Summary
Purpose
The white matter (WM) is considered critical for linking cortical processing networks necessary for cognition. The aim of this study was to assess diffusion tensor imaging (DTI) measures of regional WM in children with nonlesional localization-related epilepsy in comparison to controls, and to determine the relation between lobar WM and neuropsychological performance.
Methods
Forty children with nonlesional localization-related epilepsy and 25 healthy controls with no neurological or psychiatric disorders and normal magnetic resonance imaging (MRI) were recruited. All patients and controls underwent neuropsychological testing that evaluated intelligence, language, memory, executive function, and motor function, as well as DTI to assess regional WM measures of fractional anisotropy (FA) and mean diffusivity (MD). The regional FA and MD were compared between patients and controls, and correlated with neuropsychological function. The relations between regional FA and MD with age at seizure onset and duration of epilepsy were assessed.
Key Findings
Twenty-one patients had left-sided and 19 patients had right-sided epilepsy. There were no significant differences in seizure-related variables including age at seizure onset, duration of epilepsy, seizure frequency, and number of antiepileptic medications, as well as no significant differences in neuropsychological function and DTI measures of white matter in left-sided compared to right-sided epilepsy. Therefore, all the patients with epilepsy were treated as one group. Patients with epilepsy performed significantly worse on intelligence (p < 0.001), language (p < 0.001), and executive function (p = 0.001) evaluation than controls. Patients had significantly reduced FA in left frontal (p = 0.015), right frontal (p = 0.004), left temporal (p = 0.039), right temporal (p = 0.003), right parietal (p = 0.014), and right occipital (p = 0.025) WM relative to controls. There were no significant regional WM differences (all p > 0.05) in MD between patients and controls. There was a significant positive correlation between right temporal FA with language (r = 0.535, p < 0.001) and executive function (r = 0.617, p < 0.001), as well as between body of corpus callosum FA with intelligence (r = 0.536, p < 0.001) and language (r = 0.529, p < 0.001) in patients. Left parietal MD was significantly correlated with language (r = −0.545, p < 0.001) in patients. FA of right temporal WM was significantly associated with age at seizure onset (t = 4.97, p < 0.001).
Significance
There was widespread regional WM abnormality in children with nonlesional localization-related epilepsy, which was associated with impaired neuropsychological function. The impairment in WM may reflect disruption in the connectivity for cortical processing networks, which is necessary for the development of cognition.
Keywords: Diffusion tensor imaging, Pediatric epilepsy, Neuropsychological function
Neuropsychological impairment is an important comorbidity of epilepsy (Dodrill, 2004) and affects up to 82% of children with epilepsy (Cormack et al., 2007; Berg et al., 2008; Hoie et al., 2008). Intractable localization-related epilepsy of childhood onset can result in more generalized neuropsychological effects such as greater reduction of levels of intellectual function, memory, and executive function than would be expected from a focal epileptogenic process (Hermann et al., 2002). Although there are numerous publications on the detrimental effects of chronic intractable epilepsy on neuropsychological performance in children and adolescents (O’Leary et al., 1981, 1983), to date, there is no report on the structural correlates of neuropsychological deficits beyond brain volumetric assessment in children with epilepsy (Hermann et al., 2006).
White matter (WM) tracts are thought to play a crucial role in linking the different components of the cortical processing networks necessary for cognition (Mesulam, 1990). Hence, injury to the WM may lead to loss of “connectivity” between these cortical regions and therefore neuropsychological impairment (O’Sullivan et al., 2001). Along these lines, there is evidence to suggest that WM is vulnerable to seizure-related injury (Jorgensen et al., 1980; Dwyer & Wasterlain, 1982). Therefore, impairment in WM integrity related to epilepsy may result in reduced neuropsychological function. The integrity of the WM can be assessed with diffusion tensor imaging (DTI).
Our hypotheses were first that nonlesional intractable localization-related epilepsy is characterized by diffuse bilateral WM changes, as manifested by reduced fractional anisotropy (FA) and elevated mean diffusivity (MD); and second, FA and MD demonstrate good correlation with neuropsychological performance. The aims of this study were to assess DTI measures of regional WM in children with nonlesional localization-related epilepsy compared to controls, and to assess the relation between lobar WM and neuropsychological performance.
Methods
Subjects
This study was approved by the Hospital for Sick Children’s research ethics board, and written informed consent was obtained from parents and assent from children. Children with nonlesional localization-related epilepsy who were being worked up for epilepsy surgery were recruited into the study. Location of the epileptogenic zone was determined by ictal and interictal video–electroencephalography (EEG), magnetoencephalography (MEG), and fluorodeoxyglucose–positron emission tomography (FDG-PET) scan. All patients had normal 3T magnetic resonance imaging (MRI) with high-resolution epilepsy protocol. The control group consisted of healthy subjects with no neurologic or psychiatric disorders and were recruited through institutional website and poster publication. All control subjects had normal MRI.
Neuropsychological assessment
A comprehensive test battery assessing five domains including intelligence, language, memory, executive function, and motor function were carried out in patients and controls. Table 1 shows the details of the tests and the ability tested. A generalized neuropsychological impairment has been reported in localization-related epilepsy of childhood onset (Hermann et al., 2002). Therefore, the assessment targeted these multiple domains rather than focused on selected functions. These tests have been shown to be sensitive to cognitive deficits among children with newly diagnosed epilepsy and normal MRI scans (Hermann et al., 2008), and in children with medically refractory epilepsy (Smith et al., 2002). The tests selected allow for administration of materials across the age range included in the study.
Table 1.
Neuropsychological tests, ability tested, and domain
| Domain | Ability | Tests |
|---|---|---|
| Intelligence | Verbal | Wechsler Abbreviated Scale of Intelligence (VIQ) |
| Nonverbal | Wechsler Abbreviated Scale of Intelligence (PIQ) | |
| Language | Confrontation naming | Boston Naming Test |
| Expressive naming | Expressive Vocabulary Test-2 | |
| Receptive language | Peabody Picture Vocabulary Test-IV | |
| Memory | Verbal memory | Children’s Auditory Verbal Learning Test-II: Word list immediate recall |
| Children’s Auditory Verbal Learning Test-II: Word list delayed recall | ||
| Nonverbal memory | Children’s Memory Scale (Face recognition immediate recall) | |
| Children’s Memory Scale (Face recognition delayed recall) | ||
| Executive function | Divided attention | Delis–Kaplan Executive Function System (Verbal fluency – Category switching accuracy) |
| Response inhibition | Delis–Kaplan Executive Function System (Color-word interference test – inhibition; seconds) | |
| Problem Solving | Delis–Kaplan Executive Function System (Sorting test – confirmed correct sorts) | |
| Mental flexibility | Delis–Kaplan Executive Function System (Trail Making Test; seconds) | |
| Generative naming | Delis–Kaplan Executive Function System (verbal fluency) | |
| Inattentiveness | Conners’ Continuous Performance Test-II (omission errors) | |
| Motor function | Speeded fine motor dexterity | Grooved Pegboard (seconds) |
| Psychomotor speed | Wechsler Intelligence Scale for Children-IV (Coding) |
MRI and DTI
MRI was performed on Philips 3T scanner (Achieva, Philips Medical System, Best, The Netherlands) using an 8-channel phased array head coil in all patients and controls. The imaging in patients consisted of axial and coronal fluid-attenuated inversion recovery (FLAIR) (repetition time/echo time [TR/TE] = 10,000/140 msec, slice thickness = 3 mm, field of view [FOV] = 22 cm, matrix = 316 × 290), T2 and PD (TR/TE = 4,200/80/40 msec, slice thickness = 3 mm, FOV = 22 cm, matrix = 400 × 272), volumetric three-dimensional (3D) T1 (TR/TE = 4.9/2.3 msec, slice thickness = 1 mm, FOV = 22 cm, matrix = 220 × 220) and DTI (TR/TE = 10,000/60 msec, slice thickness = 2 mm, field of view = 22 cm, matrix = 112 × 112, number of excitation = 1, sensitivity encoding factor of 2, b = 1,000 s/mm2, 32 noncollinear directions). The imaging in controls included axial FLAIR, T2 and PD, volumetric 3D T1 and DTI, using the same parameters as those for patients.
DTI images were processed using the functional MRI brain (FMRIB) Software Library (FSL), version 4.1 (www.fmrib.ox.ac.uk/fsl; Smith et al., 2004). Briefly, raw DTI images were corrected for eddy current distortions (Jenkinson & Smith, 2001); then a diffusion tensor model was fitted to the data at each voxel (Behrens et al., 2003). Measurements of FA and MD were calculated. Each child’s T1-weighted image was classified into gray matter (GM), WM, and cerebrospinal fluid (CSF) using a priori child probability map (Wilke et al., 2003) and an automated tissue segmentation algorithm (FSL-FMRIB’s Automated Segmentation Tool [FSL-FAST] [Zhang et al., 2001]). Next, the T1-weighted scans were transformed into DTI space using a series of linear and nonlinear algorithms (Woods et al., 1998a,b) to allow region of interest (ROI) analysis using a predefined anatomic template (Kabani et al., 2002). The template, modified by Mabbott et al. (2009), consisting of bilateral frontal, temporal, parietal, and occipital lobes, was applied to each subject’s WM segmentation via affine registration (Woods et al., 1998a,b), which was then transformed into DTI space (Fig. 1). In addition to hemispheric lobar data, the corpus callosum was manually traced using Analyze 10.0 (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN, U.S.A.). Each subjects’ high-resolution T1 was transformed into stereotaxic space, and the midsagittal slice was identified. Next, the corpus callosum was manually traced in the sagittal plane and subdivided into three equal parts denoting the genu, body and splenium. FA and MD were calculated for each ROI.
Figure 1.
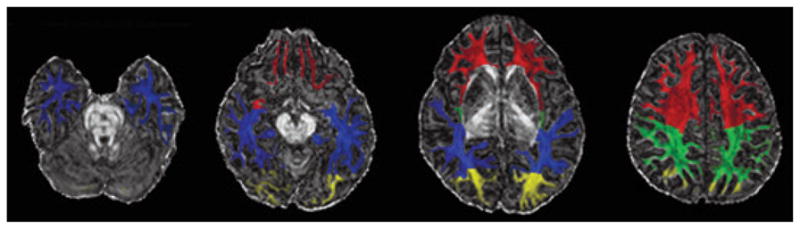
Illustration of regional white matter mask overlaid on fractional anisotropy map. Blue, temporal white matter; red, frontal white matter; green, parietal white matter; yellow, occipital white matter.
Epilepsia © ILAE
Statistical analysis
Statistical analysis was done using SAS version 9.3 (SAS Institute Inc, Cary, NC, U.S.A.). The baseline characteristics of right localization-related epilepsy compared to left localization-related epilepsy patients, and all patients with epilepsy compared to controls were done using t-tests for continuous data, and chi-square test for nominal data.
The raw neuropsychological test scores were first converted to z-scores, and a composite score for each of the five neuropsychological domains was then computed using principal component analysis. This data reduction process served to reduce the number of comparisons and therefore reduced type I error. The composite scores of neuropsychological domains of patients with left-sided epilepsy were compared with those with right-sided epilepsy using t-tests. If there were no differences in the scores between these patients, then the scores of all patients would be compared to controls using t-tests. A subgroup analysis of patients with frontal lobe epilepsy (FLE) was done by comparing the composite scores of these patients to those of controls using t-tests; the FLE sample was the only patient subgroup large enough to permit these comparisons.
The FA and MD of regional WM (frontal, temporal, parietal, occipital, and corpus callosum) were compared between patients with right-sided and left-sided epilepsy using multivariate analysis of variance (MANOVA). If there were no differences in the DTI measures of WM between right-sided and left-sided epilepsy, then the FA and MD of regional WM of all patients would be compared to controls using MANOVAs. MANOVAs were also used for subgroup analyses of FA and MD of regional WM comparing FLE patients and controls.
The FA and MD of regional WM were then correlated with neuropsychological function using Pearson correlation, with p < 0.001 considered as statistically significant. Correlations of the mean FA and MD of regional WM with neuropsychological function were also conducted in the subgroup with FLE. The FA and MD of lobar WM were regressed against clinical seizure parameters (age of seizure onset and duration of epilepsy). A p < 0.001 was considered statistically significant.
Results
Forty children with nonlesional localization-related epilepsy and 25 healthy controls were recruited. The mean age of patients was 13.5 years (range 7.0–17.4 years; standard deviation [SD] 2.9) and the mean age of controls was 14.2 years (range 8.7–18.7 years; SD 2.7; Table 2). There was no significant difference between the age of the patients and controls (p = 0.299). The mean age of seizure onset was 8.1 years (SD 3.8), mean duration of epilepsy was 5.2 years (SD 3.2), mean number of seizures per week was 12.2 (SD 22.4), and mean number of antiepileptic drugs was 2.2 (SD 0.7). Twenty-one patients had seizures arising from the left hemisphere: 11 in the left frontal lobe, 6 in the left temporal lobe, and 4 were multilobar. Nineteen patients had seizures arising from the right hemisphere: 11 in right frontal lobe, 3 in the right temporal lobe, 1 in the right parietal lobe, and 4 were multilobar. There were no significant differences in age, sex, mean age of seizure onset, duration of epilepsy, seizure frequency, and number of antiepileptic drugs between patients with right-sided and left-sided epilepsy (all p > 0.05).
Table 2.
Characteristics of patients and controls
| Left epilepsy (n = 21) | Right epilepsy (n = 19) | Controls (n = 25) | |
|---|---|---|---|
| Mean age (years) (SD) | 13.4 (2.8) | 13.5 (3.0) | 14.2 (2.7) |
| Sex (M:F) | 11:10 | 6:13 | 14:11 |
| Mean age at seizure onset (years) (SD) | 7.7 (3.4) | 8.6 (4.2) | |
| Mean duration of epilepsy (years) (SD) | 5.4 (3.5) | 4.8 (2.9) | |
| Mean seizure frequency per week (SD) | 11.6 (17.7) | 12.7 (27.1) | |
| Mean number of medications (SD) | 2.2 (0.7) | 2.1 (0.7) | |
| Location of epilepsy | L frontal (11); L temporal (6); | R frontal (11); R temporal (3); | |
| L frontotemporal (3); | R frontotemporal (1); R parietal (1); | ||
| L frontoparietal (1) | R parietooccipital (1); R hemisphere (2) |
SD, standard deviation.
Neuropsychological Tests
The mean raw test scores for patients and controls are shown in Table 3. Patients performed significantly worse on measures of intelligence, language, executive function, and the psychomotor speed component of motor function. There were no significant differences between patients and controls on all raw test scores of memory and speeded fine motor dexterity (p > 0.05).
Table 3.
Raw scores for neuropsychological tests
| Domain | Tests | Patients (n = 40) | Controls (n = 25) | p-Value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Intelligence | Wechsler Abbreviated Scale of Intelligence (VIQ) | 41.0 (12.4) | 55.0 (7.8) | <0.001 |
| Wechsler Abbreviated Scale of Intelligence (PIQ) | 21.4 (7.7) | 26.9 (3.8) | <0.001 | |
| Language | Boston Naming Test | 43.0 (7.9) | 49.3 (6.9) | 0.002 |
| Expressive Vocabulary Test-2 | 122.7 (22.1) | 147.6 (22.8) | <0.001 | |
| Peabody Picture Vocabulary Test –IV | 167.4 (22.2) | 191.7 (20.4) | 0.001 | |
| Memory | Children’s Auditory Verbal Learning Test-II: Word list immediate recall | 10.4 (3.4) | 11.4 (2.8) | 0.188 |
| Children’s Auditory Verbal Learning Test-II: Word list delayed recall | 10.0 (3.9) | 11.4 (2.8) | 0.115 | |
| Children’s Memory Scale (Face recognition immediate recall) | 36.2 (5.9) | 38.2 (4.5) | 0.149 | |
| Children’s Memory Scale (Face recognition delayed recall) | 34.8 (5.7) | 37.4 (5.5) | 0.082 | |
| Executive function | Delis–Kaplan Executive Function System (Verbal fluency – Category switching accuracy) | 10.7 (3.4) | 13.4 (3.3) | 0.003 |
| Delis–Kaplan Executive Function System (Color-word interference test – inhibition; seconds) | 76.8 (36.6) | 59.0 (18.0) | 0.010 | |
| Delis–Kaplan Executive Function System (Sorting test – confirmed correct sorts) | 8.0 (2.3) | 9.7 (1.8) | 0.003 | |
| Delis–Kaplan Executive Function System (verbal fluency) | 26.5 (13.1) | 38.3 (10.8) | <0.001 | |
| Conners’ Continuous Performance Test-II (omission errors) | 21.2 (23.2) | 7.0 (7.7) | 0.002 | |
| Motor function | Grooved Pegboard – dominant hand (seconds) | 84.6 (63.6) | 65.2 (12.1) | 0.138 |
| Grooved Pegboard – nondominant hand (seconds) | 98.9 (76.0) | 70.0 (14.7) | 0.065 | |
| Wechsler Intelligence Scale for Children-IV (Coding) | 46.5 (17.7) | 64.4 (20.7) | <0.001 |
SD, standard deviation.
Principal component analysis explained 0.897 of the variation in intelligence domain, 0.913 of the variation in language domain, 0.714 of the variation in memory domain, 0.630 of the variance in executive function domain, and 0.737 of the variation in motor domain (Table S1).
There were no significant differences in intelligence (p = 0.717), language (p = 0.581), executive function (p = 0.641), memory (p = 0.885), and motor function (p = 0.493) between patients with right- and left-sided epilepsy. Overall patients performed significantly worse than controls on intelligence (p < 0.001), language (p < 0.001), and executive function (p = 0.001; Fig. 2). There was a trend for poorer performance in motor function (p = 0.018) in patients relative to controls. There was no significant difference in memory performance (p = 0.073) in patients relative to controls. Subgroup analysis showed FLE patients performed significantly worse than controls on intelligence (p < 0.001), language (p < 0.001), and executive function (p < 0.001), and a trend for poorer performance in memory (p = 0.044) and motor function (p = 0.025).
Figure 2.
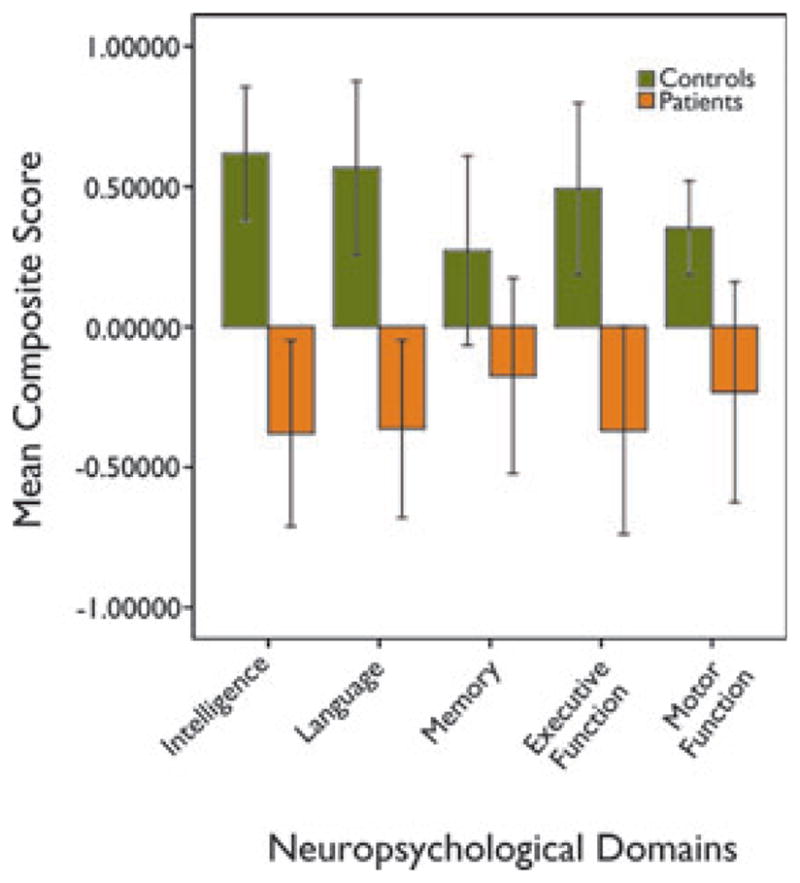
Bar chart showing mean composite scores of intelligence, language, memory, executive function, and motor function of patients and controls. Whiskers show 95% confidence interval of the composite scores.
Epilepsia © ILAE
Diffusion tensor imaging
There were no significant differences in overall FA (F = 0.415, p = 0.936), and also no significant regional differences in FA (all p > 0.05) between patients with right-and left-sided epilepsy. There were significant group differences in FA between all patients and controls (F = 2.03, p = 0.037). Patients with epilepsy had significantly reduced FA in the left frontal (p = 0.015), right frontal (p = 0.004), left temporal (p = 0.039), right temporal (p = 0.003), right parietal (p = 0.014), and right occipital (p = 0.025) WM relative to controls (Fig. S1). There were no significant differences in FA in the left parietal and left occipital WM, genu, body, and splenium of corpus callosum in patients relative to controls. There were no significant overall group differences (F = 1.27, p = 0.261), and also no significant regional differences (all p > 0.05) in MD between patients and controls (Fig. S2).
In the subgroup with FLE, there was no significant overall difference in FA between patients and controls (F = 1.99, p = 0.0610), but FLE patients had significant reduction in FA in the left frontal (p = 0.028), right frontal (p = 0.009), right temporal (p = 0.029), right parietal (p = 0.009), genu (p = 0.012), body (p = 0.009), and splenium of corpus callosum (p = 0.044). There were no significant overall differences (F = 1.03, p = 0.444) or regional differences in MD between FLE patients and controls (all p > 0.05).
Relation between DTI parameters and neuropsychological domains
There was a significant positive correlation between right temporal FA and language (r = 0.535, p < 0.001) and executive function (r = 0.617, p < 0.001), as well as between the body of corpus callosum FA and intelligence (r = 0.536, p < 0.001) and language (r = 0.529, p < 0.001) in patients (Table S2). There were weaker correlations between right and left frontal, left temporal, right parietal, body of corpus callosum FA, and executive function (r = 0.500–0.542, all p value between 0.001 and 0.005), as well as between right temporal FA and intelligence (r = 0.465, p < 0.005) in patients. In the subgroup with FLE, there was a weak positive correlation between body of corpus callosum FA and executive function (r = 0.660, p = 0.004).
There was a significant negative correlation between left parietal MD and language (r = −0.545, p < 0.001) in patients. There was a weaker negative correlation between left parietal MD and intelligence (r = −0.478, p = 0.003); as well as between right and left frontal and temporal MD, right parietal MD, and language (r = −0.485 to −0.496, all p-value between 0.001 and 0.005) in patients. In the subgroup with FLE, there was a weak negative correlation between left frontal MD (r = −0.640, p = 0.002), left temporal MD (r = −0.667, p = 0.001), left parietal MD (r = −0.617, p = 0.003), and language.
There was a weak correlation between right parietal FA and language (r = 0.540, p = 0.004) in controls. There was no significant correlation between either lobar FA or MD with neuropsychological function in controls.
Relation between DTI and clinical seizure parameters
There was a significant relation between FA of right temporal WM with age at seizure onset (t = 4.97, p < 0.001). The relations between FA of left frontal (t = 3.27, p = 0.002), right frontal (t = 3.40, p = 0.002), left temporal (t = 3.21, p = 0.003), left parietal (t = 3.225, p = 0.003), and right parietal (t = 3.053, p = 0.004) WM and body of corpus callosum (t = 3.378, p = 0.002) with age at seizure onset were weak. There was a weak relation between FA of right temporal WM (t = 3.593, p = 0.001) and body of corpus callosum (t = 3.246, p = 0.003) with duration of epilepsy. Similarly, the relations between MD of left frontal (t = −3.504, p = 0.001), right frontal (t = −3.309, p = 0.002), right temporal (t = −3.245, p = 0.003), left parietal (t = −3.577, p = 0.001), right parietal (t = −2.895, p = 0.006), and right occipital (t = −2.814, p = 0.008) WM with age at seizure onset were also weak. No relation was detected between lobar MD and duration of epilepsy (all p > 0.05).
Discussion
We found that patients with nonlesional localization-related epilepsy performed worse than healthy subjects in multiple domains of neuropsychological performance including intelligence, language, and executive function. Patients had reduced FA in multiple lobes including bilateral frontal, bilateral temporal, right parietal, and right occipital WM relative to healthy controls. There was a significant correlation between reduced right temporal FA and impaired language and executive function, as well as between reduced body of corpus callosum FA and decreased intelligence and language in patients. Although there was no significant difference in MD between patients and controls, there was a significant correlation between elevated left parietal MD and impaired language in patients. Neither FA nor MD was correlated with neuropsychological function in controls.
Considerable neuropsychological impairment has been reported in children and adolescents with chronic epilepsy (Farwell et al., 1985; Schoenfeld et al., 1999; Smith et al., 2002; Germano et al., 2005). Impairment in a wide range of cognitive functions including intelligence, language, attention, executive function, and psychomotor speed was also evident in children with newly diagnosed epilepsy (Oostrom et al., 2003; Hermann et al., 2006), including children with localization-related epilepsy (Oostrom et al., 2003; Hermann et al., 2006). Childhood-onset localization-related epilepsy has been associated with more generalized cognitive effects than would be expected from a focal epileptogenic process (Hermann et al., 1997; Oyegbile et al., 2004a, b). A wide range of cognitive deficits including intelligence, language, executive function, and memory has been reported in childhood-onset temporal lobe epilepsy compared to more specific deficits seen in adult-onset temporal lobe epilepsy (Hermann et al., 2002). We have found similar findings in that, relative to controls, children with localization-related epilepsy have widespread cognitive impairment involving intelligence, language, and executive function, including the subgroup with FLE.
We found widespread abnormal WM in both cerebral hemispheres in children with localization-related epilepsy, including the subgroup with FLE. The WM may be vulnerable to seizure-related changes in the pediatric brain. Dwyer and Wasterlain (1982) have examined the effects of electro-convulsive-induced seizures in rats at different developmental stages. They reported that seizures occurring early in development selectively impaired myelin accumulation out of proportion to their overall effect on brain growth. Hermann et al. (2002) examined patients with childhood-onset chronic temporal lobe epilepsy and found greater reduction in WM volume compared to GM volume. During normal development in children and adolescents, there are greater age-related linear increases in WM volumes compared to GM volumes as demonstrated by in vivo quantitative MR (Pfefferbaum et al., 1994; Giedd et al., 1996; Courchesne et al., 2000; De Bellis et al., 2001; Paus et al., 2001). The rapid growth of the WM in childhood may predispose the WM to seizure-related changes.
The widespread WM changes in patients with localization-related epilepsy may be related to spread of seizures. The kainate model of epilepsy in rats has shown widespread DTI abnormalities (Sierra et al., 2011), involving dorsal endopiriform nucleus, external capsule, corpus callosum, dentate gyrus, thalamus, optic tract, horizontal limb of the diagonal band, stria medullaris, habenula, entorhinal cortex, and superior colliculus. Histology targeted to the abnormal FA showed altered myelination, neurodegeneration, and/or calcification of the tissue. DTI abnormalities have also been reported outside the hippocampus in temporal lobe epilepsy, including the internal capsule, external capsule, corpus callosum, fornix, and cingulum (Arfanakis et al., 2002; Concha et al., 2005; Thivard et al., 2005; Gross et al., 2006; Focke et al., 2008). These findings appear to implicate a larger dysfunctional network, reaching well beyond the seizure focus in chronic unilateral epilepsy (Thivard et al., 2005; Nilsson et al., 2008). The treatment implication of the widespread WM impairment remains to be elucidated. Further study is needed to clarify if this widespread impairment in WM is predictive of poor neuropsychological outcome following epilepsy surgery.
Previous studies in adults with epilepsy have found a relation between impaired WM integrity and neuropsychological function. In one study of temporal lobe epilepsy, tractography of the parahippocampal gyrus demonstrated that reduced FA in left parahippocampal gyrus, and FA in the left and right parahippocampal gyri correlated with verbal and nonverbal memory, respectively (Yogarajah et al., 2008). McDonald et al. (2008) found that reduced FA and elevated MD of multiple fiber tracts (including uncinate fasciculus, arcuate fasciculus, parahippocampal cingulum, and inferior frontooccipital fasciculus) were related to verbal memory and naming performances but not with nonverbal memory or fluency. Diehl et al. (2008) evaluated the uncinate fasciculus of patients with temporal lobe epilepsy and found reduced FA of right uncinate fasciculus was correlated with visual delayed memory, whereas MD of left uncinate fasciculus was negatively correlated with auditory immediate and delayed memory. O’Muircheartaigh et al. (2011) found that reduced FA in the WM of supplementary motor area predicted word naming tasks and expression scores, and reduced FA in posterior cingulate predicted cognitive inhibitions scores on mental flexibility task in adults with juvenile myoclonic epilepsy. A more recent study in adults with nonlesional localization-related epilepsy found that IQ and cognitive impairment were strongly associated with clustering and path lengths as assessed using WM connectivity network (Vaessen et al., 2012). To our knowledge, the present study is the first to assess the relation between WM integrity in children with nonlesional localization-related epilepsy and neuropsychological function. Children with nonlesional localization-related epilepsy demonstrated a significant correlation between reduced right temporal FA and impaired language and executive function, between reduced body of corpus callosum FA and decreased intelligence and language, and between elevated left parietal MD and impaired language. The impaired WM integrity did not appear to be tightly linked to specific cognitive abilities, for instance poor language function was related to impaired WM integrity in right temporal, body of corpus callosum, and right parietal WM. This may be related to using lobar WM rather than specific white matter tracts, and using composite scores of neuropsychological function rather than specific neuropsychological abilities. The association between impaired language and reduced right temporal FA could be related to the role of the right anterior temporal lobe in language. Early in life, both left and right hemispheres contribute to language processing, with an increase in left hemisphere contribution with increasing age (Holland et al., 2001; Brown et al., 2005; Ressel et al., 2008; Kadis et al., 2011). It may be that the disturbance in networks for language processing in children with epilepsy alters the timing or degree of the contribution of right hemisphere structures for language. There is also evidence that the right temporal lobe plays a role in certain aspects of language such as semantic processing, object naming (Lambon Ralph et al., 2001), and language comprehension (Crinion & Price, 2005).
The severity of abnormal WM was related to age at seizure onset, with greater reduction in FA associated with earlier age at seizure onset. Riley et al. (2010) have also found that lower FA in the posterior corpus callosum was significantly correlated to earlier age at seizure onset but not epilepsy duration. In addition, animal studies have demonstrated repeated electroconvulsive-induced seizures in the immature rat resulted in reduced brain growth, an effect found to be dependent on the developmental stage of the animals when seizures were induced, being more severe when seizures occurred at younger ages (Wasterlain & Plum, 1973; Jorgensen et al., 1980). These data suggest that the immature brain may be more susceptible to changes related to recurrent seizures. The relation between abnormal WM and duration of epilepsy was weaker.
We have examined children with a variety of seizures. The heterogeneity in the seizure semiology of our patient population reflects the heterogeneity of the clinical population of pediatric patients being evaluated for epilepsy surgery. However, within our patient population, there was a greater predominance of FLE; hence, the subgroup analyses were conducted. We found that patients with FLE have widespread reduced FA involving the frontal and extrafrontal WM, including bilateral frontal, right temporal, right parietal, and genu, body, and splenium of corpus callosum. We also found a trend for correlation between FA of the body of corpus callosum and executive function, and between MD of the left frontal, left temporal, and left parietal WM with language in patients with FLE. These findings confirm that there are widespread effects of a focal epileptogenic process on structural and cognitive function in pediatric epilepsy.
In summary, we found widespread abnormal WM in children with nonlesional localization-related epilepsy, which was associated with impaired neuropsychological function. Impaired WM integrity may be related to the direct effect of seizures. Alternatively, the data may reflect an underlying structural abnormality that is responsible for both the seizures and the neuropsychological deficits, or a combination of these factors. The impairment in WM integrity observed in our cohort of children with nonlesional localization-related epilepsy may reflect disruption in the connectivity for cortical processing networks, which is necessary for the development of cognition.
Supplementary Material
Boxplot of regional fractional anisotropy of patients with epilepsy relative to controls. The box representing 25–75th percentile and the bar representing 50th percentile. Patients had significantly reduced FA (*) in left and right frontal and temporal white matter, right parietal, and right occipital white matter relative to controls.
Boxplot of regional mean diffusivity of patients with epilepsy relative to controls. The box representing 25th to 75th percentile and the bar representing 50th percentile. There was no significant reduction in mean diffusivity in patients relative to controls.
Table S1. Principal components analysis of neuropsychological domains.
Table S2. Correlation of neuropsychological function versus fractional anisotropy and mean diffusivity of white matter in patients with localization-related epilepsy.
Acknowledgments
We would like to thank Rahim Moineddin from University of Toronto for his assistance in statistical analysis.
Footnotes
Disclosure
This work was supported by Sickkids Foundation/CIHR Institute of Human Development, Child and Youth Health. None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, Kulas J. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57:188–196. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- Cormack F, Cross JH, Isaacs E, Harkness W, Wright I, Vargha-Khadem F, Baldeweg T. The development of intellectual abilities in pediatric temporal lobe epilepsy. Epilepsia. 2007;48:201–204. doi: 10.1111/j.1528-1167.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–2871. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–S24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Dwyer BE, Wasterlain CG. Electroconvulsive seizures in the immature rat adversely affect myelin accumulation. Exp Neurol. 1982;78:616–628. doi: 10.1016/0014-4886(82)90079-6. [DOI] [PubMed] [Google Scholar]
- Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–737. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Germano E, Gagliano A, Magazu A, Sferro C, Calarese T, Mannarino E, Calamoneri F. Benign childhood epilepsy with occipital paroxysms: neuropsychological findings. Epilepsy Res. 2005;64:137–150. doi: 10.1016/j.eplepsyres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–376. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth R, Ruggles K, Wendt G, O’Leary D, Magnotta V. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43:1062–1071. doi: 10.1046/j.1528-1157.2002.49901.x. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Jones JE, Sheth R, Koehn M, Becker T, Fine J, Allen CA, Seidenberg M. Growing up with epilepsy: a two-year investigation of cognitive development in children with new onset epilepsy. Epilepsia. 2008;49:1847–1858. doi: 10.1111/j.1528-1167.2008.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoie B, Sommerfelt K, Waaler PE, Alsaker FD, Skeidsvoll H, Mykletun A. The combined burden of cognitive, executive function, and psychosocial problems in children with epilepsy: a population-based study. Dev Med Child Neurol. 2008;50:530–536. doi: 10.1111/j.1469-8749.2008.03015.x. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen OS, Dwyer B, Wasterlain CG. Synaptic proteins after electroconvulsive seizures in immature rats. J Neurochem. 1980;35:1235–1237. doi: 10.1111/j.1471-4159.1980.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Kabani NJ, Sled JG, Chertkow H. Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer’s type. Neuroimage. 2002;15:604–610. doi: 10.1006/nimg.2001.0992. [DOI] [PubMed] [Google Scholar]
- Kadis DS, Pang EW, Mills T, Taylor MJ, McAndrews MP, Smith ML. Characterizing the normal developmental trajectory of expressive language lateralization using magnetoencephalography. J Int Neuropsychol Soc. 2011;17:896–904. doi: 10.1017/S1355617711000932. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC, III, Raybaud CR, Widjaja E. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008;81:128–135. doi: 10.1016/j.eplepsyres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Seidenberg M, Berent S, Boll TJ. Effects of age of onset of tonic-clonic seizures on neuropsychological performance in children. Epilepsia. 1981;22:197–204. doi: 10.1111/j.1528-1157.1981.tb04102.x. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Lovell MR, Sackellares JC, Berent S, Giordani B, Seidenberg M, Boll TJ. Effects of age of onset of partial and generalized seizures on neuropsychological performance in children. J Nerv Ment Dis. 1983;171:624–629. doi: 10.1097/00005053-198310000-00006. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P, Duncan JS, Koepp MJ, Richardson MP. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology. 2011;76:34–40. doi: 10.1212/WNL.0b013e318203e93d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”–a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–1344. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oyegbile T, Hansen R, Magnotta V, O’Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004a;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004b;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Ressel V, Wilke M, Lidzba K, Lutzenberger W, Krageloh-Mann I. Increases in language lateralization in normal children as observed using magnetoencephalography. Brain Lang. 2008;106:167–176. doi: 10.1016/j.bandl.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–545. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, Hermann B. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–731. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- Sierra A, Laitinen T, Lehtimaki K, Rieppo L, Pitkanen A, Grohn O. Diffusion tensor MRI with tract-based spatial statistics and histology reveals undiscovered lesioned areas in kainate model of epilepsy in rat. Brain Struct Funct. 2011;216:123–135. doi: 10.1007/s00429-010-0299-0. [DOI] [PubMed] [Google Scholar]
- Smith ML, Elliott IM, Lach L. Cognitive skills in children with intractable epilepsy: comparison of surgical and nonsurgical candidates. Epilepsia. 2002;43:631–637. doi: 10.1046/j.1528-1157.2002.26101.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, Baulac M, Dupont S. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JF, Vlooswijk MC, Hofman PA, Majoie HJ, Aldenkamp AP, Backes WH. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex. 2012;22:2139–2147. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Plum F. Vulnerability of developing rat brain to electroconvulsive seizures. Arch Neurol. 1973;29:38–45. doi: 10.1001/archneur.1973.00490250056006. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Normative pediatric brain data for spatial normalization and segmentation differs from standard adult data. Magn Reson Med. 2003;50:749–757. doi: 10.1002/mrm.10606. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Powell HW, Parker GJ, Alexander DC, Thompson PJ, Symms MR, Boulby P, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Neuroimage. 2008;40:1755–1764. doi: 10.1016/j.neuroimage.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Boxplot of regional fractional anisotropy of patients with epilepsy relative to controls. The box representing 25–75th percentile and the bar representing 50th percentile. Patients had significantly reduced FA (*) in left and right frontal and temporal white matter, right parietal, and right occipital white matter relative to controls.
Boxplot of regional mean diffusivity of patients with epilepsy relative to controls. The box representing 25th to 75th percentile and the bar representing 50th percentile. There was no significant reduction in mean diffusivity in patients relative to controls.
Table S1. Principal components analysis of neuropsychological domains.
Table S2. Correlation of neuropsychological function versus fractional anisotropy and mean diffusivity of white matter in patients with localization-related epilepsy.


