10.1007/978-90-481-9078-2_14. [DOI] [PubMed] [Google Scholar]
- 19.Abraham D, Grieve RB, Holy JM, Christensen BM. Immunity to larval Brugia malayi in BALB/c mice: protective immunity and inhibition of larval development. Am J Trop Med Hyg. 1989;40:598–604. doi: 10.4269/ajtmh.1989.40.598. [DOI] [PubMed] [Google Scholar]
- 20.Hem SL, Johnston CT, HogenEsch H. Imject® Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25(27):4985–6. doi: 10.1016/j.vaccine.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 21.Harris JR, Soliakov A, Lewis RJ, Depoix F, Watkinson A, Lakey JH. Alhydrogel(R) adjuvant, ultrasonic dispersion and protein binding: a TEM and analytical study. Micron. 2012;43(2-3):192–200. doi: 10.1016/j.micron.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Cain DW, Sanders SE, Cunningham MM, Kelsoe G. Disparate adjuvant properties among three formulations of “alum”. Vaccine. 2013 Jan 11;31(4):653–60. doi: 10.1016/j.vaccine.2012.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin BR, Kim SJ, Lee JM, Kang SH, Han HJ, Jang YS, et al. Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype. Immune Netw. 2013 Feb;13(1):10–5. doi: 10.4110/in.2013.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed SG. Vaccine adjuvants. Expert Review of Vaccines. 2013 Jul;12(7):705–706. doi: 10.1586/14760584.2013.811201. [DOI] [PubMed] [Google Scholar]
- 25.McKee AS, Burchill MA, Munks MW, Jin L, Kappler JW, Friedman RS, Jacobelli J, Marrack P. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):E1122–31. doi: 10.1073/pnas.1300392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012 Mar;26(3):1272–9. doi: 10.1096/fj.11-184556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 28.Richards RL, Rao M, Wassef NM, Glenn GM, Rothwell SW, Alving CR. Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS,S malaria antigen. Infect Immun. 1998;66(6):2859–65. doi: 10.2307/1366431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babu S, Ganley LM, Klei TR, Shultz LD, Rajan TV. Role of Gamma Interferon and Interleukin-4 in host defense against the human filarial parasite Brugia malayi. Infect Immun. 2000;68:3034–5. doi: 10.1128/iai.68.5.3034-3035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharmila S, Christiana I, Kiran P, Reddy MV, Kaliraj P. The adjuvant-free immunoprotection of recombinant filarial protein Abundant Larval Transcript-2 (ALT-2) in Mastomys coucha and the immunoprophylactic importance of its putative signal sequence. Exp Parasitol. 2011;129(3):247–53. doi: 10.1016/j.exppara.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Dash Y, Ramesh M, Kalyanasundaram R, Munirathinam G, Shultz LD, Rajan TV. Granuloma formation around filarial larvae triggered by host responses to an excretory/secretory antigen. Infect Immun. 2011;79(2):838–45. doi: 10.1128/IAI.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu XS, Leerberg J, MacDonald K, Leggatt GR, Frazer IH. IFN-gamma promotes generation of IL-10 secreting CD4+ T cells that suppress generation of CD8 responses in an antigen-experienced host. J Immunol. 2009;183(1):51–8. doi: 10.4049/jimmunol.0802047. [DOI] [PubMed] [Google Scholar]
- 33.Hansen B, Sokolovska A, HogenEsch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine. 2007;25(36):6618–24. doi: 10.1016/j.vaccine.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 34.Alving CR, Detrick B, Richards RL, Lewis MG, Shafferman A, Eddy GA. Novel adjuvant strategies for experimental malaria and AIDS vaccines. Ann N Y Acad Sci. 1993;690:265–75. doi: 10.1111/j.1749-6632.1993.tb44015.x. [DOI] [PubMed] [Google Scholar]
- 35.Edelman R. Vaccine adjuvants. Rev Infect Dis. 1980;2(3):370–83. doi: 10.1093/clinids/2.3.370. [DOI] [PubMed] [Google Scholar]