Abstract
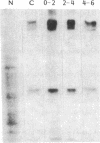
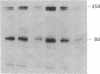
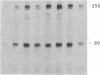
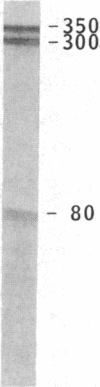
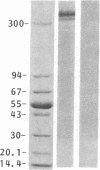
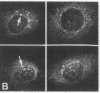
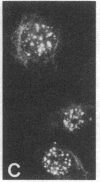
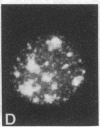
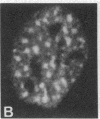
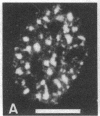
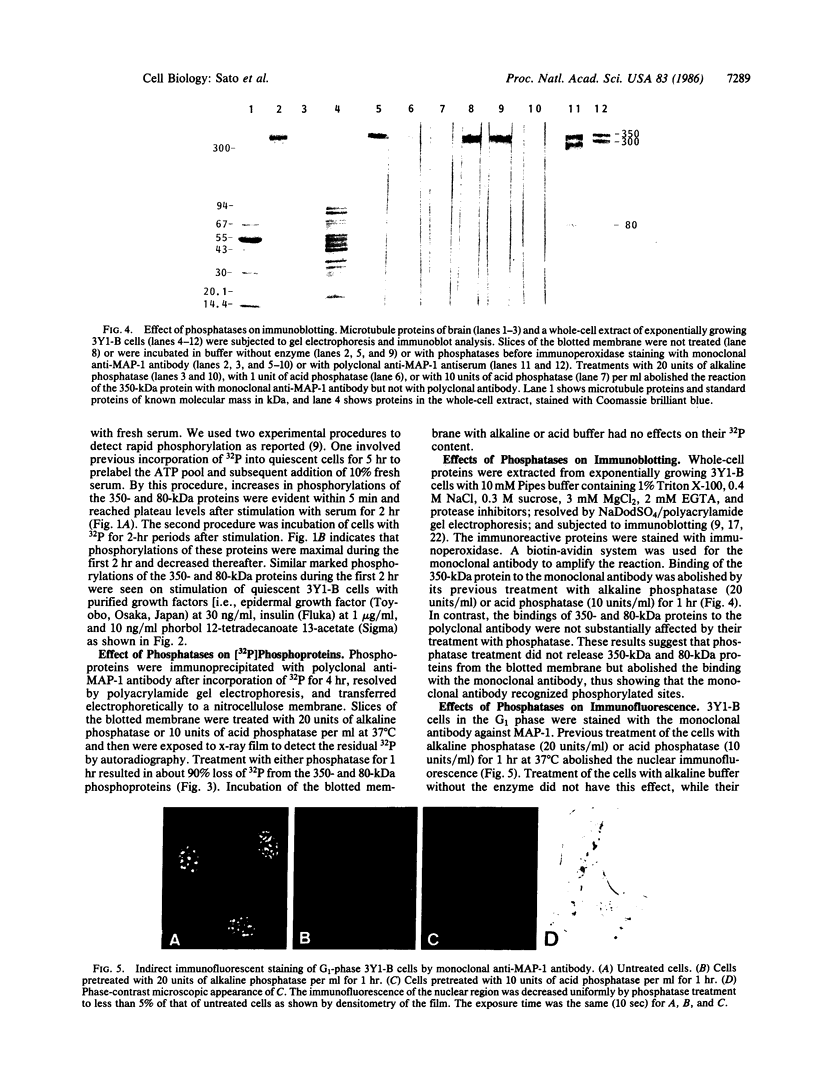
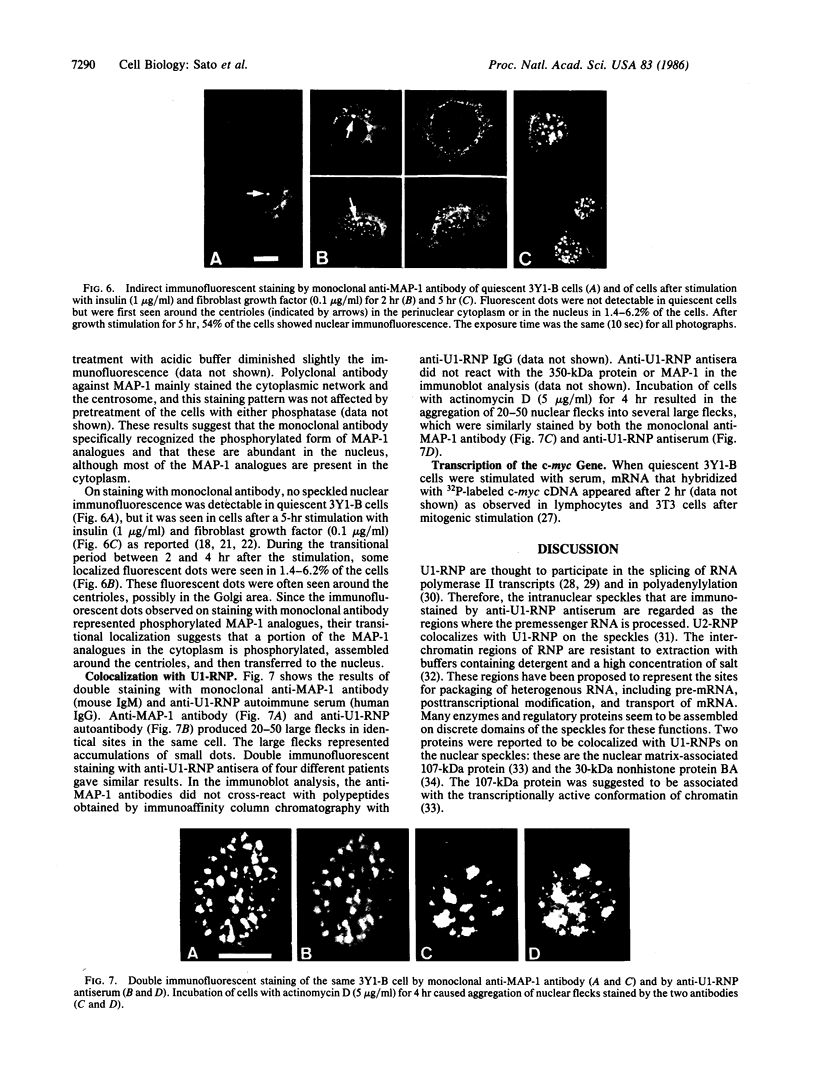
Cytoskeleton-associated 350-kDa and 80-kDa polypeptides, which were immunoprecipitated with polyclonal antibody against microtubule-associated protein 1 (MAP-1), were rapidly phosphorylated on mitogenic stimulation of quiescent fibroblasts with serum or growth factors. The enhanced phosphorylation was evident within 5 min and reached a maximum 2 hr after the stimulation. Phosphorylated MAP-1 analogues were first detected in the cytoplasm around the microtubule-organizing center and then in the nucleus by immunofluorescent staining with a monoclonal antibody that recognized the phosphorylated form of MAP-1. The monoclonal antibody reacted with the 350-kDa protein in immunoblot analysis and immunostained intranuclear speckles; both immunoreactions were abolished by treatment with alkaline or acid phosphatase. The nuclear speckles stained by the monoclonal antibody were also stained by anti-U1 small nuclear ribonucleoprotein antibodies on double immunofluorescence, suggesting that the stained regions are sites of maturation of messenger RNA. These results support the idea that part of the cytoskeleton-associated 350-kDa protein is phosphorylated and transferred to the nuclear region of mRNA modification as a common early process after growth stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai D. J., Thompson W. C., Wilson L., Dresden C. F., Schulman H., Purich D. L. Microtubule-associated proteins (MAPs): a monoclonal antibody to MAP 1 decorates microtubules in vitro but stains stress fibers and not microtubules in vivo. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1434–1438. doi: 10.1073/pnas.82.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett F. C., Yeoman L. C. Co-localization of non-histone protein BA with U-snRNPs to the same regions of the cell nucleus. Exp Cell Res. 1985 Apr;157(2):379–386. doi: 10.1016/0014-4827(85)90123-5. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockus B. J., Stiles C. D. Regulation of cytoskeletal architecture by platelet-derived growth factor, insulin and epidermal growth factor. Exp Cell Res. 1984 Jul;153(1):186–197. doi: 10.1016/0014-4827(84)90460-9. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Klausner R. D., Sandoval I. V. A widely distributed nuclear protein immunologically related to the microtubule-associated protein MAP1 is associated with the mitotic spindle. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1146–1150. doi: 10.1073/pnas.82.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Corces V. G., Manso R., De La Torre J., Avila J., Nasr A., Wiche G. Effects of DNA on microtubule assembly. Eur J Biochem. 1980 Mar;105(1):7–16. doi: 10.1111/j.1432-1033.1980.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Erikson E., Shealy D. J., Erikson R. L. Evidence that viral transforming gene products and epidermal growth factor stimulate phosphorylation of the same cellular protein with similar specificity. J Biol Chem. 1981 Nov 25;256(22):11381–11384. [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985 Mar;100(3):764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Dastidar P., Fox C. F. Epidermal growth factor and epidermal growth factor receptor-dependent phosphorylation of a Mr = 34,000 protein substrate for pp60src. J Biol Chem. 1983 Feb 10;258(3):2041–2044. [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Halegoua S., Patrick J. Nerve growth factor mediates phosphorylation of specific proteins. Cell. 1980 Nov;22(2 Pt 2):571–581. doi: 10.1016/0092-8674(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Site-specific polyadenylation in a cell-free reaction. Cell. 1984 Mar;36(3):581–591. doi: 10.1016/0092-8674(84)90337-4. [DOI] [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohno T., Ohkawa A., Sato C. Factors controlling the appearance of the immunofluorescent nuclear dots revealed with monoclonal antibody against microtubule-associated protein-1. Exp Cell Res. 1985 Jun;158(2):558–562. doi: 10.1016/0014-4827(85)90480-x. [DOI] [PubMed] [Google Scholar]
- Okuda A., Kimura G. Effects of serum deprivation on the initiation of DNA synthesis in the second generation in rat 3Y1 cells. J Cell Physiol. 1982 Mar;110(3):267–270. doi: 10.1002/jcp.1041100308. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakamura H., Komagoe Y., Shimada K., Ueda R., Suzuki S. Monoclonal antibody against microtubule associated protein-1 produces immunofluorescent spots in the nucleus and centrosome of cultured mammalian cells. Cell Struct Funct. 1983 Sep;8(3):245–254. doi: 10.1247/csf.8.245. [DOI] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakamura H., Ueda R. Nuclear immunofluorescence by a monoclonal antibody against microtubule-associated protein-1 as it is associated with cell proliferation and transformation. Exp Cell Res. 1984 Nov;155(1):33–42. doi: 10.1016/0014-4827(84)90765-1. [DOI] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Kobayashi T. Effect upon mitogenic stimulation of calcium-dependent phosphorylation of cytoskeleton-associated 350,000- and 80,000-mol-wt polypeptides in quiescent 3Y1 cells. J Cell Biol. 1985 Mar;100(3):748–753. doi: 10.1083/jcb.100.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Yamaguchi N. Co-localization of SV40 T antigen and p53 with immunological analogues of microtubule-associated protein-1 on the nuclear skeleton. Cell Struct Funct. 1984 Sep;9(3):305–309. doi: 10.1247/csf.9.305. [DOI] [PubMed] [Google Scholar]
- Sato C., Tanabe K., Nishizawa K., Nakayma T., Kobayashi T., Nakamura H. Localization of 350K molecular weight and related proteins in both the cytoskeleton and nuclear flecks that increase during G1 phase. Exp Cell Res. 1985 Sep;160(1):206–220. doi: 10.1016/0014-4827(85)90249-6. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Spector D. L., Woodcock C. L., Ochs R. L., Bhorjee J. Alterations in chromatin conformation are accompanied by reorganization of nonchromatin domains that contain U-snRNP protein p28 and nuclear protein p107. J Cell Biol. 1985 Aug;101(2):560–567. doi: 10.1083/jcb.101.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Colocalization of U1 and U2 small nuclear RNPs by immunocytochemistry. Biol Cell. 1984;51(1):109–112. doi: 10.1111/j.1768-322x.1984.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Werth D. K., Pastan I. Vinculin phosphorylation in response to calcium and phorbol esters in intact cells. J Biol Chem. 1984 Apr 25;259(8):5264–5270. [PubMed] [Google Scholar]