Abstract
Angiogenesis is central to many physiological and pathological processes. Here we show two potent bioinformatically-identified peptides, one derived from collagen IV and translationally optimized, and one from a somatotropin domain-containing protein, synergize in angiogenesis and lymphangiogenesis assays including cell adhesion, migration and in vivo Matrigel plugs. Peptide-peptide combination therapies have recently been applied to diseases such as human immunodeficiency virus (HIV), but remain uncommon thus far in cancer, age-related macular degeneration and other angiogenesis-dependent diseases. Previous work from our group has shown that the collagen IV-derived peptide primarily binds β1 integrins, while the receptor for the somatotropin-derived peptide remains unknown. We investigate these peptides’ mechanisms of action and find both peptides affect the vascular endothelial growth factor (VEGF) pathway as well as focal adhesion kinase (FAK) by changes in phosphorylation level and total protein content. Blocking of FAK both through binding of β1 integrins and through inhibition of VEGFR2 accounts for the synergy we observe. Since resistance through activation of multiple signaling pathways is a central problem of anti-angiogenic therapies in diseases such as cancer, we suggest that peptide combinations such as these are an approach that should be considered as a means to sustain anti-angiogenic and anti-lymphangiogenic therapy and improve efficacy of treatment.
Keywords: Angiogenesis, Synergy, Combination therapy, Peptide, Inhibitor
Introduction
Angiogenesis is essential to many physiological processes and pathologies including cancer, inflammatory disorders and eye disease [1]. Angiogenesis therapies have been estimated to potentially benefit over a half a billion people worldwide [2]; angiogenesis is an active area of fundamental and translational research [3]. Initial inhibitors targeting neovasculature formation in tumor models were met with high expectations but often failed clinically due to redundancy in growth factor signaling pathways and acquired tumor resistance [4, 5]. Despite these limitations, to date hundreds of thousands of patients have benefited from inhibitors of vascular endothelial growth factor (VEGF) [2, 6]; however, so far the strategy has been effective only in selected types of cancer and in sub-population of patients. Paradoxically, VEGF inhibitors may reduce primary tumor size but increase tumor aggression and metastasis by driving tumor hypoxia and invasion through the epithelial-mesenchymal transition (EMT) [7]. Thus, there remains an unmet need to develop more potent and diverse angiogenesis inhibitors and to devise strategies to overcome tumor resistance. Lymphangiogenesis is critical to cancer dissemination and metastasis, whereby functional lymphatic vessels are one route for invasive tumor cells to leave a primary tumor site and establish distant metastases [8]. A functional lymphatic endothelium is also critical for host immune tolerance, and may promote tumor immune escape and contribute to tumor progression [9]. Thus, targeting the tumor endothelium and lymphatic endothelium simultaneously may more effectively sustain tumor inhibition than anti-angiogenic therapies alone.
Asystematic, bioinformatics methodology for identifying peptides within conserved protein domains was introduced by Karagiannis and Popel [10] identifying more than 80 angiogenesis inhibitors. One sequence derived from type IV collagen, we refer to as SP2000 (seq: LRRFSTMPFMFCNI NNVCNF), showed anti-angiogenic activity in vitro in cell proliferation and migration assays [10, 11] and in vivo in mouse xenograft models [12–14]. Interestingly, this peptide appeared to antagonize the activity of the CXC chemokines-derived peptide family and thrombospondin-1 domain-derived peptides and had not shown to be synergistic with other peptide families. To improve the sequence for translation, we removed the cysteines in SP2000 and replaced with Abu (l-α-amino-n-butyric acid), a cysteine analog, and named the new sequence SP2012 (seq: LRRFSTMPFMF-Abu-NINNV-Abu-NF) [15], which has been extensively characterized [16, 17]. A separate group of somatotropin-domain derived peptides had also been reported but untested [10]. Lee et al. [18] verified the collection of 14-mer somatotropin sequences were active and found a potent inhibitor of angiogenesis and lymphangiogenesis named SP5031 (seq: LLRSSLILLQGSWF), derived from trans-membrane protein 45A.
It was reported that anti-angiogenic compounds could be combined with dual and trimodal combinations of chemotherapy and/or radiotherapy with added efficacy [19]. Many conventional chemotherapeutics for late-stage metastatic disease have failed to add significant therapeutic benefit to these patients without the combined anti-angiogenic agents [20]. Combination therapies with anti-angiogenic drugs and chemotherapeutics in cancer are increasing in number with 200 adjuvant clinical trials planned or underway [4, 21]; however, anti-angiogenic peptide–peptide combinations have received little attention as a strategy to enhance anti-angiogenic treatments and limit deleterious side effects from chemotherapeutics such as toxicity and drug resistance. Peptides present certain advantages over small molecules such as lower toxicity and high selectivity to target receptors; they have a significantly higher rate of success in clinical trials [22]. A combination of Her-2 and VEGF peptide mimics was demonstrated to increase anti-angiogenic and anti-tumor properties and could be advantageous over monoclonal antibody treatments for the same receptors [23]. We thus envision similar peptide combination strategies to increase efficacy of treatment and avoid potential toxicities associated with combinations of chemotherapeutics, small molecules and monoclonal antibodies.
In the present study we show SP2012 synergizes with SP5031 in angiogenesis and lymphangiogenesis assays according to the Chou-Talalay method for drug synergy analysis [24, 25]. We define synergy by the combination index (CI) where CI < 1 indicates synergy, CI = 1 indicates additivity, and CI > 1 indicates antagonism. We investigate the peptides’ mechanisms of action and show redundancy in focal adhesion kinase (FAK) inhibition by SP2012 binding to β1 integrins and blocking VEGFR2 phosphorylation. Limiting VEGFR2 phosphorylation with both SP2012 and SP5031 and inducing protein degradation of VEGFR2, FAK and PLCγ for peptides accounts in part for the synergistic effect. Furthermore, we show that although SP2012 and SP5031 block VEGFR2 activation SP2012 has the added effect of Akt survival factor activation through FMS-related tyrosine kinase-3 (Flt-3), indicating potential multiple target receptors. SP5031 also attenuates the effect of SP2012 on pAkt, giving a stronger net anti-angiogenic effect by limiting the cell survival factor Akt. Both peptides are anti-angiogenic and show significant activity in vivo. The peptides appear strongest together as angiogenesis and lymphangiogenesis inhibitors as the combination gives an enhanced response over each peptide treatment alone. This study shows the significant potential for peptide–peptide combinations to treat angiogenesis- and lymphangiogenesis-associated pathologies and provides the rationale for combining angiogenesis and lymphangiogenesis inhibitors through knowledge of their mechanisms of action.
Results
In vitro validation of synergistic activity
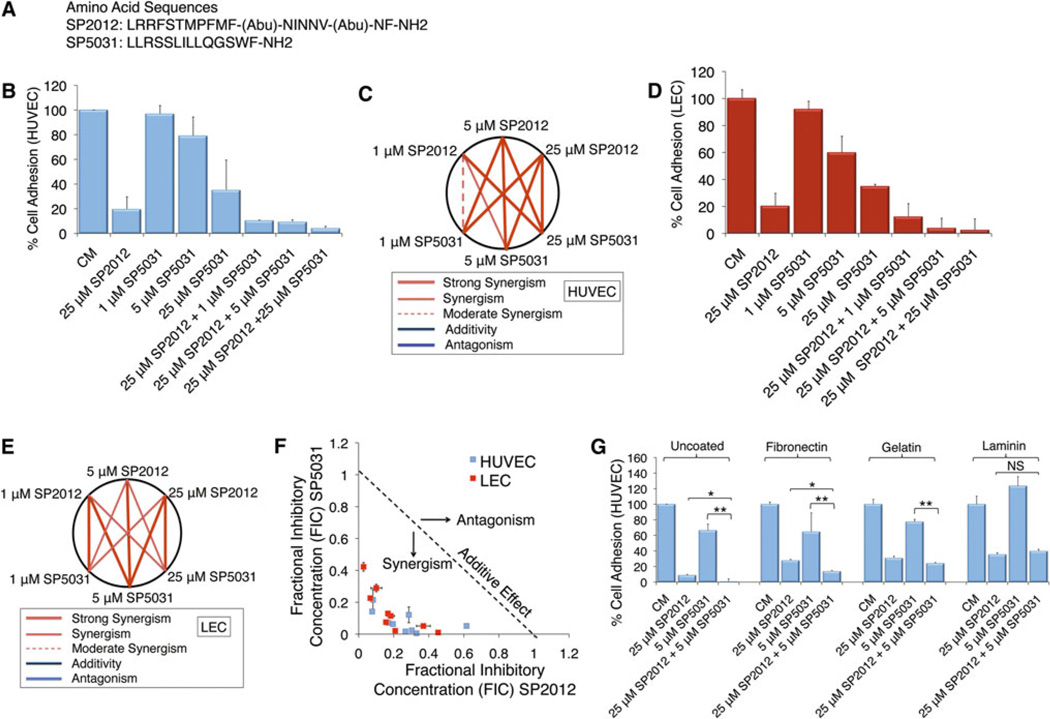
Adhesion is necessary for the formation of both blood and lymphatic vessels inside and around tumors. Work by our group had shown both SP2012 and SP5031 individually block endothelial cell adhesion and migration in human umbilical vein endothelial cells (HUVECs), microvascular endothelial cells (MECs) and lymphatic endothelial cells (LECs) [15, 18] (sequences shown in Fig. 1a). Here we show a synergistic response when SP2012 and SP5031 are applied simultaneously to HUVECs (Fig. 1b) on cell-culture treated plates (no extracellular matrix). We hypothesized based on previous work these peptides would behave similarly in lymphatic cell adhesion assays and show similar synergy (Fig. 1d). All CI are < 1 in tested doses indicating synergy by the Chou-Talalay method, and tested doses show an improvement over single doses. These results are shown graphically in Fig. 1c (HUVECs) and Fig. 1e (LECs) and are highly synergistic with all CI < 1. Figure 1f shows a normalized isobologram for experimentally tested values in both HUVECs and LECs. We additionally tested the effect of SP2012 and SP5031 on adhesion assays on different extracellular matrix coatings, including fibronectin, gelatin and laminin (Fig. 1g). At 25 µM SP2012 and 5 µM SP5031 the peptide combination enhances adhesion inhibition on uncoated plates, fibronectin and gelatin. We did not see this effect on laminin-coated plates; SP5031 does not block adhesion of HUVECs at 5 µM.
Fig. 1.
SP2012 and SP5031 block endothelial cell adhesion alone and synergistically in combination. a Sequences of selected peptides: SP2012, a collagen IV mimetic peptide and SP5031, a somatotropin-domain derived peptide. b SP2012 and SP5031 both block endothelial cell adhesion on cell-culture treated plates (no extracellular matrix coating) and show synergy. SD of the mean (±) is shown. c Graphical representation of combinatorial dosing. All dosing combinations show synergy as determined by the Chou-Talalay method. d SP2012 and SP5031 synergistically block LEC adhesion on cell-culture treated plates. e Graphical representation of peptide doses on LECs. f Isobologram showing experimental values demonstrating synergy. g Different extracellular matrices shown including fibronectin, gelatin, and laminin coatings. SP2012 and SP5031 synergistically block cell adhesion on uncoated plates, fibronectin, and gelatin-coated plates. SP5031 does not block cell attachment to laminin at 5 µM
As a separate control, we tested a homologous sequence to SP5031, named SP5001 (seq: RLRLLTLQSWLL) as a sham peptide in the adhesion assay. SP5001 is similar to SP5031 and had been previously shown to be inactive in adhesion. Fig S1a shows this peptide is inactive and does not increase the synergistic response of SP2012 in adhesion on cell-culture treated plates. We tested SP2012 and SP5031 in MECs to further confirm the effect on endothelial cells. Similar to HUVECs, Fig S1b and c show SP2012 and SP5031 are highly synergistic when tested against MECs at indicated doses. Since there appeared to be little difference between MECs and HUVECs, we continued to use HUVECs for their ease of propagation.
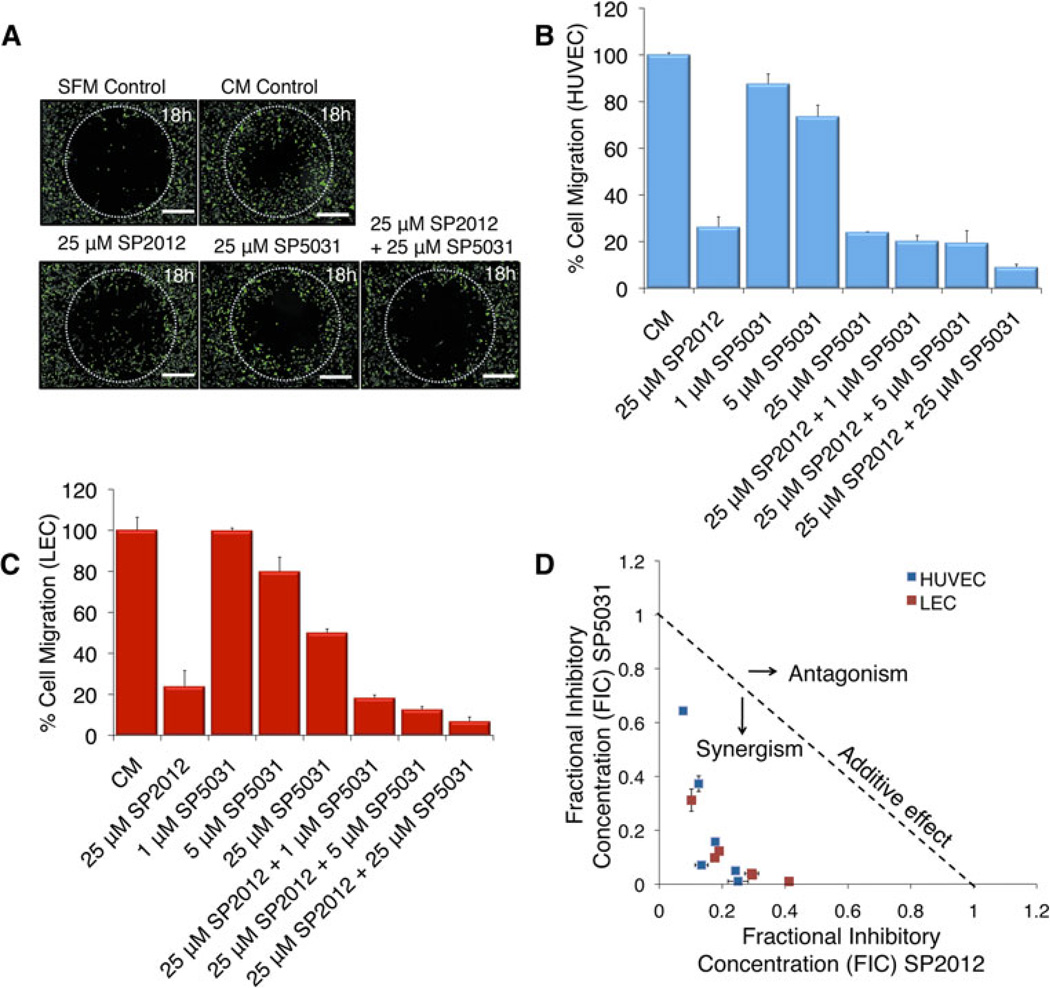
After observing the synergistic effect of the two peptides in adhesion we then tested SP2012 and SP5031 in HUVEC migration on fibronectin-coated transwell plates and in a modified wound healing-type assay. A growing body of evidence indicates adhesion is necessary for proper migration of endothelial cells. Figure 2a shows a modified wound healing-type assay showing a synergistic response for the combination of SP2012 and SP5031 in HUVEC migration after 18 h. Scale bars shown are 500 µM. Cells were allowed to adhere and were then plated with peptides at indicated doses. Images shown are representative of tested conditions and demonstrate an enhanced response for the combination than each peptide tested separately. Figure 2b shows quantification of the inhibitory migration effect on HUVECs in a transwell-based assay coated with fibronectin. All combinatorial doses tested are highly synergistic. Figure 2c shows a similar synergy in LECs with SP20102 and SP5031 tested alone or in combination at indicated doses. We next constructed a normalized isobologram showing all experimentally tested values are synergistic in inhibition of HUVEC and LEC migration measured by the Chou-Talalay combination index (Fig. 2d).
Fig. 2.
SP2012 and SP5031 synergistically block migration of HUVECs and LECs. a Modified wound-healing type assay showing visually the effects of SP2012 and SP5031 tested in combination. The fluorescent cells inside the circle have migrated for 18 h. Scale bars shown represent 500 µM. b Transwell migration HUVEC data, where SP2012 and SP5031 are synergistic at blocking cell migration on fibronectin-coated plates. SD of the mean (±) is shown. c LEC data showing SP2012 and SP5031 are synergistic at blocking cell migration on fibronectin-coated plates in a similar transwell-based assay. d Normalized isobologram showing experimentally tested combinations are synergistic
VEGF pathway analysis
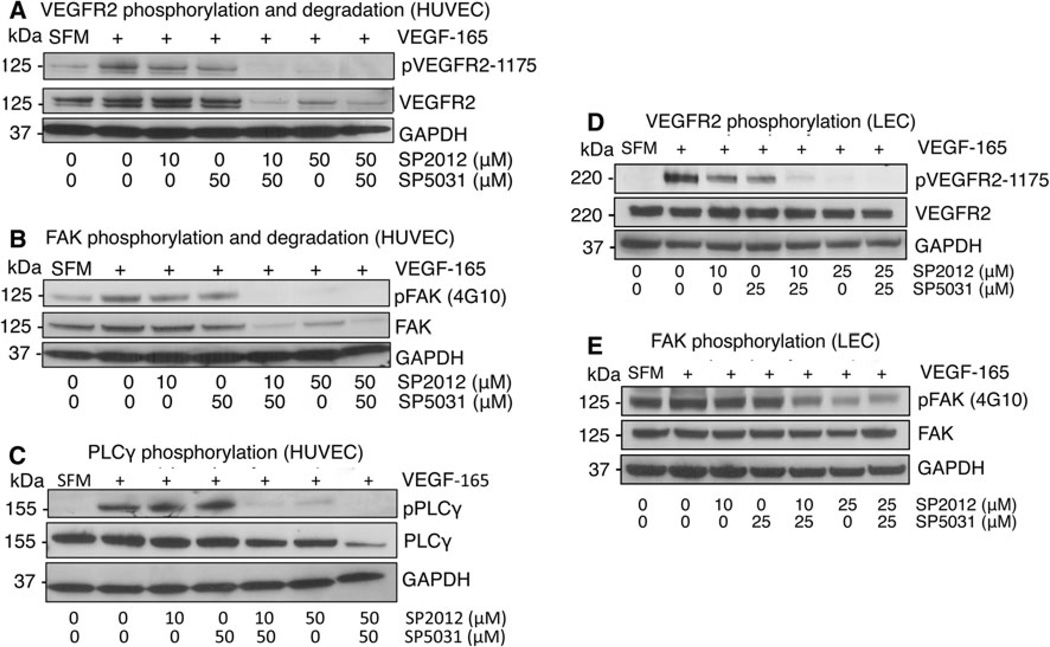
To understand how the synergistic effect occurs, we next began investigating the peptides’ mechanisms of action. We do not use the Chou Talalay method for synergy calculations here as we have not measured median effects for each compound due to limitations in these assays; instead we consider additive effects on the inhibition of phosphorylation and the effect of the compounds on total protein content. Previously we had shown SP2012 binds to β1 integrins [26]. The receptor for SP5031 remains unknown. We now show both SP2012 and SP5031 block VEGF signaling by blocking phosphorylation of VEGFR2 Tyr1175 in the presence of VEGF. This effect appears to be enhanced when peptides are tested in combination in HUVECs at 10 µM SP2012 or 50 µM SP2012, and 50 µM SP5031 (Fig. 3a). Focal adhesion kinase (FAK) is activated by both β1 integrins [27] and VEGFR2 [28] and is central to adhesion [29]. Endothelial FAK has been shown to be crucial to the pathophysiology of a variety of diseases and is a significant mediator of angiogenesis [30]. We show here SP2012 and SP5031 block activation of FAK alone or in combination (Fig. 3b). Both VEGFR2 and FAK partially degrade after 90 min peptide treatment compared to the GAPDH loading control in HU-VECs; this phenomenon contributes to the net synergistic anti-angiogenic effect from the combination. Another downstream effector molecule of VEGF signaling is phospholipase C-gamma (PLCγ) and is activated upon VEGFR2 Tyr1175 phosphorylation [31]. We next showed SP2012 and SP5031 block PLCγ phosphorylation alone or in combination, as part of the VEGF pathway (Fig. 3c) and through blockage of VEGFR2. The total PLCγ protein content was slightly degraded at high concentrations of both peptides versus the GAPDH loading control.
Fig. 3.
Effects of SP2012 and SP5031 on vascular endothelial growth factor receptor 2 (VEGFR2) and focal adhesion kinase (FAK). a SP2012 and SP5031 block induction of VEGFR2 phosphorylation and in combination degrade total VEGFR2 in VEGF-stimulated HUVECs. b SP2012 and SP5031 block phosphorylation of focal adhesion kinase (FAK) additively and induce FAK degradation. c Downstream from VEGFR2, SP2012 and SP5031 block phosphor-ylation of pPLCγ. The peptides together show additivity in the presence of VEGF. d SP2012 and SP5031 block phosphorylation of VEGFR2 in LECs. This effect is additive at low and high doses of SP2012. e SP2012 and SP5031 block phosphorylation of FAK in LECs at low and high doses of SP2012
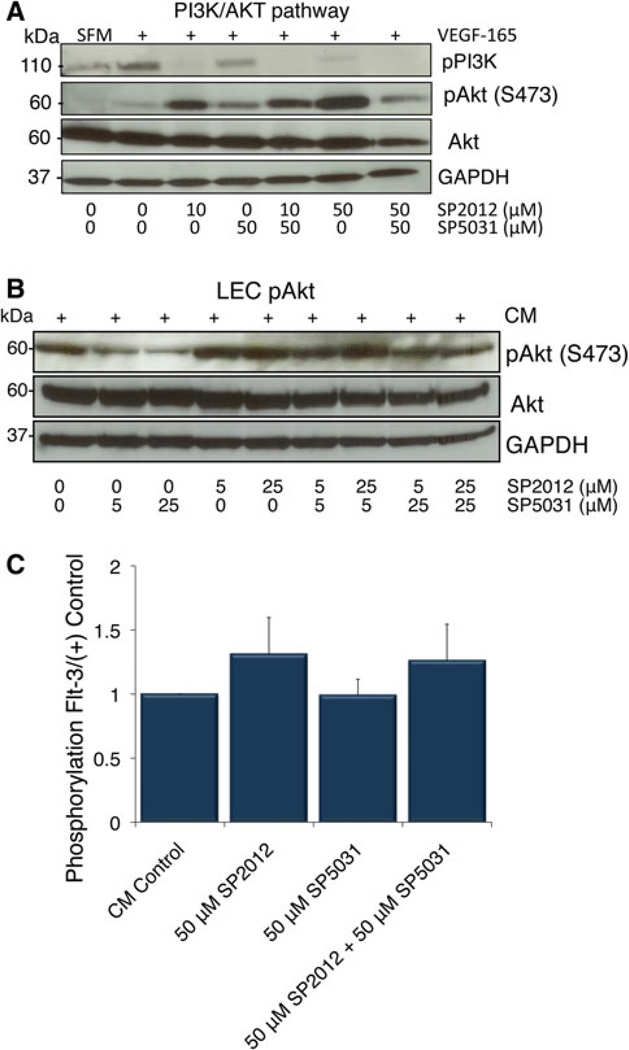
In addition to PLCγ phosphorylation, upon binding of VEGF to VEGFR2 phosphatidylinositol 3-kinase (PI3K) is phosphorylated and signals to Akt. We show in Fig. 4a PI3K phosphorylation is also blocked by SP2012, SP5031 or together in combination in the presence of VEGF. Pathway results are similar for LECs as expected based on adhesion data where SP2012 and SP5031 block VEGFR2 and FAK phosphorylation alone or together in combination (Fig. 3d, e). Experimental conditions were similar between the two cell lines, however, LECs did not exhibit any VEGFR2 or FAK total protein degradation.
Fig. 4.
SP2012 and SP5031 effects on the PI3K/Akt pathway. a SP2012 and SP5031 together inhibit phosphorylation of PI3K alone or in combination. SP2012 phosphorylates Akt at S473. Adding SP5031 attenuates the effect of SP2012 at the level of pAkt showing a compensatory effect in HUVECs. b Similarly, SP5031 blocks induction of pAkt at S473 while SP2012 slightly activates pAkt in LECs. c SP2012 activates Flt-3 but the effects are independent of SP5031. Flt-3 activation leads to activation of pAkt
SP5031 compensation of SP2012 Akt activation
As we further investigated the VEGF pathway downstream of PI3K, we found surprisingly SP2012 activates the phosphorylation of Akt at S473 shown in both HUVECs (Fig. 4a) and LECs (Fig. 4b), an atypical result for a potent angiogenesis inhibitor. Although prior results had shown SP2012 is a potent anti-angiogenic agent against HUVEC viability [26], the activation of Flt-3 by SP2012 and activation of Akt may reduce the anti-viability response. SP5031 appears to diminish Akt phosphorylation in LECs and modulate the response in both HUVECs and LECs. To explain this phenomenon we tested a reverse tyrosine kinase angiogenesis array to identify other potential target receptors and found SP2012 caused enhanced tyrosine phosphorylation of FMS-related tyrosine kinase 3 (Flt-3) upon peptide treatment (Fig. 4c). Flt-3 is a class 3-receptor tyrosine kinase, a structurally similar molecule to platelet-derived growth factor receptor (PDGFR). Upon ligand binding tyrosine residues are phosphorylated, causing cell survival and differentiation through multiple signaling molecules [32]. The effect only appears to be true for SP2012. SP5031 does not activate Flt-3 phosphorylation and in combination with SP2012, it does not reduce SP2012 induced Flt-3 phosphorylation, indicating this effect is unique only to the collagen-derived peptide. Previously published results show Flt-3 phosphorylation activates pAkt [33]. SP5031 blocks activation of Akt through inhibition of VEGFR2 phosphorylation. These results suggest in addition to direct synergistic effects on FAK and VEGFR2, SP2012, an Akt inducer, may be combined with SP5031, an Akt inhibitor, to enhance the overall therapeutic response.
In vitro capillary-like tubule formation and in vivo Matrigel plug assays
We then tested the combination of SP2012 and SP5031 in in vitro tube formation and in vivo Matrigel plug assays. For tubules and networks to form in vitro and in vivo endothelial cells must adhere, migrate and remain viable. Note that in this two-dimensional in vitro Matrigel assay, capillary lumen is not formed, thus the structures are also referred to as cords or tubules. In vitro, we show a mild response for SP2012 25 µM and SP5031 5 µM. Tubules are most fragmented and broken in HUVECs (Fig. 5a) and in LECs (Fig. 5b) when the peptides are applied in combination at tested doses. Next, we implanted Matrigel plugs with or without peptide into athymic nude mice with VEGF and heparin as the positive control. Importantly, this assay is disease-independent and the results are indicative of the potential effects in specific disease conditions. Both peptides limited the invasion of microvessels into Matrigel plugs when applied separately, however, the response is enhanced when the peptides are used in combination. FITC-dextran vessel perfusion and imaging confirmed a significant inhibition of vessel growth (**p < 0.01). Results were quantified and the combination shows improved activity over each peptide separately (Fig. 5e). Normalized FITC positive area is shown where the percent FITC-positive vessels are scaled to the positive control. Additionally, to show the anti-lymphangiogenic response we performed immunohistochemistry by staining cross sections for LYVE-1, a marker for lymphatic vessels. Figure 5f shows representative cross sections, quantified in Fig. 5g. SP2012 and SP5031 together show a significant decrease in lymphatic vessel density, statistically lower than each peptide alone (*p < 0.01).
Fig. 5.
In vitro capillary-like tubule formation assay and in vivo Matrigel Plug assay. a SP2012 and SP5031 moderately block formation of endothelial sprouts on Matrigel but show enhanced response in combination on HUVECs. Scale bars represent 200 µM. b LEC tube formation assay. SP2012 and SP5031 in combination show enhanced activity in blocking tubule formation. c Photograph of excised Matrigel plugs implanted for 10 days in nude mice. Positive (+) control contains VEGF165. SP5031, and SP2012 block formation of tubes and show additivity in combination. Scale bars represent 10 mm. d FITC-dextran fluorescent antibody staining to mark endothelial cell vessels. Plugs were excised and perfused with FITC-dextran. Scale bars represent 100 µM. e Quantification of FITC in excised plugs scaled to the VEGF (+) control. Results are statistically significant for **p < 0.01 for SP2012 25 µM and SP5031 50 µM, and show (±) standard deviation (SD) of the mean. f LYVE-1 immunohistochemical staining for lymphatic cell vasculature. SP2012 and SP5031 applied in combination significantly reduce lymphatic vessel formation (brown color). Images shown are at × 10 magnification, and scale bars represent 100 µM. g Quantification of LYVE-1 staining. The combination of SP2012 25 µM and SP5031 50 µM statistically significantly reduce lymphatic vessel formation into plugs over either compound alone (**p < 0.01, and *p < 0.05). The SD of the mean (±) is shown
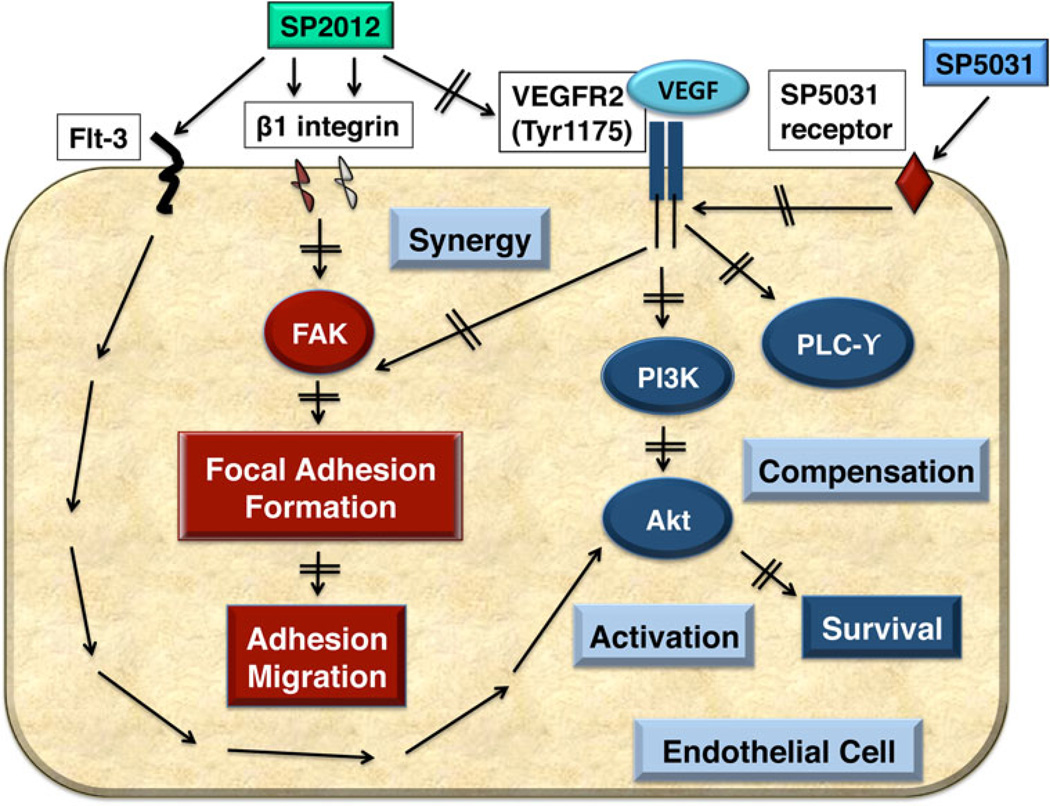
Based on the above data it is apparent there are functional molecular differences between the two sequences, SP2012 and SP5031. Both peptides are potent inhibitors of angiogenesis and lymphangiogenesis in endothelial cell adhesion and migration assays and act synergistically when applied in combination. SP2012 also causes Flt-3 activation, which may account for the activation of Akt downstream of VEGFR2. Pathway results are summarized in the Fig. 6 signaling schematic, where the peptides act synergistically at the level of VEGFR2 and FAK. SP5031 compensates for the activation of Akt by SP2012. We see similarities among HUVECs, MECs and LECs in cell adhesion assays. SP2012 and SP5031 have the additional effect of total FAK and VEGFR2 protein degradation in HUVECs, which was not apparent in LECs. In vivo, the net anti-angiogenic and anti-lymphangiogenic effects are greatest when both peptides are applied in combination, as evidenced by capillary-like tubule formation and in vivo Matrigel plug assays.
Fig. 6.
Schematic of signaling pathways blocked by SP2012 collagen IV peptide and SP5031 somatotropin peptide in combination. Both SP2012 and SP5031 modulate VEGFR2 signaling through inhibition of phosphorylation and protein turnover. FAK may be blocked directly by binding β1 integrins and through inhibition of VEGFR2 signaling. Both peptides additively block induction of PLCγ. SP2012 induces pAkt by activation of Flt3, but is compensated by an Akt inhibitor such as SP5031
Discussion
Angiogenesis is regulated by many endogenous regulatory proteins containing active epitopes and domains. We showed previously two active fragments derived from collagen IV and a transmembrane 45A somatotropin-domain containing protein are active in vitro and in vivo xenograft models. A similar somatotropin-domain derived peptide, SP5001, tested as a separate control did not show a synergistic response with SP2012. Here we show in endothelial cell adhesion and migration assays the synergistic response of SP2012 and SP5031 applied in combination. SP2012 and SP5031 block cell attachment on uncoated, fibronectin and gelatin-coated plates, but not laminin-coated plates. The capillary-like tubule formation assay in HUVECs and LECs shows the strong net anti-angiogenic and anti-lymphangiogenic effect of the peptides applied in combination. In vivo, the Matrigel plugs show a synergistic response when applied in combination as seen by FITC-dextran perfusion for blood vessels and LYVE-1 immunohistochemistry for lymphatic vessels.
We show these peptides synergize in endothelial cell adhesion and migration assays through FAK and VEGFR2 phosphorylation inhibition and total protein turnover. Previous work in our group has shown SP2012 binds to β1 integrins. Although complex, integrin receptor biology is central to endothelial cell attachment to the extracellular matrix, and is regulated by different combinations of alpha and beta integrin subunits to different matrices including fibronectin and laminin. For instance, endothelial cells attach to fibronectin primarily via α4β1 and α5β1 integrins, while they attach to laminin via α3β1, α6β1 and α6β4 integrins [34]. Since SP5031 does not block endothelial cell attachment to laminin alone or in combination but blocks adhesion in the presence of gelatin and fibronectin, more comprehensive studies to elucidate the peptide’s receptor should be explored based on these results. Furthermore, although SP2012 activates Akt through Flt-3, SP5031 attenuates this process not by directly inhibiting Flt-3 whose activation appears to be responsible for Akt activation but by inhibition by VEGFR2 phosphorylation and downstream signaling molecules, yielding a stronger net anti-angiogenic effect. Additional studies should be carried out to investigate whether SP2012 may be further combined with other Akt inhibitors or Flt-3 phosphorylation inhibitors in addition to SP5031. This study describes how combining two compounds with knowledge of their mechanisms of action can produce a therapeutic combination with enhanced activity. Optimal peptide–peptide dosing combinations, dose intervals and which disease-specific angiogenesis-based models to apply SP2012 and SP5031 treatment are further areas that should be tested and refined in subsequent studies.
Anti-angiogenic therapies have been tested extensively in clinical trials in combination with standard chemotherapeutics. Peptide-peptide combination therapies may be advantageous to these chemotherapeutic regimens or to monoclonal antibodies and small molecules due to lower toxicity and enhanced specificity. Furthermore, targeting angiogenesis through endothelial cell adhesion, migration and proliferation, in addition to lymphangiogenesis may yield the more efficacious result of limiting functional vessel formation in tumors and should be considered as a treatment strategy in angiogenesis and lymphangiogenesis-dependent diseases. As angiogenesis and lymphangiogenesis are central to metastatic cell dissemination and metastatic disease is the primary cause of death in cancer, these compounds applied in combination should be considered as treatment for metastatic disease.
Methods
Cell culture
Human Umbilical Vein Endothelial Cells (HUVECs) were purchased from Lonza (Walkersville, MD), and grown under standard conditions at 37 °C and 5 % CO2 with basal medium (EBM-2) containing a bullet kit with 2 % fetal bovine serum, growth factors, and antibiotics. All experiments were performed under cell passages 2–6.
Microvascular endothelial cells (MECs) and lymphatic endothelial cells (LECs) were also purchased from Lonza. We propagated these cells in Microvascular Endothelial Cell Medium-2 (EGM-2MV, Lonza) from passages 2–6. Cells were split at constant ratios.
Peptide synthesis and storage
A commercial provider (New England Peptide, Gardner, MA) produced the collagen IV sequence SP2012 (seq: LRRFSTMPFMF-Abu-NINNV-Abu-NF). New England Peptide provided HPLC and MS data to show >95 % purity.
SP5031 (seq: LLRSSLILLQGSWF) was produced by Bachem Peptide (Torrance, CA). HPLC and MS data were provided to show >95 % purity. All sequences tested were solubilized in 5 % DMSO and 95 % water while percent DMSO was <0.1 % on cells and had no effect on cell viability. DMSO percentage was held at parity across all experiments and conditions tested.
Adhesion assay
We measured the inhibitory potential of peptides in combination with a real-time electrical impedance RT-CIM assay from Roche/ACEA Biosciences (San Diego, CA). 25,000 cells/well were plated in 16-well E-plates (Roche, Indianapolis, IN) in the presence or absence of the peptides and allowed to adhere over 3 h. Sensors integrated into the bottom of the wells measure cell contact through changing impedance readings in real time, correlating to adhesion. Values were scaled to a negative control (no compound) containing complete endothelial cell media, and expressed as a percentage of control. We performed assays in two independent replicates, and each replicate contained two experimental wells per condition. Plates were pre-coated with fibronectin (20 µg/mL fibronectin), laminin (20 µg/mL) or gelatin (1 mg/mL) for 1 h prior to treatment where indicated.
Migration assay
Similar to the adhesion assay, we used the same RT-CIM electrical impedance system from Roche/ACEA to measure cell migration. Transwell CIM plates allowed for migration between top and bottom compartments, separated by an 8-µm microporous polycarbonate membrane (Roche, Indianapolis, IN). The membrane was coated with 20 µg/mL fibronectin, then 45,000 HUVECs/well or 100,000 LECs/well were added with or without the peptides in serum free media to the top compartment. The bottom compartment contained full endothelial cell media as chemoattractant. The assay ran for 20 h, and data shown are at the 20 h time point. Values were scaled to a negative control (no compound), with only serum free media in the top compartment and complete endothelial cell media as the chemoattractant. Sensors integrated into the bottom side of the membranes recorded impedance changes and correlated to the number of migrated cells. We performed assays in two independent replicates and each replicate contained two experimental wells per condition.
Synergy calculations
The Chou-Talalay method [25] commonly used in drug combination studies provided the standard for drug synergy in cell assays using the calculation by combination index (CI). CI < 1 indicates synergy, CI = 1 indicates additivity, and CI > 1 indicates antagonism. The CI can be expressed as:
(D)1 is the tested dose of SP2012 (1, 5 or 25 µM) and (D)2 is the tested dose of SP5031 (1, 5 or 25 µM). (Dx)1/2 = (D)m[fa/(1 – fa)]1/m where (D)m is the median-effect dose (IC50 value), m = the slope or hill coefficient, and fa is the fraction affected, in this case activity of the compound in adhesion, migration or proliferation. We used IC50 values and slope values measured for each cell type (HUVECs, LECs) for each compound (SP2012, SP5031) in each cell assay (adhesion, migration, and proliferation), calculated with GraphPad Prism 5 Software (GraphPad Software, Inc. San Diego, CA). Normalized isobolograms were constructed using the dose reduction index (DRI) for SP2012 and SP5031, defined as (DRI)1 = (DX)1/(D)1 and (DRI)2 = (DX)2/(D)2, plotted on the x- and y-axes.
Modified wound-healing assay
A modified wound-healing type assay, Oris™ Pro, was used for HUVECs to see whether the compounds inhibited cell migration after adhering (Platypus Technologies, Madison, WI). A special 96-well plate contains a biodegradable hydrogel that dissolves shortly after media is added, and cell migration commences upon dissolution of the gel. Cells were plated at 20,000 cells/well in the presence or absence of peptide, and cells allowed to migrate for 18 h. Cells were then stained with calcein AM (0.5 µg/mL) (Invitrogen, CA), and imaged with a CCD Sensicam camera in a Nikon Eclipse T-100 inverted microscope (Nikon Instruments, Inc., Meliville, NY). Scale bars shown represent 500 µM. The experiment was performed with four replicates per condition and two independent experiments were performed. Images shown are representative of each condition.
Western blotting
HUVECs and LECs were grown in complete endothelial cell media and plated in tissue culture-treated 6-well plates at 360,000 cells/well in complete media (CM) or serum free media (SFM). For CM experiments, peptide was added alone or in combination at different doses—10, 25 or 50 µM for SP2012, and 5, 25 or 50 µM for SP5031 for 90 min. VEGF was then added at 20 ng/mL (Cell Signaling Technology, Inc., Danvers, MA) for 10 min to the indicated wells. Reactions were stopped by adding cold PBS and lysis buffer (150 mM NaCl, 1 mM EDTA, 100 µL/mL Protease Inhibitors (Sigma, St. Louis, MO), 10 µL/mL Phosphatase inhibitors (Sigma) and 1 % Triton) for 2 h, and then scraped. Cell lysates were then spun at 14,000 g for 15 min to remove cell membranes and debris, separated by SDS-PAGE, and transferred to nitrocellulose blots (Invitrogen, Carlsbad, CA). We blocked for 1 h with 5 % milk and 1 % BSA in TBST and probed with antibodies of interest in milk including anti-pAkt, anti-Akt, and anti-GAPDH (Cell Signaling Technology, Inc.).
Experiments in which SFM was used had the added step of serum-starving cells for 24 h before peptide and growth factor addition. We tested similar doses of SP2012 and SP5031, and probed with 4G10 pan-tyrosine phosphorylation antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-pVEGFR2-1175, anti-VEGFR2, anti-FAK, anti-pPLCγ, anti-PLCγ, anti-pPI3K, anti-pAkt, and anti-Akt antibodies (Cell Signaling Technology, Inc.) overnight. Anti-GAPDH was used as a loading control. The next day secondary antibodies were added at 1:2,000 dilution, and protein bands detected with chemiluminescence detection reagent (GE Healthcare, United Kingdom). Blots could then be stripped and probed for additional antibodies. Experiments were repeated at least twice. Each blot shown is representative of one complete experiment.
Reverse tyrosine kinase array
To assess if any other receptor tyrosine kinases were phosphorylated or blocked with peptide treatment we used a Proteome Profiler Human phospho-RTK Array Kit (R&D Systems, Minneapolis, MN). Cell lysate was washed over the array containing spotted antibodies. Four experimental conditions were tested in HUVECs: an untreated negative control, equivalent to normal cell viability, in complete media (CM), SP2012 50 µM peptide treatment, SP5031 50 µM peptide treatment, and SP2012 50 µM + SP5031 50 µM combination. Peptide treatment was 90 min in complete media. Cells were lysed similar to western blots and developed with appropriate secondary antibodies with chemiluminescent detection reagent. The array was tested twice, and the results show the average of the two experiments. Blots were then quantified and phosphorylation changes determined with ImageJ Software.
Capillary-like tubule formation assay
To assess the effect of the collagen IV and somatotropin peptides on angiogenesis and lymphangiogenesis we used a HUVEC and LEC capillary-like tubule formation assay with Matrigel™ (BD Biosciences, San Jose, CA). Matrigel was thawed overnight at 4 °C and kept on ice over the experiment. We loaded 50 µL Matrigel into each tested well in a 96-well plate and incubated for 30 min at 37 °C to allow solidification. We then added 100 µL of cells at 15,000 cells/well, then peptide solution on top of the cells in complete endothelial cell media. The formation of capillary-like tubules could be seen after 20 h, at which point the tubules were imaged with a Nikon microscope. For HUVECs and LECs 25 µM SP2012 with 5 µM SP5031 were tested. Scale bars shown are at 200 µM.
In vivo Matrigel plug assay
A Matrigel plug assay was performed to evaluate the inhibitory effect of the peptides in angiogenesis and lymphangiogenesis in vivo. Basement Membrane Matrix (Matrigel, growth factor reduced, high concentration, LDEV-free, BD Biosciences) containing VEGF-165 (380 ng/mL) and heparin (10 unit) was mixed with or without the peptides making a total volume of 400 µL. The final concentration of the peptides was 25 µM of SP2012, 50 µM of SP5031 and their combination (25 µM of SP2012 + 50 µM of SP5031). DMSO contents were controlled to be identical within all experimental groups. The Matrigel mixtures were subcutaneously injected on both flanks on the abdominal side of athymic nude mice under anesthesia (25 % ketamine + 25 % acepromazine (vol/vol) in PBS giving a concentration of 50 mg/kg of ketamine and 5 mg/kg of acepromazine). Animals were housed and treated according to the approved animal protocol of the Institutional Care and Use Committee at Johns Hopkins Medical Institution (JHMI). After 10 days mice were euthanized and the gels were removed and weighed. Images of the gels are shown with scale bars of 10 mm. At the end of the study, two mice were infused intravenously with 200 µL of FITC (fluorescein isothiocyanate)-Dextran (20 mg/mL; Santa Cruz Biotechnology, Santa Cruz, CA) Representative sections were imaged using a Nikon microscope to show the amount of vascular invasion into the gel. Scale bars shown are at 100 µM. One hour later, selected Matrigel plugs were surgically removed and placed in 10 % formalin (BD Biosciences, San Diego, CA) for 16 h, then washed in PBS, homogenized, and quantified in a 96-well fluorescent plate reader.
Quantification of lymphatic vessels was performed with immunohistochemistry. The samples were sent to a commercial vendor (Covance Inc., Princeton NJ) for immunohistochemical staining with LYVE-1 antibodies to assess lymphatic vessels. Upon receipt of images, we quantified LYVE-1 by the FRiDA software (Johns Hopkins University, Baltimore, MD) measuring pixel intensity/4X frame. Images shown are magnified to 10× to visually show greater surface area. Scale bars are shown at 100 µM. The FITC intensity and specific antibody positive areas in immunohistochemistry were normalized by the weight of the Matrigel plugs.
Supplementary Material
Acknowledgments
This work was supported by NIH CA R01 138264, R21 CA131931, R21 CA 152473, The Safeway Foundation for Breast Cancer, The Thome Memorial Foundation and TEDCO Maryland Technology Development Corporation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10456-012-9308-7) contains supplementary material, which is available to authorized users.
Ethical Standards All experiments described herein complied with US legal and ethical standards.
Conflict of interest The authors declare no conflict of interest. ASP serves as the CSO of AsclepiX Therapeutics, LLC; the terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Contributor Information
Jacob E. Koskimaki, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA, Jkoskimaki@gmail.com; jkoskim1@jhmi.edu
Esak Lee, Department of Chemical and Biomolecular Engineering, Johns Hopkins University, 221 Maryland Hall, 3400 N. Charles St., Baltimore, MD 21218, USA.
William Chen, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA.
Corban G. Rivera, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA
Elena V. Rosca, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA
Niranjan B. Pandey, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA
Aleksander S. Popel, Department of Biomedical Engineering, School of Medicine, Johns Hopkins University, 613 Traylor Bldg, 720 Rutland Avenue, Baltimore, MD 21205, USA Department of Chemical and Biomolecular Engineering, Johns Hopkins University, 221 Maryland Hall, 3400 N. Charles St., Baltimore, MD 21218, USA; Department of Oncology and the Sidney Kimmel Comprehensive Cancer Center, School of Medicine, Johns Hopkins University, Baltimore, MD 21231, USA.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3(114) doi: 10.1126/scitranslmed.3003149. 114rv113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8(4):210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerbel RS. Issues regarding improving the impact of antiangiogenic drugs for the treatment of breast cancer. Breast. 2009;18(Suppl 3):S41–S47. doi: 10.1016/S0960-9776(09)70271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht I, Christofori G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol. 2011;55(4–5):483–494. doi: 10.1387/ijdb.103226ia. [DOI] [PubMed] [Google Scholar]
- 9.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1(3):191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannis ED, Popel AS. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc Natl Acad Sci USA. 2008;105(37):13775–13780. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karagiannis ED, Popel AS. Identification of novel short peptides derived from the alpha 4, alpha 5, and alpha 6 fibrils of type IV collagen with anti-angiogenic properties. Biochem Biophys Res Commun. 2007;354(2):434–439. doi: 10.1016/j.bbrc.2006.12.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koskimaki JE, Karagiannis ED, Rosca EV, Vesuna F, Winnard PT, Jr., Raman V, Bhujwalla ZM, Popel AS. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia. 2009;11(12):1285–1291. doi: 10.1593/neo.09620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, Popel AS. Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model. BMC Cancer. 2010;10:29. doi: 10.1186/1471-2407-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosca EV, Lal B, Koskimaki JE, Popel AS, Laterra J. Collagen IV and CXC chemokine-derived antiangiogenic peptides suppress glioma xenograft growth. Anticancer Drugs. 2012 doi: 10.1097/CAD.0b013e3283531041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosca EV, Koskimaki JE, Pandey NB, Wolff AC, Popel AS. Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer. Cancer Biol Ther. 2011;12(9):808–817. doi: 10.4161/cbt.12.9.17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rivera CG, Rosca EV, Pandey NB, Koskimaki JE, Bader JS, Popel AS. Novel peptide-specific quantitative structure-activity relationship (QSAR) analysis applied to collagen IV peptides with antiangiogenic activity. J Med Chem. 2011;54(19):6492–6500. doi: 10.1021/jm200114f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosca EV, Koskimaki JE, Pandey NB, Tamiz AP, Popel AS. Structure-activity relationship study of collagen derived anti-angiogenic biomimetic peptides. Chem Biol Drug Des. 2012 doi: 10.1111/j.1747-0285.2012.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E, Rosca EV, Pandey NB, Popel AS. Small peptides derived from somatotropin domain-containing proteins inhibit blood and lymphatic endothelial cell proliferation, migration, adhesion and tube formation. Int J Biochem Cell Biol. 2011;43(12):1812–1821. doi: 10.1016/j.biocel.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber PE, Bischof M, Jenne J, Heiland S, Peschke P, Saffrich R, Grone HJ, Debus J, Lipson KE, Abdollahi A. Trimodal cancer treatment: beneficial effects of combined antiangiogenesis, radiation, and chemotherapy. Cancer Res. 2005;65(9):3643–3655. doi: 10.1158/0008-5472.CAN-04-1668. [DOI] [PubMed] [Google Scholar]
- 20.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13(1–2):16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12(8):1101–1116. doi: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs. 2009;12(12):779–784. [PubMed] [Google Scholar]
- 23.Foy KC, Liu Z, Phillips G, Miller M, Kaumaya PT. Combination treatment with HER-2 and VEGF peptide mimics induces potent anti-tumor and anti-angiogenic responses in vitro and in vivo. J Biol Chem. 2011;286(15):13626–13637. doi: 10.1074/jbc.M110.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 26.Rosca EV, Koskimaki JE, Pandey NB, Wolff AC, Popel AS. Development of a biomimetic peptide derived from collagen IV with anti-angiogenic activity in breast cancer. Cancer Biol Ther. 2011;12(9):808–817. doi: 10.4161/cbt.12.9.17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92(13):6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem. 2004;279(37):39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63(8):610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Infusino GA, Jacobson JR. Endothelial FAK as a therapeutic target in disease. Microvasc Res. 2011 doi: 10.1016/j.mvr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong Y, Huo Y, Chen C, Zeng H, Lu X, Wei C, Ruan C, Zhang X, Hu Z, Shibuya M, Luo J. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-gamma 1- and protein kinase A-dependent pathways. J Biol Chem. 2009;284(35):23217–23224. doi: 10.1074/jbc.M109.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21(16):2555–2563. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 33.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009;12(3):81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28(10):1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.