Abstract
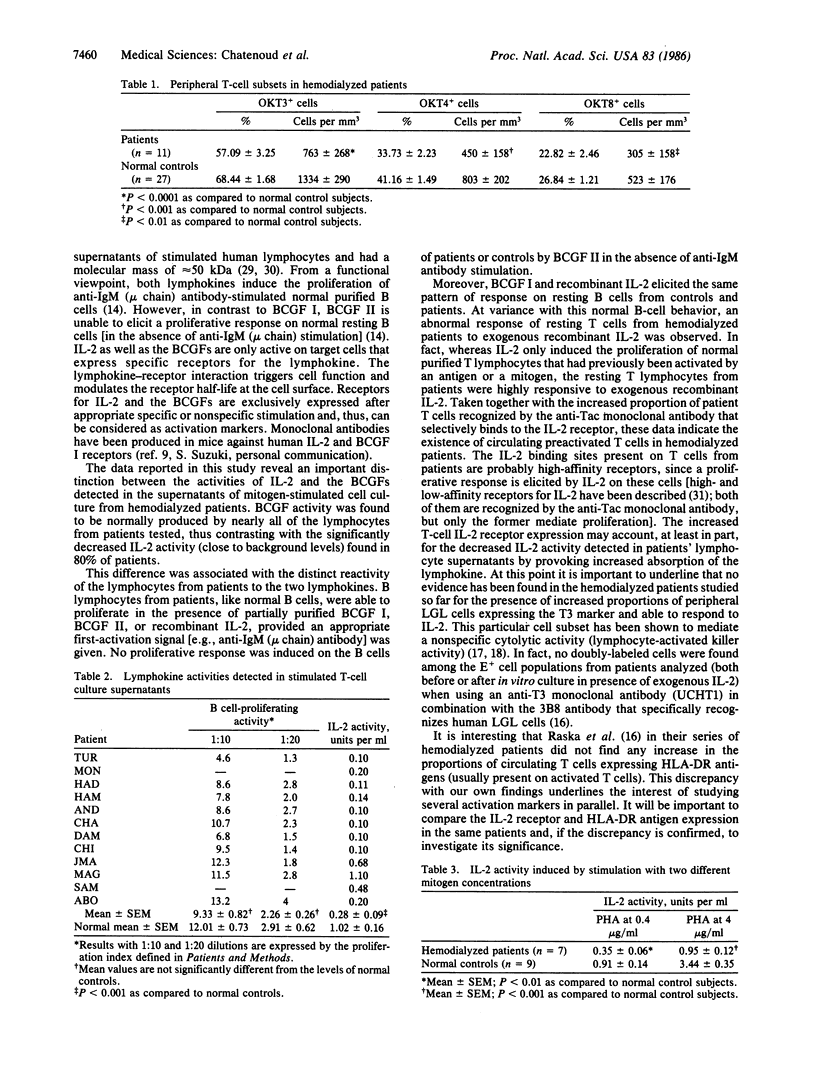
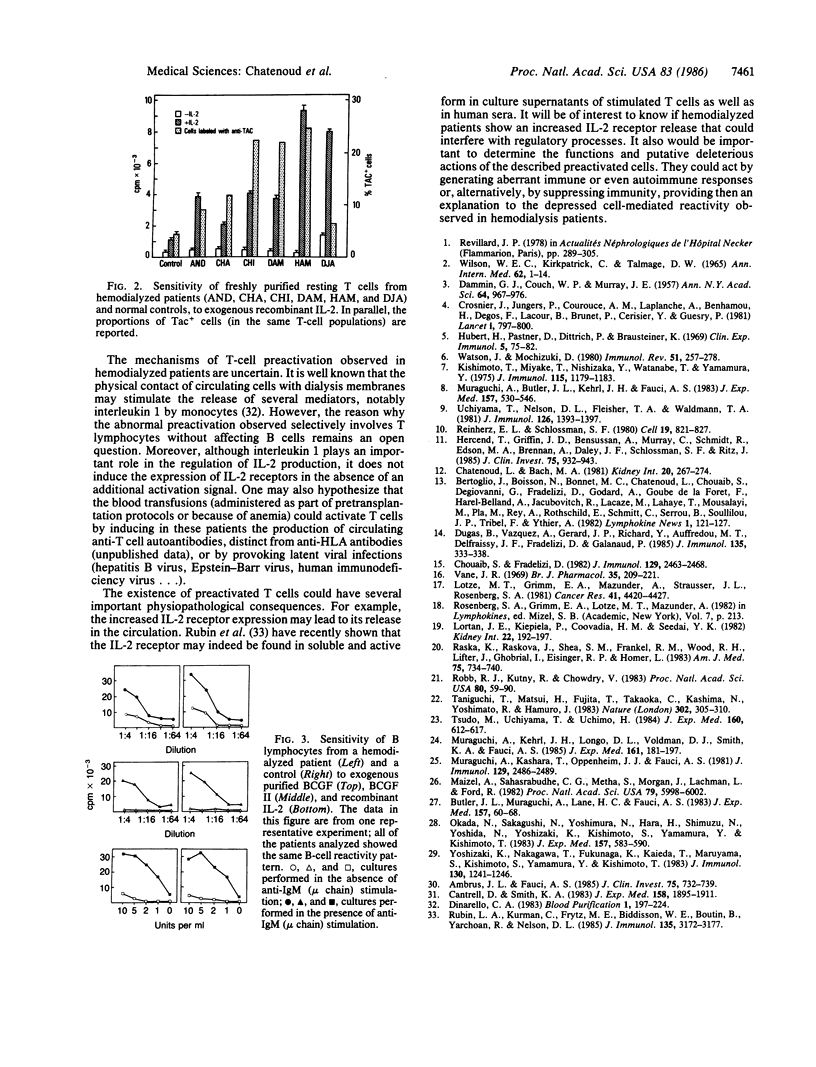
Interleukin 2 (IL-2) and B-cell growth factors I and II (BCGF I and BCGF II) are lymphokines produced by T cells that play a major role in T- and B-cell cooperation. Peripheral blood lymphocytes from 12 uremic patients undergoing intermittent hemodialysis were tested for their capacity to produce IL-2 and BCGFs and to respond to these soluble mediators. IL-2 and BCGF activities were determined by means of two biological assays (proliferation of IL-2-dependent cytotoxic T-cell line CTLL-2 and of anti-human IgM (mu chain)-stimulated normal B cells, respectively) in the supernatants of phytohemagglutinin A-stimulated T-cell cultures. IL-2 activity was significantly decreased in patients as compared to normal controls (mean +/- SEM, 0.28 +/- 0.09 unit per ml) in hemodialyzed patients versus 1.02 +/- 0.16 units per ml in normal controls). This profound abnormality contrasted with the normal activity of the BCGFs that was invariably observed in the same supernatants. A similar dissociation was detected when analyzing the sensitivity of uremic B and T cells to exogenous purified lymphokines. Anti-IgM (mu chain)-stimulated uremic B cells exhibited a normal response to recombinant IL-2 and to chromatography-purified BCGF I and BCGF II. Resting B cells did not show any increased reactivity to these lymphokines. In contrast, whereas in normal controls recombinant IL-2 exclusively induced the proliferation of T cells that had been previously activated by a mitogen, resting T cells from uremic patients were highly responsive to exogenous IL-2. This abnormal response was paralleled by significantly increased proportions of peripheral T cells recognized by the anti-Tac monoclonal antibody that specifically binds to the IL-2 receptor. These data clearly show the existence in hemodialyzed patients of abnormally high proportions of T cells presenting phenotypic and functional signs of preactivation. This increased T-cell IL-2 receptor expression may offer an explanation to the deficient IL-2 activity observed in patients' supernatants (by inducing increased absorption of the lymphokine). The potential relevance of these preactivated T cells to the depressed cell-mediated immunity observed in hemodialyzed patients is outlined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Jr, Fauci A. S. Human B lymphoma cell line producing B cell growth factor. J Clin Invest. 1985 Feb;75(2):732–739. doi: 10.1172/JCI111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. L., Muraguchi A., Lane H. C., Fauci A. S. Development of a human T-T cell hybridoma secreting B cell growth factor. J Exp Med. 1983 Jan 1;157(1):60–68. doi: 10.1084/jem.157.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L., Bach M. A. Abnormalities of T-cell subsets in glomerulonephritis and systemic lupus erythematosus. Kidney Int. 1981 Aug;20(2):267–274. doi: 10.1038/ki.1981.130. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Fradelizi D. The mechanism of inhibition of human IL 2 production. J Immunol. 1982 Dec;129(6):2463–2468. [PubMed] [Google Scholar]
- Crosnier J., Jungers P., Couroucé A. M., Laplanche A., Benhamou E., Degos F., Lacour B., Prunet P., Cerisier Y., Guesry P. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in french haemodialysis units: II, Haemodialysis patients. Lancet. 1981 Apr 11;1(8224):797–800. doi: 10.1016/s0140-6736(81)92679-9. [DOI] [PubMed] [Google Scholar]
- DAMMIN G. J., COUCH N. P., MURRAY J. E. Prolonged survival of skin homografts in uremic patients. Ann N Y Acad Sci. 1957 Mar 22;64(5):967–976. doi: 10.1111/j.1749-6632.1957.tb52488.x. [DOI] [PubMed] [Google Scholar]
- Dugas B., Vazquez A., Geŕard J. P., Richard Y., Auffredou M. T., Delfraissy J. F., Fradelizi D., Galanaud P. Functional properties of two human B cell growth factor species separated by lectin affinity column. J Immunol. 1985 Jul;135(1):333–338. [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Pastner D., Dittrich P., Braunsteiner H. In vitro reactivity of human lymphocytes in uraemia--a comparison with the impairment of delayed hypersensitivity. Clin Exp Immunol. 1969 Jul;5(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Miyake T., Nishizawa Y., Watanabe T., Yamamura Y. Triggering mechanism of B lymphocytes. I. Effect of anti-immunoglobulin and enhancing soluble factor on differentiation and proliferation of B cells. J Immunol. 1975 Nov;115(5):1179–1184. [PubMed] [Google Scholar]
- Lortan J. E., Kiepiela P., Coovadia H. M., Seedat Y. K. Suppressor cells assayed by numerical and functional tests in chronic renal failure. Kidney Int. 1982 Aug;22(2):192–197. doi: 10.1038/ki.1982.152. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Grimm E. A., Mazumder A., Strausser J. L., Rosenberg S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981 Nov;41(11 Pt 1):4420–4425. [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kasahara T., Oppenheim J. J., Fauci A. S. B cell growth factor and T cell growth factor produced by mitogen-stimulated normal human peripheral blood T lymphocytes are distinct molecules. J Immunol. 1982 Dec;129(6):2486–2489. [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Longo D. L., Volkman D. J., Smith K. A., Fauci A. S. Interleukin 2 receptors on human B cells. Implications for the role of interleukin 2 in human B cell function. J Exp Med. 1985 Jan 1;161(1):181–197. doi: 10.1084/jem.161.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M., Sakaguchi N., Yoshimura N., Hara H., Shimizu K., Yoshida N., Yoshizaki K., Kishimoto S., Yamamura Y., Kishimoto T. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med. 1983 Feb 1;157(2):583–590. doi: 10.1084/jem.157.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska K., Jr, Raskova J., Shea S. M., Frankel R. M., Wood R. H., Lifter J., Ghobrial I., Eisinger R. P., Homer L. T cell subsets and cellular immunity in end-stage renal disease. Am J Med. 1983 Nov;75(5):734–740. doi: 10.1016/0002-9343(83)90401-1. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H. Expression of Tac antigen on activated normal human B cells. J Exp Med. 1984 Aug 1;160(2):612–617. doi: 10.1084/jem.160.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Mochizuki D. Interleukin 2: a class of T cell growth factors. Immunol Rev. 1980;51:257–278. doi: 10.1111/j.1600-065x.1980.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Nakagawa T., Fukunaga K., Kaieda T., Maruyama S., Kishimoto S., Yamamura Y., Kishimoto T. Characterization of human B cell growth factor (BCGF) from cloned T cells or mitogen-stimulated T cells. J Immunol. 1983 Mar;130(3):1241–1246. [PubMed] [Google Scholar]


