Abstract
Fatigue is a debilitating and pervasive complication of cancer and cancer care. Clinical research investigating potential therapies is hindered by variability in patient histories, different metrics for measuring fatigue, and environmental factors that may affect fatigue. The purpose of this study was to establish an animal model of chemotherapy-related fatigue. Female HSD:ICR mice were treated with doxorubicin (2.5 mg/kg) or saline in 2 cycles (days 1 through 3 and 10 through 12). After treatment, mice were individually housed in cages equipped with running wheels. Open-field activity and motor coordination were examined after each cycle of treatment and after each week of wheel running. In a separate cohort, modafinil (50 mg/kg) was assessed as a potential treatment for fatigue. Doxorubicin administration resulted in greater than 30% less wheel running compared with that of saline controls. Activity differences were specific to wheel running: neither distance traveled in the open field nor motor coordination according to the rotarod test differed between groups. Compared with control values, RBC counts in the doxorubicin group were decreased on days 15 and 22 but recovered to control levels by study completion. Modafinil was efficacious in increasing wheel running in the doxorubicin group. The current results establish an animal model of chemotherapy-related fatigue that recapitulates the physical symptoms of cancer-related fatigue as manifested as decreased voluntary activity. This model is sensitive to pharmaceutical intervention and can be used to screen potential treatments for fatigue.
Abbreviations: CRF, cancer-related fatigue
Lasting and debilitating fatigue is among the most common side effects of cancer and its treatment.12,20 It has a profoundly negative effect on patients’ quality of life and is among the most distressing symptoms reported by those undergoing care.20,31,50 Frustrating for patients and providers is the lack of approved treatment. One hindrance to better understanding the pathogenesis of cancer-related fatigue (CRF), as well as to the development of an effective intervention, has been the lack of an effective translatable animal model of the condition.
Few models of CRF have been reported. One study62 found that female C57Bl/6J mice displayed significantly reduced wheel running activity after repeated etoposide administration. Because activity was examined only during the course of treatment and one day afterward, the observed changes may have been at least partly attributable to anemia. In addition, the duration of fatigue was not established, given the short examination period after treatment. In another study,49 reductions in wheel running activity lasted for as long 3 wk after 5 consecutive administrations of paclitaxel or nab-paclitaxel in female BALB/cJ mice; these changes were independent of anemia. The ability of the model to assess potential interventions was not reported. Some investigators43 reported reduced home cage activity in rats after cisplatin treatment. In a follow-up study, dexamethasone, a synthetic corticosteroid, was found to protect against cisplatin-induced reductions in activity.42 Whether anemia contributed to the cisplatin-induced reduction in activity is unclear. In addition, fatigue was short-lived in this model,41 lasting only 7 d, whereas CRF in patients is often long-lasting and can affect them months to years after treatment.8
The objective of the current study was to establish a murine model of CRF that replicates the condition in humans by using endpoints that are translatable to clinical trials and that discriminate the effectiveness of a pharmacologic intervention. Such an objective presents 2 major challenges in animal model design. First, whereas fatigue in humans typically is measured by self-report and quantitative scales,35,45 in rodents the subjective state of the animal must be inferred from their behavior. As a fatigue surrogate, physical activity appears to be a good indicator.29 Voluntary physical activity is particularly appropriate because fatigue most commonly is diagnosed by self-report, and voluntary activity, unlike forced treadmill running, is independent of stressful stimuli that may interact with fatigue. Second, translational validation of a new animal model is dependent on demonstrating that agents that produce an efficacy signal in humans produce a similar response in the model. The absence of an approved treatment for CRF precludes this requirement to some degree. However, several compounds, including methylphenidate and modafinil, have been tested in clinical trials. Although the results for methylphenidate have been mixed,10,11,28,41,44,46,53 the results for modafinil have been more promising.6,57 In a large double-blind phase 3 clinical trial, modafinil was more effective than placebo in treating patients with severe fatigue.37 Therefore, we selected modafinil as a positive control in the current study to assess the predictive validity of the model.
Several methods for assessing voluntary activity in rodents have been established, including wheel-running, home-cage activity, burrowing, and open-field activity.22,51,64 In the current study, we used wheel-running as the primary endpoint for evaluating fatigue. This decision was made in part because wheel-running captures the entire circadian rhythm of activity, and circadian rhythm disruptions are a possible symptom of CRF.33 Home-cage activity as measured by video recording or telemetry device also captures circadian rhythm.49,64 However, we chose wheel-running activity over home-cage activity assessments because previous studies have suggested that wheel-running may be more sensitive than is home-cage activity in detecting fatigue.49 In addition, baseline wheel-running activity generally is greater than is home-cage activity,17,64 allowing for a larger window of detection to assess activity reductions.
Materials and Methods
Animals.
All procedures were approved by the IACUC and adhered to NIH guidelines. Female Hsd-ICR (CD1) mice (n = 60; age, 7 wk; Harlan Sprague–Dawley, Indianapolis, IN) were used. Mice initially were housed in groups of 3 in ventilated cages containing corncob bedding (Bed-O-Cob, The Andersons, Maumee, OH) and sunflower seeds as enrichment. Sterile commercial rodent chow (LabDiet 5053, Purina, St Louis, MO) and water were provided ad libitum throughout the course of the study. The vivarium was maintained at a temperature of 21 ± 2 °C with a reverse light:dark cycle (lights on, 1800; lights off, 0600). The vivarium was lit with dim red lights to allow researchers to manipulate the mice during their active period (the dark period) to minimally disturb the circadian rhythm of the mice.
Chemotherapy treatment.
Doxorubicin (Henry Schein, Melville, NY) was diluted in sterile saline (0.9%) to a concentration of 1 mg/4 mL. Intraperitoneal injections of doxorubicin (2.5 mg/kg) or saline were administered on days 1 through 3 and 10 through 12, resulting in a cumulative dose of 15 mg/kg in the doxorubicin-treated group.
Wheel-running.
On day 15 (day 1 was the first day of doxorubicin dosing), mice were housed individually in standard shoebox-type cages that were equipped with running wheels. Each wheel was 11.5-cm in diameter and was mounted in the cage top. Wheel rotations were monitored continuously in 15-min increments via magnetic reed switches that interfaced with a personal computer running VitalView software (Respironics, Bend, OR). Wheel rotations were converted into distance by calculating the number of rotations multiplied by the circumference of the wheel, to facilitate comparisons across studies with differing wheel diameters.
Rotarod.
On days on which mice were scheduled to be euthanized for blood testing, additional behavioral tests were conducted. To determine whether changes in wheel-running were attributable to motor dysfunction, a subset of mice from each group was tested for motor coordination by using a rotarod. Rotarod testing was performed under the same lighting conditions as in the vivarium (dim red light). During the morning (0900 to 1100) of days 5, 15, and 22 and at study completion at day 30, mice (n = 6 per group for all days except days 22 and 30, when n = 5 for the doxorubicin group) were placed on a stationary dowel that then accelerated at a rate of 1 rpm/3 s until the mouse fell off the dowel or until 3 min had elapsed. The time until the mouse fell was recorded automatically by using photobeams at the base of the rotarod. If the mouse did not fall within 3 min, it was removed from the dowel, and the time was recorded as 180 s. This procedure was repeated for 4 consecutive trials with a 30-s intertrial interval.
Open field.
After rotarod testing, the same mice (n = 6 per group for all days except days 22 and 30, when n = 5 for the doxorubicin group) were tested in an open-field apparatus in the afternoon (1300 to 1500). Open-field chambers (61 × 61 × 30 cm) were lit with diffuse white light. Mice were placed in the middle of the open field and removed 5 min later. While the mouse was in the open field, the distance it traveled was recorded automatically by using TopScan (Clever Sys, Reston, VA) video-tracking software. The open-field chamber was cleaned with commercial detergent after the testing of each mouse.
CBC count.
After the open-field test on days 15, 22, and 30, mice were euthanized via CO2 inhalation. Cardiocentesis was performed, and whole blood was placed in a heparinized tube. WBC and RBC counts were obtained by using an automated hematology system (PCE-Vet90, High Technology, Walpole, MA).
Modafinil.
An additional 48 female CD1 mice (age, 7 wk; n = 12 per group) obtained from the same vendor were used to test the predictive validity of the fatigue model. The doxorubicin and wheel-running procedures were performed as described earlier. The saline and doxorubicin-treated groups were further allocated into vehicle (0.25% methylcellulose) and modafinil (50 mg/kg)-treated groups. Both compounds were administered via oral gavage at 1200 once daily on days 15 through 30. The rotarod and open-field tests were not conducted on day 30.
Statistics.
Statistical analysis was performed by using Prism 5 (GraphPad, La Jolla, CA) and SigmaPlot 11.0 (Systat Software, San Jose, CA). Open field, blood count, and area under the curve data were all analyzed by using the Student t test. Wheel-running activity was binned by week and analyzed by using 2-way repeated-measures ANOVA with week and treatment as factors. Rotarod data were analyzed by 2-way repeated-measures ANOVA for each test day and with treatment and trial as factors. Modafinil data were analyzed by 2-way ANOVA with each treatment (doxorubicin and modafinil) as factors. All post hoc comparisons were made by using the Holm–Sidak method.
Results
Health and weight changes.
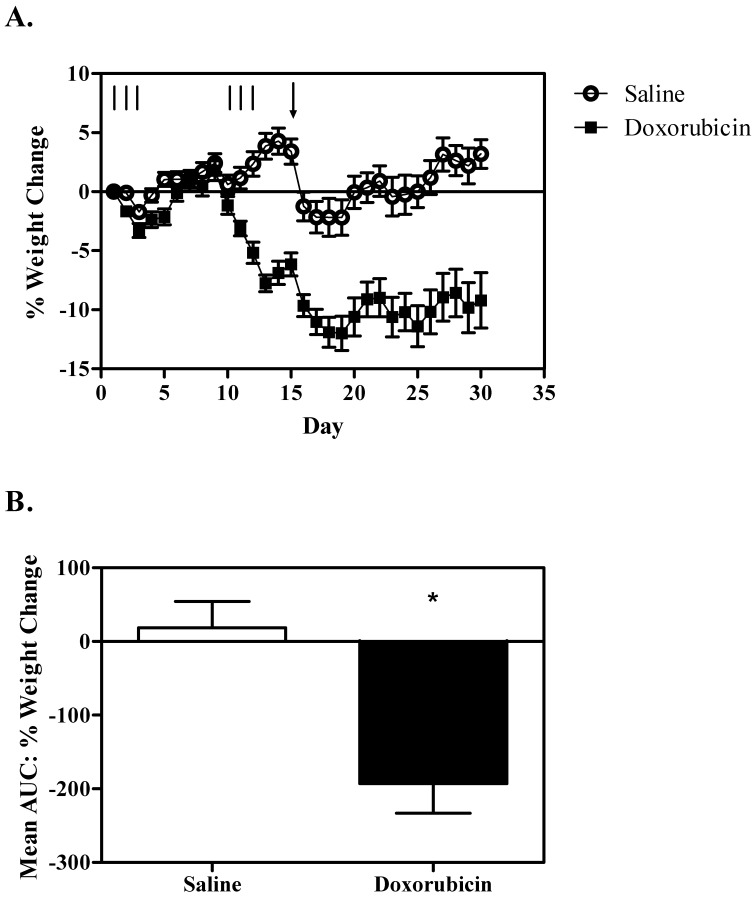
During the course of the study, 3 mice in the doxorubicin treatment group were found moribund and were euthanized on study days 20, 21, and 25, respectively. As expected, weight loss was observed in mice treated with doxorubicin compared with control mice (Figure 1) , particularly after the second cycle (AUC t22 = 4.1, P < 0.001).
Figure 1.
Weight change. (A) Mean percentage weight change by day. The number of mice per group is variable across the graph due to cohorts being sampled for behavioral and blood analyses. The lines represent each day of treatment (2.5 mg/kg doxorubicin or saline). The arrow indicates the day when mice were housed in cages equipped with running wheels. Note the approximately 5% weight loss in both groups after wheel access. (B) Mean area under the curve for the weight change in mice sampled on day 30. Overall, mice treated with doxorubicin (n = 10) lost more weight than did saline-treated animals (n = 12). *, Significant (P < 0.05) difference between values; error bars, SEM.
Blood profile.
CBC counts were assessed from blood samples taken from mice euthanized on days 15, 22, and 30. Lower RBC counts were observed in doxorubicin treated mice compared with control mice on days 15 (t10 = 2.4, P < 0.05) and 22 (t9 = 3.4, P < 0.01), but this difference had resolved by the end of the study on day 30 (Figure 2 B). No differences in WBC count were observed between groups on any of the days examined (Figure 2 C).
Figure 2.
(A) Mean open-field activity. No differences in distance traveled in the open field between saline- and doxorubicin treated groups were noted. (B) Mean RBC count. Compared with controls, doxorubicin-treated mice showed significant (*, P < 0.05) decreases in RBC count on days 15 and 30. (C) Mean WBC count. No significant differences in WBC count were noted on any of the sampling days. Error bars represent SEM (n = 6 per group except for days 22 and 30, when n = 5 for the doxorubicin group).
Wheel-running activity.
Compared with baseline levels, wheel-running activity increased during the first week of wheel access in both the doxorubicin and control groups (Figure 3 A; main effect of week, F1,43 = 86.9, P < 0.001; post hoc comparison, P < 0.001). Activity was greater in the control group than in the doxorubicin treated group (main effect of treatment, F1,43 = 4.6, P < 0.05; post hoc comparison, P < 0.05). This effect was greatest during the second week, as indicated by a significant interaction between treatment and week (F1,43 = 4.4, P < 0.05). Holm–Sidak post hoc comparison within weeks revealed greater activity in the control group as compared with the doxorubicin-treated group during week 2 (P < 0.05) but not week 1.
Figure 3.
Wheel-running activity. (A) Mean distance run daily for the duration of the experiment. The number of mice per group varies across days due to sampling of cohorts for behavioral and blood analyses. In both groups, activity was greater from days 23 through 29 than during with the acclimation week (days 16 through 22). Compared with control mice, cumulative activity during the second week of wheel access was significantly (P < 0.05) lower in the doxorubicin-treated group. (B) Plots of the circadian rhythm of activity during 15-min intervals for day 23 (saline group, n = 12; doxorubicin group, n = 11). The black bar at the top of the graph represents the dark period of the photocycle. Note that the separation in activity between groups was greatest in the afternoon period (between 1200 to 1800). Error bars represent SEM.
In addition to the 24-h wheel-running activity analysis, activity was examined in 15-min epochs for the purpose of assessing the circadian rhythm of activity. Qualitative analysis of the data reveals the active phase of doxorubicin-treated mice was unchanged relative to that of controls; in both groups, activity was largely limited to the 12-h dark period. Within this time, the reductions in wheel-running activity were most pronounced in the second half of the dark period (1200 to 1800; Figure 3 B).
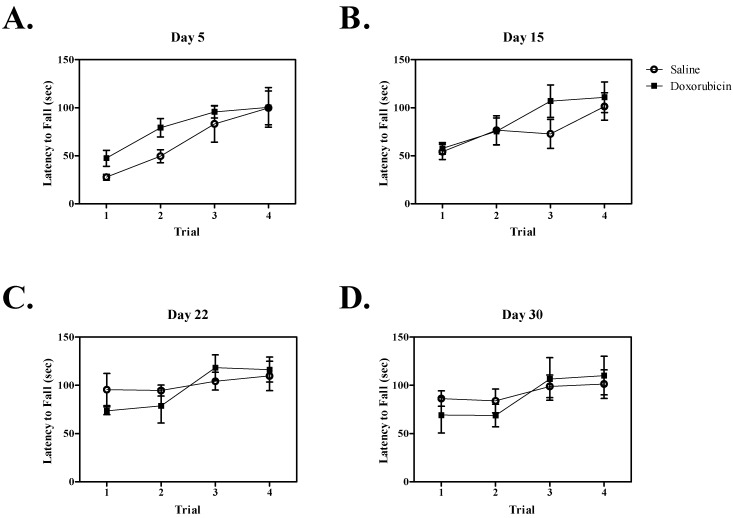
Rotarod.
Both control and doxorubicin-treated mice learned the rotarod test, as indicated by a significant increase in latency to fall across sequential trials on each test day (day 5, F3,46 = 8.9, P < 0.001; day 15, F3,46 = 8.5, P < 0.001; day 22, F3,43 = 4.8, P < 0.01) except day 30, for which there was a trend (F3,42 = 2.7, P = 0.067). No significant effect of doxorubicin treatment on rotarod performance was observed on any of the test days (Figure 4). The interaction of treatment and trial also was not significant.
Figure 4.
Mean latency to fall during the rotarod trials on each test day. Each day represents a separate cohort of mice (n = 6/group for all days except days 22 and 30, when n = 5 for the doxorubicin group). Latency to fall was significantly (P < 0.05) increased across trials in both the doxorubicin- and saline-treated groups on all days except day 30, suggesting that motor learning remained intact. No significant difference between doxorubicin and saline groups was present, suggesting that motor function was not impaired by doxorubicin treatment. Error bars represent SEM.
Open field.
Activity in the open field was not impaired by doxorubicin treatment, as evidenced by no significant difference in distance traveled between the doxorubicin and control groups on any of the test days (Figure 2 A).
Modafinil.
Because the initial experiment revealed activity differences between doxorubicin and control groups were greatest in the afternoon, modafinil was administered at 1200. During the course of the experiment, one mouse in the saline–modafinil treatment group was euthanized after a gavage injury. Seven mice were euthanized (2 in the doxorubicin-treated group and 5 in the doxorubicin-and-modafinil–treated group) after observations of poor health (that is, moribund). Because the primary effect of modafinil was during the afternoon period, total activity between 1200 and 1800 each day was analyzed (Figure 5). As before, doxorubicin significantly (F1,39 = 6.4, P < 0.05) reduced wheel-running activity. Modafinil was equally efficacious in increasing activity in both saline and doxorubicin-treated mice, as evidenced by a significant main effect of modafinil treatment (F1,39 = 4.2, P < 0.05) but no significant interaction of the 2 treatments. Posthoc tests revealed a significant (P < 0.05) difference between the doxorubicin-vehicle treated group and all other groups.
Figure 5.
Wheel-running activity in the afternoon (1200 to 1800) was summed across days, and mean distance per group was plotted. Doxorubicin significantly (*, P < 0.05) reduced wheel-running activity, whereas the activity of mice given both modafinil and doxorubicin did not differ from that of control animals. Saline–vehicle, n = 12; doxorubicin–vehicle, n = 10; saline–modafinil, n = 11; and doxorubicin–modafinil group, n = 7; error bars represent SEM.
Discussion
Administration of doxorubicin to mice significantly decreased their voluntary wheel-running activity. The reduction was not a result of motor ataxia, given the lack of significant differences in rotarod performance between doxorubicin-treated and control mice. The reduction in wheel running activity was greatest during the afternoon, a finding that is consistent with clinical observations, in which patients often report fatigue to be most severe in the afternoon.52 In addition, the finding that morning wheel-running activity was similar between groups suggests that the decreased activity of doxorubicin-treated mice in the afternoon is due to fatigue rather than a lack of motivation to run. The open-field data further support this idea. Open-field activity is thought to measure exploratory behavior and the motivation to explore a novel environment,19 and the distance traveled in the open field was equivalent between control and doxorubicin-treated mice. Furthermore, we performed the open field test in the afternoon, when the difference in activity between groups was greatest.
We selected female mice for our study because several clinical studies of fatigue have assessed patients with breast cancer1-3,5,7,9,36,58 and because fatigue may be more prevalent in women.61 We chose doxorubicin as the test drug in light of previous inhouse studies and because it often is used in treating breast cancer. Furthermore, activity levels, as measured by wrist actigraphy, were significantly lower in women receiving doxorubicin-based protocols as compared with those receiving nondoxorubicin-based protocols.4 A possible explanation for greater susceptibility to fatigue after doxorubicin is that it is known to cause cardiac toxicity and muscular weakness.15,48 Indeed, reports of limb ‘heaviness’ and general weakness are defining features of cancer-related fatigue.14 In rodents, acute doxorubicin administration leads to decreased maximal force and increased rate of fatigue in skeletal muscle.27,32,56 However, in the previous studies,4,14 doxorubicin was administered as a single, high dose rather than as smaller, multiple doses over several days, as in the current study. Whether the current paradigm would result in muscle impairments similar to those seen previously is unknown.
The circadian rhythm of activity was not disrupted in doxorubicin-treated mice. Although sleep disruptions occur in cancer patients,5,55 data regarding circadian rhythm disturbances are inconsistent.47 Doxorubicin-treated mice may have experienced disrupted sleep that was not reflected in wheel-running activity. Ray and colleagues49 examined sleep patterns in C57Bl/6J and BALB/cJ mice after treatment with the chemotherapy agents paclitaxel and nab-paclitaxel. No differences were noted in the time mice spent asleep, as measured by electroencephalography and electromyography during the course of or after treatment.
Anemia is a known side effect of several chemotherapy agents, including doxorubicin, and can contribute to fatigue.13,23,39 Compared with controls, mice treated with doxorubicin had significantly lower RBC counts on days 15 and 22. However, this difference was no longer present at study completion on day 30, despite a continued attenuation in wheel running behavior. Consequently, anemia cannot completely account for activity difference between groups. Clinically, fatigue symptoms in humans often persist even after anima resolves;30 therefore, the current model recapitulates the clinical condition.
Cachexia is another side effect of cancer and its therapy. Cachexia is characterized by a loss of weight in both adipose tissue and skeletal muscle; these losses are not intentional (that is, due to a weight loss program) and are not reversed by nutritional changes such as increased caloric intake.59,60 In the current study, mice treated with doxorubicin lost significantly more weight than did control mice. The observed weight loss may be due to cachexia or anorexia. Examination of food and water intake may help to explain the causes of the weight loss associated with doxorubicin treatment. Regardless, weight loss is an indicator of general health and may contribute to fatigue.24
Modafinil administration significantly increased wheel-running activity in both saline- and doxorubicin-treated mice. This finding is consistent with other reports of increased activity after modafinil administration in rodents.34,63 Modafinil is a stimulant that is used to treat narcolepsy, but its precise mechanism of action is unknown. It is differentiated in part from other stimulants such as amphetamine and methylphenidate, which are associated with different patterns of c-Fos activation in the brain than that of mondafinil.40 In the present study, modafinil was successful in ameliorating the reduction in activity after doxorubicin administration. These findings buttress clinical studies suggesting that modafinil may be efficacious in treating cancer-related fatigue.6,37,57 However, modafinil administration also resulted in increased weight loss, such that 5 mice that received both doxorubicin and modafinil became moribund. Given the increased mortality, these results should be interpreted with caution, and additional supportive care to maintain body weight is recommended for future studies.
Meta-analyses has indicated that exercise can be beneficial in treating cancer-related fatigue in humans.18,26,38 In rodents, wheel running is used as a model of exercise.16 Therefore, wheel-running activity may have altered the true extent of measurable fatigue in our doxorubicin-treated mice. This hypothesis can be tested in additional studies by incorporating additional assays to assess fatigue and comparing the duration of symptoms with those after wheel-running.
The construct validity (whether the model displays the same mechanisms as the human condition) of the current model of chemotherapy-induced fatigue remains to be tested. Although a comprehensive understanding of the pathogenesis of CRF is not yet available, several hypotheses regarding causes of fatigue could be tested in an animal model of fatigue. Growing clinical evidence suggests that increases in proinflammatory cytokines may promote fatigue. IL6, IL1 receptor antagonist, TNFα, and neopterin have been shown to positively correlate with fatigue in cancer patients.25,54 Animal models have demonstrated that increases in cytokine expression induce sickness behavior (that is, listlessness, lethargy, and decreased food and water intake).21 Additional studies might examine the role of cytokines in chemotherapy-induced fatigue in mice.
Collectively, our current results demonstrate a murine model for the study of CRF in which mice display behaviors analogous to the symptoms of clinical fatigue in human patients. Specifically, voluntary activity in mice was reduced similarly to reports of reduced activity in breast cancer patients4 and modeled the physical symptoms of fatigue (that is, increased need to rest, diminished energy, decreased motivation to engage in activity, and postexertional malaise). Lasting fatigue8 is captured in our model, because the reduction in activity persisted beyond the cessation of treatment and was present in the absence of motor ataxia and anemia. The potential utility of the model in the development of an effective CRF intervention was suggested by the finding that modafinil administration was efficacious in reversing the reduction in wheel-running activity caused by doxorubicin treatment of our mice. Additional studies can validate the model by examining the mechanisms of fatigue.
Acknowledgments
We thank Ryan Dell and Jenny Tsui for assistance with data collection and animal care. Special thanks to the remainder of our animal care staff—Scott Anderson, Samantha Rogers, Ingrid Rankin, Sean Graham, and Martina Rosado—without whom this work would have not been possible. This work was supported by internal funding from Biomodels. Authors declare competing interests because of their employment at Biomodels.
References
- 1.Alexander S, Minton O, Andrews P, Stone P. 2009. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer 45:384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dimsdale J, Cohen-Zion M, Fiorentino L. 2006. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 14: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrykowski MA, Donovan KA, Jacobsen PB. 2009. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. J Pain Symptom Manage 37:341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AM. 1998. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum 25:51–62 [PubMed] [Google Scholar]
- 5.Berglund G, Bolund C, Fornander T, Rutqvist LE, Sjoden PO. 1991. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Eur J Cancer 27:1075–1081 [DOI] [PubMed] [Google Scholar]
- 6.Blackhall L, Petroni G, Shu J, Baum L, Farace E. 2009. A pilot study evaluating the safety and efficacy of modafinal for cancer-related fatigue. J Palliat Med 12:433–439 [DOI] [PubMed] [Google Scholar]
- 7.Bower JE, Ganz PA, Aziz N. 2005. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 67:277–280 [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. 2006. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 106:751–758 [DOI] [PubMed] [Google Scholar]
- 9.Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. 1998. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol 16:1689–1696 [DOI] [PubMed] [Google Scholar]
- 10.Bruera E, Valero V, Driver L, Shen L, Willey J, Zhang T, Palmer JL. 2006. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol 24:2073–2078 [DOI] [PubMed] [Google Scholar]
- 11.Butler JM, Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, Lesser G, McMullen K, McQuellon R, Naughton M, Rapp S, Stieber V, Shaw EG. 2007. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 69:1496–1501 [DOI] [PubMed] [Google Scholar]
- 12.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. 2011. Cancer-related fatigue: a practical review. Ann Oncol 22:1273–1279 [DOI] [PubMed] [Google Scholar]
- 13.Cella D. 1997. The functional assessment of cancer therapy–anemia (FACT–An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 34:13–19 [PubMed] [Google Scholar]
- 14.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. 1998. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 12:369–377 [PubMed] [Google Scholar]
- 15.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. 2007. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 7:147–156 [DOI] [PubMed] [Google Scholar]
- 16.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. 2008. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155:1048–1058 [DOI] [PubMed] [Google Scholar]
- 17.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. 2011. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav 10:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cramp F, Daniel J. 2008. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev CD006145. [DOI] [PubMed] [Google Scholar]
- 19.Crawley JN. 1985. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev 9:37–44 [DOI] [PubMed] [Google Scholar]
- 20.Curt GA. 2000. The impact of fatigue on patients with cancer: overview of FATIGUE 1 and 2. Oncologist 5 Suppl 2:9–12 [DOI] [PubMed] [Google Scholar]
- 21.Dantzer R. 2009. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29:247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deacon RM. 2009. Burrowing: a sensitive behavioural assay, tested in 5 species of laboratory rodents. Behav Brain Res 200:128–133 [DOI] [PubMed] [Google Scholar]
- 23.Demetri GD, Kris M, Wade J, Degos L, Cella D. 1998. Quality-of-life benefit in chemotherapy patients treated with epoetin-α is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol 16: 3412–3425 [DOI] [PubMed] [Google Scholar]
- 24.Foltz C, Ullman-Cullere M. 1999. Guidelines for assessing the health and condition of mice. Lab Anim (NY) 28:28–32 [PubMed] [Google Scholar]
- 25.Fung FY, Li M, Breunis H, Timilshina N, Minden MD, Alibhai SM. 2013. Correlation between cytokine levels and changes in fatigue and quality of life in patients with acute myeloid leukemia. Leuk Res 37:274–279 [DOI] [PubMed] [Google Scholar]
- 26.Galvao DA, Newton RU. 2005. Review of exercise intervention studies in cancer patients. J Clin Oncol 23: 899–909 [DOI] [PubMed] [Google Scholar]
- 27.Gilliam LA, Ferreira LF, Bruton JD, Moylan JS, Westerblad H, St Clair DK, Reid MB. 2009. Doxorubicin acts through tumor necrosis factor receptor subtype 1 to cause dysfunction of murine skeletal muscle. J Appl Physiol 107:1935–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanna A, Sledge G, Mayer ML, Hanna N, Einhorn L, Monahan P, Daggy J, Bhatia S. 2006. A phase II study of methylphenidate for the treatment of fatigue. Support Care Cancer 14: 210–215 [DOI] [PubMed] [Google Scholar]
- 29.Harrington ME. 2012. Neurobiological studies of fatigue. Prog Neurobiol 99:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzner B, Kemmler G, Greil R, Kopp M, Zeimet A, Raderer M, Hejna M, Zochbauer S, Krajnik G, Huber H, Fleischhacker WW, Sperner-Unterweger B. 2002. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol 13:965–973 [DOI] [PubMed] [Google Scholar]
- 31.Hurny C, Bernhard J, Joss R, Schatzmann E, Cavalli F, Brunner K, Alberto P, Senn HJ, Metzger U. 1993. ‘Fatigue and malaise’ as a quality-of-life indicator in small-cell lung cancer patients. The Swiss Group for Clinical Cancer Research (SAKK). Support Care Cancer 1: 316–320 [DOI] [PubMed] [Google Scholar]
- 32.Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R. 2011. Characterization of the effect of in vivo doxorubicin treatment on skeletal muscle function in the rat. Anticancer Res 31:2023–2028 [PubMed] [Google Scholar]
- 33.Innominato PF, Mormont MC, Rich TA, Waterhouse J, Levi FA, Bjarnason GA. 2009. Circadian disruption, fatigue, and anorexia clustering in advanced cancer patients: implications for innovative therapeutic approaches. Integr Cancer Ther 8:361–370 [DOI] [PubMed] [Google Scholar]
- 34.Ishizuka T, Murakami M, Yamatodani A. 2008. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol 578:209–215 [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen PB. 2004. Assessment of fatigue in cancer patients. J Natl Cancer Inst Monogr 2004:93–97 [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen PB, Donovan KA, Small BJ, Jim HS, Munster PN, Andrykowski MA. 2007. Fatigue after treatment for early stage breast cancer: a controlled comparison. Cancer 110:1851–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jean-Pierre P, Morrow GR, Roscoe JA, Heckler C, Mohile S, Janelsins M, Peppone L, Hemstad A, Esparaz BT, Hopkins JO. 2010. A phase III randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer 116:3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kangas M, Bovbjerg DH, Montgomery GH. 2008. Cancer-related fatigue: a systematic and metaanalytic review of nonpharmacological therapies for cancer patients. Psychol Bull 134:700–741 [DOI] [PubMed] [Google Scholar]
- 39.Kirshner J, Hatch M, Hennessy DD, Fridman M, Tannous RE. 2004. Anemia in stage II and III breast cancer patients treated with adjuvant doxorubicin and cyclophosphamide chemotherapy. Oncologist 9:25–32 [DOI] [PubMed] [Google Scholar]
- 40.Lin JS, Hou Y, Jouvet M. 1996. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci USA 93:14128–14133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lower EE, Fleishman S, Cooper A, Zeldis J, Faleck H, Yu Z, Manning D. 2009. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage 38:650–662 [DOI] [PubMed] [Google Scholar]
- 42.Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. 2007. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica, and gastric function in rats. Eur J Pharmacol 555:164–173 [DOI] [PubMed] [Google Scholar]
- 43.Malik NM, Moore GB, Smith G, Liu YL, Sanger GJ, Andrews PL. 2006. Behavioural and hypothalamic molecular effects of the anticancer agent cisplatin in the rat: a model of chemotherapy-related malaise? Pharmacol Biochem Behav 83:9–20 [DOI] [PubMed] [Google Scholar]
- 44.Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, Tannock IF. 2008. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 16: 577–583 [DOI] [PubMed] [Google Scholar]
- 45.Minton O, Stone P. 2009. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 20:17–25 [DOI] [PubMed] [Google Scholar]
- 46.Moraska AR, Sood A, Dakhil SR, Sloan JA, Barton D, Atherton PJ, Suh JJ, Griffin PC, Johnson DB, Ali A, Silberstein PT, Duane SF, Loprinzi CL. 2010. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol 28: 3673–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne JK. 2011. Altered circadian rhythms and cancer-related fatigue outcomes. Integr Cancer Ther 10:221–233 [DOI] [PubMed] [Google Scholar]
- 48.Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD, Parry JD, Lyon JJ, Marczylo EL, Gant TW. 2010. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 5:e12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. 2011. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med 61:119–130 [PMC free article] [PubMed] [Google Scholar]
- 50.Redmond K. 1996. Advances in supportive care. Eur J Cancer Care (Engl) 5:1–7 [DOI] [PubMed] [Google Scholar]
- 51.Rhodes JS, Garland T. 2003. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 167:242–250 [DOI] [PubMed] [Google Scholar]
- 52.Richardson A, Ream E, Wilson-Barnett J. 1998. Fatigue in patients receiving chemotherapy: patterns of change. Cancer Nurs 21:17–30 [DOI] [PubMed] [Google Scholar]
- 53.Roth AJ, Nelson C, Rosenfeld B, Scher H, Slovin S, Morris M, O'Shea N, Arauz G, Breitbart W. 2010. Methylphenidate for fatigue in ambulatory men with prostate cancer. Cancer 116:5102–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. 2007. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun 21:413–427 [DOI] [PubMed] [Google Scholar]
- 55.Silberfarb PM, Hauri PJ, Oxman TE, Schnurr P. 1993. Assessment of sleep in patients with lung cancer and breast cancer. J Clin Oncol 11: 997–1004 [DOI] [PubMed] [Google Scholar]
- 56.Smuder AJ, Kavazis AN, Min K, Powers SK. 2011. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol 111:1190–1198 [DOI] [PubMed] [Google Scholar]
- 57.Spathis A, Dhillan R, Booden D, Forbes K, Vrotsou K, Fife K. 2009. Modafinil for the treatment of fatigue in lung cancer: a pilot study. Palliat Med 23:325–331 [DOI] [PubMed] [Google Scholar]
- 58.Swenson KK, Nissen MJ, Henly SJ. 2010. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncol Nurs Forum 37:321–330 [DOI] [PubMed] [Google Scholar]
- 59.Tisdale MJ. 2002. Cachexia in cancer patients. Nat Rev Cancer 2:862–871 [DOI] [PubMed] [Google Scholar]
- 60.Tisdale MJ. 2003. Pathogenesis of cancer cachexia. J Support Oncol 1:159–168 [PubMed] [Google Scholar]
- 61.Vestergaard S, Nayfield SG, Patel KV, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik JM. 2009. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci 64:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. 2006. The cancer chemotherapy drug etoposide (VP16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs 8:157–169 [DOI] [PubMed] [Google Scholar]
- 63.Young JW, Kooistra K, Geyer MA. 2011. Dopamine receptor mediation of the exploratory–hyperactivity effects of modafinil. Neuropsychopharmacology 36: 1385–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zombeck JA, Deyoung EK, Brzezinska WJ, Rhodes JS. 2011. Selective breeding for increased home cage physical activity in collaborative cross and Hsd:ICR mice. Behav Genet 41:571–582 [DOI] [PubMed] [Google Scholar]