Abstract
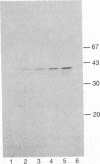
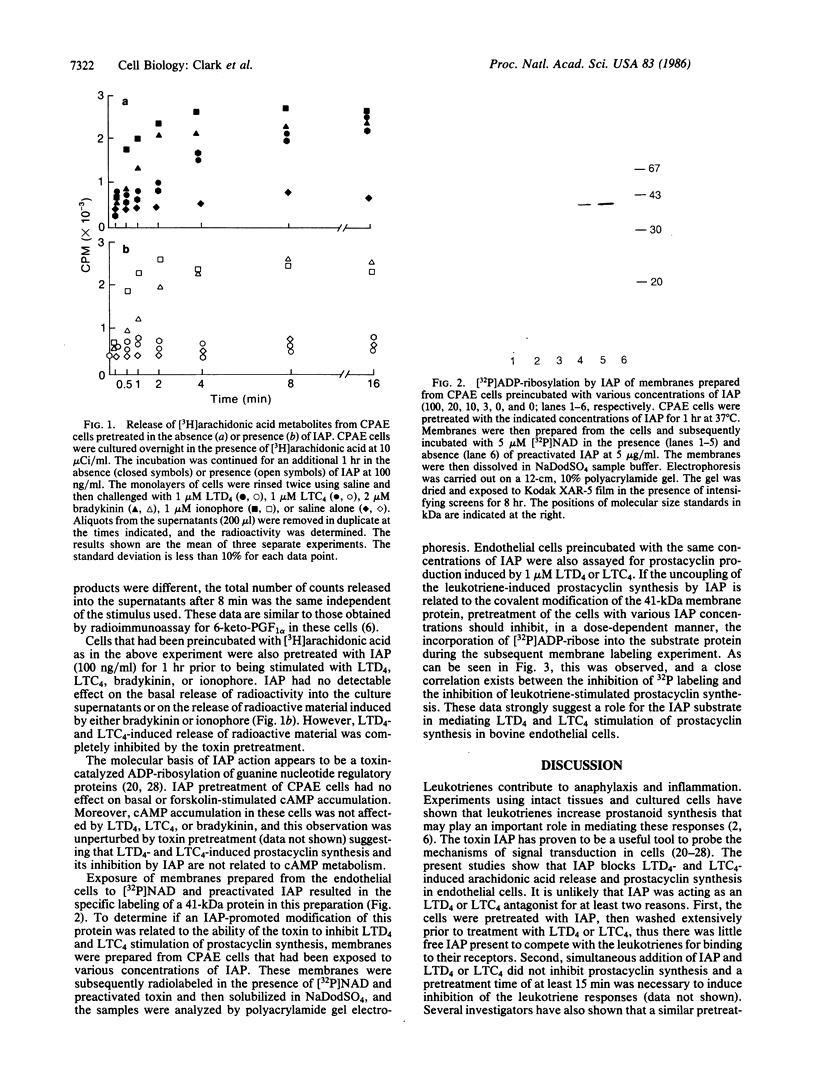
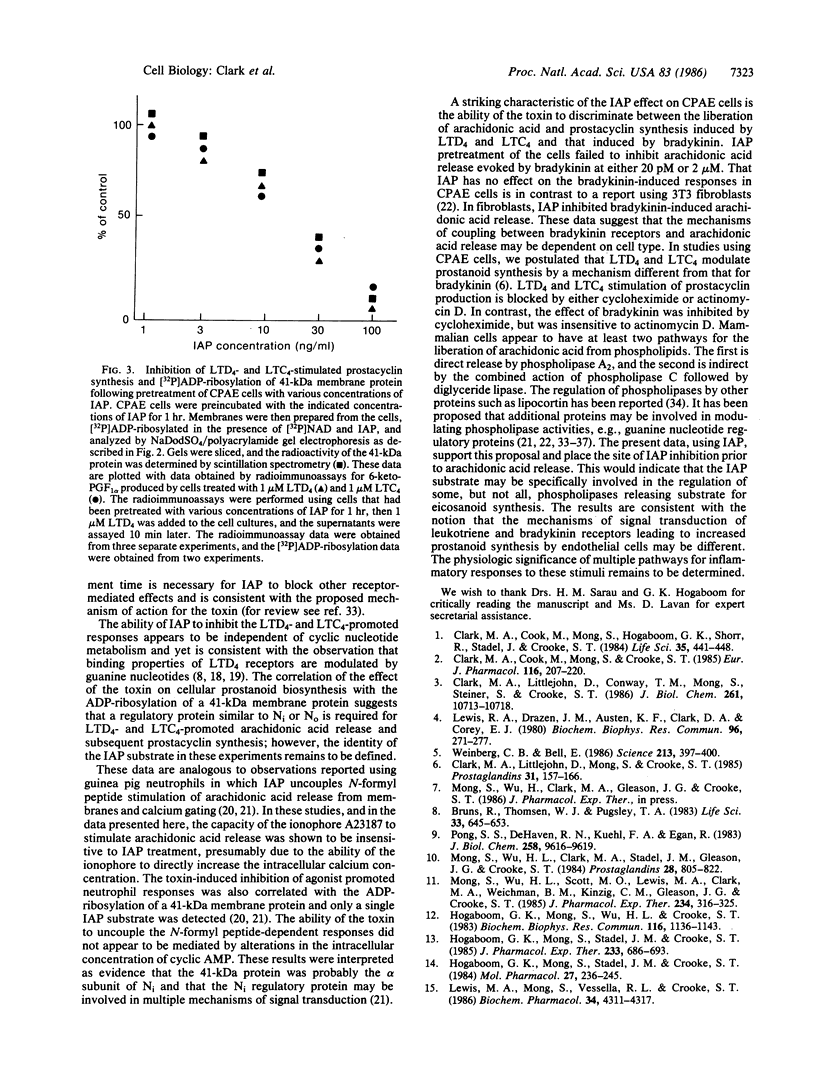
Incubation of the bovine endothelial cell line, CPAE, with leukotriene D4, leukotriene C4, bradykinin, or the calcium ionophore A23187 results in the release of arachidonic acid metabolites including 6-keto-prostaglandin F1 alpha, the stable metabolite of prostacyclin. Pretreatment of these cells with the pertussis toxin islet-activating protein (IAP) results in a dose-dependent inhibition of the release of arachidonic acid metabolites and prostacyclin in response to leukotriene D4 and leukotriene C4. In contrast, similar responses evoked by bradykinin or ionophore were not significantly altered by the IAP pretreatment of the cells. IAP in the presence of [32P]NAD specifically [32P]ADP-ribosylates a 41-kDa protein in membranes prepared from CPAE cells. Pretreatment of the intact cells with IAP resulted in a dose-dependent inhibition of subsequent 32P labeling of the toxin substrate in the membranes and correlates with the uncoupling of the leukotriene responses. These results suggest that the 41-kDa IAP substrate, presumably a guanine nucleotide regulatory protein, mediates the response of CPAE cells to leukotriene D4 and leukotriene C4, but not to bradykinin or the calcium ionophore.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Thomsen W. J., Pugsley T. A. Binding of leukotrienes C4 and D4 to membranes from guinea pig lung: regulation by ions and guanine nucleotides. Life Sci. 1983 Aug 15;33(7):645–653. doi: 10.1016/0024-3205(83)90253-9. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Cook M., Mong S., Crooke S. T. The binding of leukotriene C4 and leukotriene D4 to membranes of a smooth muscle cell line (BC3H1) and evidence that leukotriene induced contraction in these cells is mediated by thromboxane, protein and RNA syntheses. Eur J Pharmacol. 1985 Oct 22;116(3):207–220. doi: 10.1016/0014-2999(85)90155-4. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Cook M., Mong S., Hogaboom G. K., Shorr R., Stadel J., Crooke S. T. Leukotriene C4 ([3H]-LTC4) binding to membranes isolated from a hamster smooth muscle cell line (DDT1MF2). Life Sci. 1984 Jul 23;35(4):441–448. doi: 10.1016/0024-3205(84)90655-6. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Conway T. M., Mong S., Steiner S., Crooke S. T. Leukotriene D4 treatment of bovine aortic endothelial cells and murine smooth muscle cells in culture results in an increase in phospholipase A2 activity. J Biol Chem. 1986 Aug 15;261(23):10713–10718. [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Mong S., Crooke S. T. Effect of leukotrienes, bradykinin and calcium ionophore (A 23187) on bovine endothelial cells: release of prostacyclin. Prostaglandins. 1986 Jan;31(1):157–166. doi: 10.1016/0090-6980(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboom G. K., Mong S., Stadel J. M., Crooke S. T. Characterization of [3H]leukotriene D4 binding sites in guinea-pig ventricular myocardium. J Pharmacol Exp Ther. 1985 Jun;233(3):686–693. [PubMed] [Google Scholar]
- Hogaboom G. K., Mong S., Stadel J. M., Crooke S. T. Characterization of guinea pig myocardial leukotriene C4 binding sites. Regulation by cations and sulfhydryl-directed reagents. Mol Pharmacol. 1985 Feb;27(2):236–245. [PubMed] [Google Scholar]
- Hogaboom G. K., Mong S., Wu H. L., Crooke S. T. Peptidoleukotrienes: distinct receptors for leukotriene C4 and D4 in the guinea-pig lung. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1136–1143. doi: 10.1016/s0006-291x(83)80261-7. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilis S., Lewis R. A., Corey E. J., Austen K. F. Specific receptors for leukotriene C4 on a smooth muscle cell line. J Clin Invest. 1983 Oct;72(4):1516–1519. doi: 10.1172/JCI111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H., Katada T., Amano T., Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. A., Mong S., Vessella R. L., Crooke S. T. Identification and characterization of leukotriene D4 receptors in adult and fetal human lung. Biochem Pharmacol. 1985 Dec 15;34(24):4311–4317. doi: 10.1016/0006-2952(85)90290-4. [DOI] [PubMed] [Google Scholar]
- Lewis M. A., Mong S., Vessella R. L., Hogaboom G. K., Wu H. L., Crooke S. T. Identification of specific binding sites for leukotriene C4 in human fetal lung. Prostaglandins. 1984 Jun;27(6):961–974. doi: 10.1016/s0090-6980(84)80013-1. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Drazen J. M., Austen K. F., Clark D. A., Corey E. J. Identification of the C(6)-S-conjugate of leukotriene A with cysteine as a naturally occurring slow reacting substance of anaphylaxis (SRS-A). Importance of the 11-cis-geometry for biological activity. Biochem Biophys Res Commun. 1980 Sep 16;96(1):271–277. doi: 10.1016/0006-291x(80)91210-3. [DOI] [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Mong S., Wu H. L., Clark M. A., Stadel J. M., Gleason J. G., Crooke S. T. Identification of leukotriene D4 specific binding sites in the membrane preparation isolated from guinea pig lung. Prostaglandins. 1984 Dec;28(6):805–822. doi: 10.1016/0090-6980(84)90036-4. [DOI] [PubMed] [Google Scholar]
- Mong S., Wu H. L., Hogaboom G. K., Clark M. A., Stadel J. M., Crooke S. T. Regulation of ligand binding to leukotriene D4 receptors: effects of cations and guanine nucleotides. Eur J Pharmacol. 1984 Nov 13;106(2):241–253. doi: 10.1016/0014-2999(84)90711-8. [DOI] [PubMed] [Google Scholar]
- Mong S., Wu H. L., Scott M. O., Lewis M. A., Clark M. A., Weichman B. M., Kinzig C. M., Gleason J. G., Crooke S. T. Molecular heterogeneity of leukotriene receptors: correlation of smooth muscle contraction and radioligand binding in guinea-pig lung. J Pharmacol Exp Ther. 1985 Aug;234(2):316–325. [PubMed] [Google Scholar]
- Mong S., Wu H. L., Stadel J. M., Clark M. A., Crooke S. T. Solubilization of [3H]leukotriene D4 receptor complex from guinea pig lung membranes. Mol Pharmacol. 1986 Mar;29(3):235–243. [PubMed] [Google Scholar]
- Murayama T., Katada T., Ui M. Guanine nucleotide activation and inhibition of adenylate cyclase as modified by islet-activating protein, pertussis toxin, in mouse 3T3 fibroblasts. Arch Biochem Biophys. 1983 Mar;221(2):381–390. doi: 10.1016/0003-9861(83)90157-1. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Pong S. S., DeHaven R. N., Kuehl F. A., Jr, Egan R. W. Leukotriene C4 binding to rat lung membranes. J Biol Chem. 1983 Aug 25;258(16):9616–9619. [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Weinberg C. B., Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986 Jan 24;231(4736):397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]