Abstract
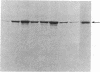
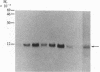
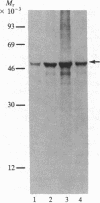
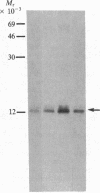
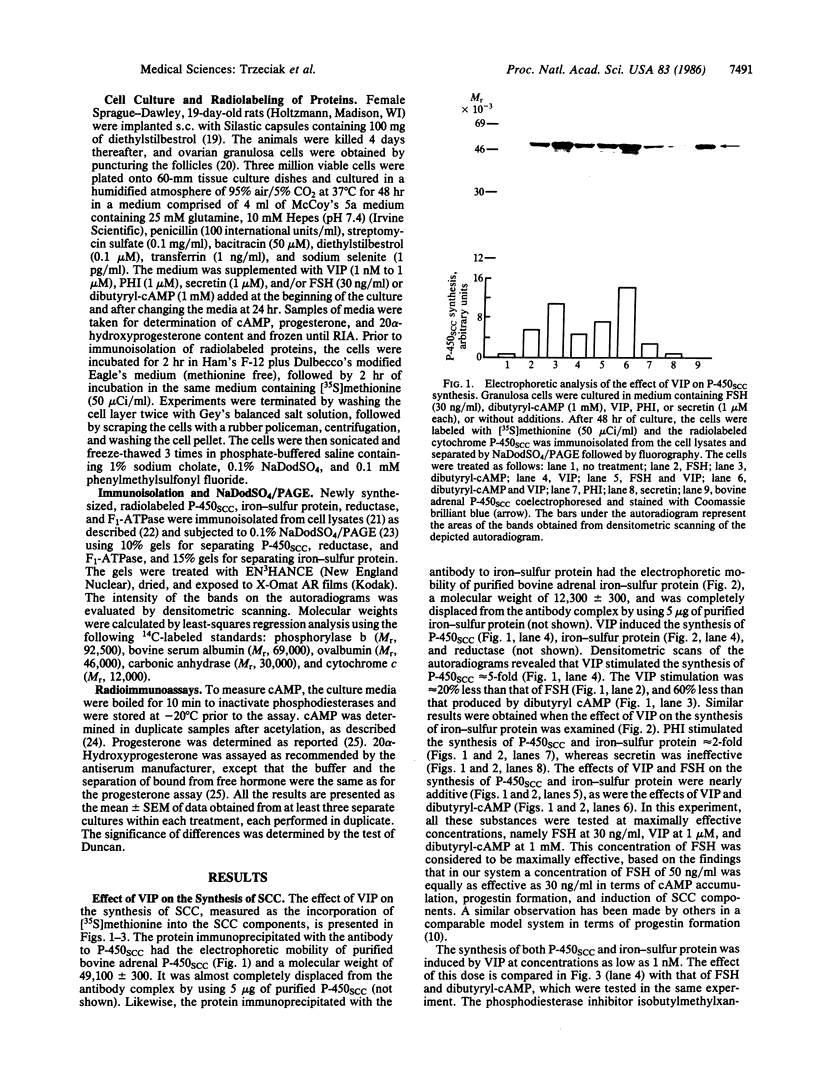
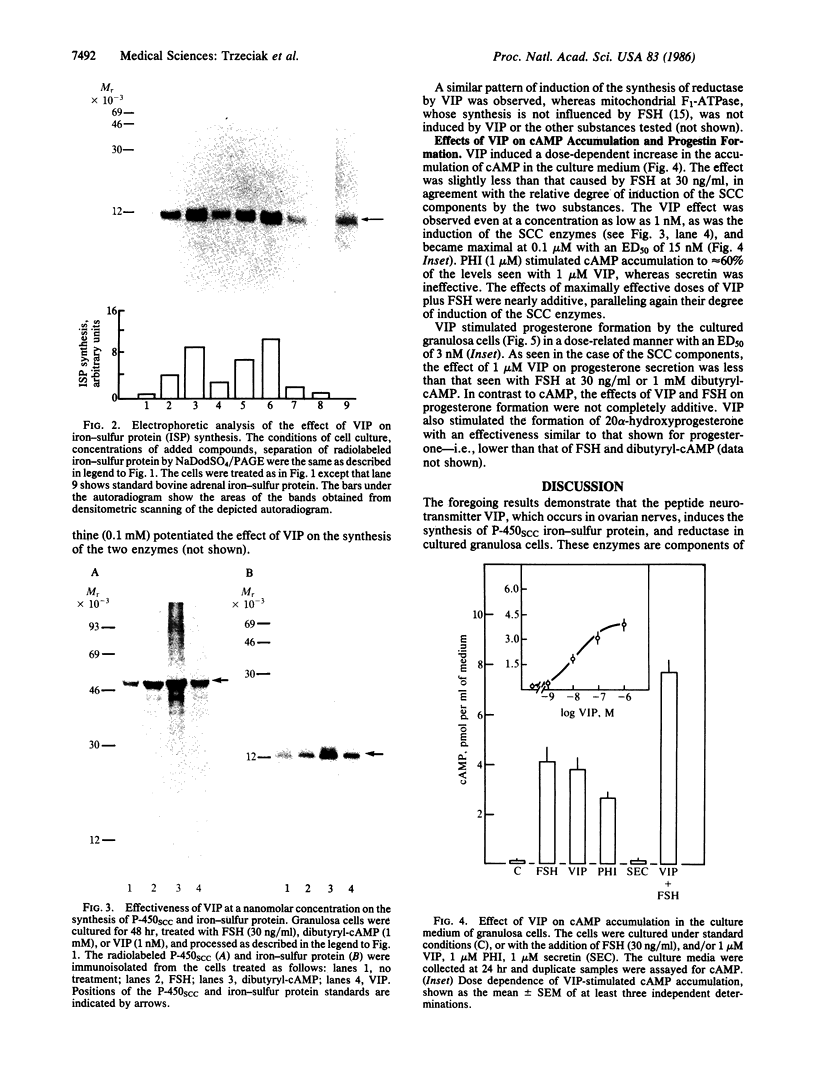
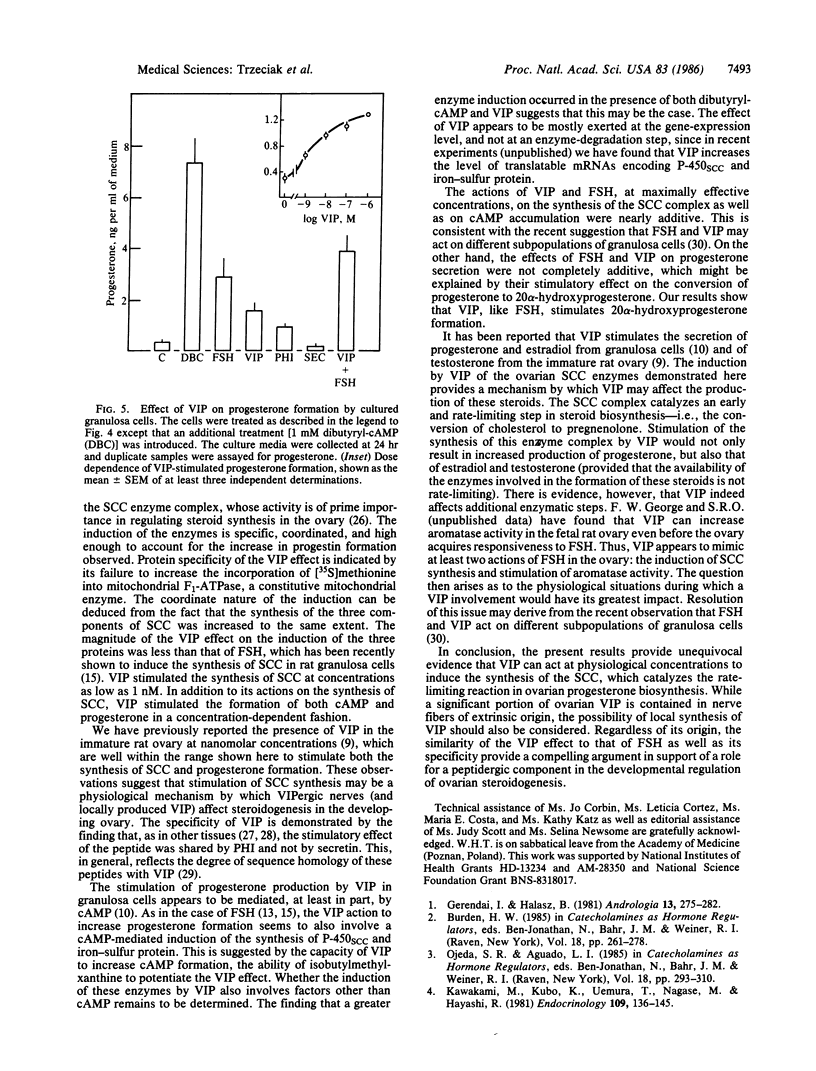
Vasoactive intestinal peptide (VIP) has been identified in ovarian nerves and stimulates steroid secretion from immature ovaries. To gain insight into its mechanism of action, the effect of VIP on the synthesis of the cholesterol side-chain cleavage enzyme complex was studied in ovarian granulosa cells from immature estrogen-primed rats. The cells were cultured for 48 hr in serum-free medium; the proteins were labeled with [35S]methionine; and the synthesis of cytochrome P-450, iron-sulfur protein, and NADPH:iron-sulfur protein reductase was evaluated by electrophoretic analysis after immunoisolation with polyclonal antibodies directed against the bovine adrenal enzymes. VIP at concentrations ranging from 0.001 to 1 microM stimulated 3- to 5-fold the synthesis of cytochrome P-450 and iron-sulfur protein. Peptide NH2-terminal histidine, COOH-terminal isoleucine, which has greater than 50% sequence homology of VIP, stimulated the synthesis of both proteins at approximately 50% of VIP effectiveness. Secretin, another member of the glucagon-secretin family of peptides, which has only 30% sequence homology to VIP, was without effect. Similar results were observed with the NADPH:iron-sulfur protein reductase. VIP-induced synthesis of the cholesterol side-chain cleavage enzyme complex was accompanied by a dose-related increase in cAMP accumulation and progestin formation. It is concluded that VIP regulates the synthesis of the ovarian cholesterol side-chain cleavage enzyme complex, which catalyzes the rate-limiting reaction in progesterone biosynthesis, and that the VIP effect is at least partially mediated through cAMP. It is suggested that a stimulatory action of VIP on the synthesis of ovarian progesterone may contribute to regulating the functional development of the ovary.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Advis J. P., Ojeda S. R. Hyperprolactinemia-induced precocious puberty in the female rat: ovarian site of action. Endocrinology. 1978 Sep;103(3):924–935. doi: 10.1210/endo-103-3-924. [DOI] [PubMed] [Google Scholar]
- Aguado L. I., Ojeda S. R. Prepubertal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 1984 May;114(5):1845–1853. doi: 10.1210/endo-114-5-1845. [DOI] [PubMed] [Google Scholar]
- Ahmed C. E., Dees W. L., Ojeda S. R. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986 Apr;118(4):1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Ledwitz-Rigby F. Methods for assessing hormone-mediated differentiation of ovarian cells in culture and in short-term incubations. Methods Enzymol. 1975;39:183–230. doi: 10.1016/s0076-6879(75)39020-4. [DOI] [PubMed] [Google Scholar]
- Davoren J. B., Hsueh A. J. Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol Reprod. 1985 Aug;33(1):37–52. doi: 10.1095/biolreprod33.1.37. [DOI] [PubMed] [Google Scholar]
- Dees W. L., Kozlowski G. P., Dey R., Ojeda S. R. Evidence for the existence of substance P in the prepubertal rat ovary. II. Immunocytochemical localization. Biol Reprod. 1985 Sep;33(2):471–476. doi: 10.1095/biolreprod33.2.471. [DOI] [PubMed] [Google Scholar]
- Fredericks C. M., Lundquist L. E., Mathur R. S., Ashton S. H., Landgrebe S. C. Effects of vasoactive intestinal polypeptide upon ovarian steroids, ovum transport and fertility in the rabbit. Biol Reprod. 1983 Jun;28(5):1052–1060. doi: 10.1095/biolreprod28.5.1052. [DOI] [PubMed] [Google Scholar]
- Funkenstein B., Waterman M. R., Masters B. S., Simpson E. R. Evidence for the presence of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin in fresh granulosa cells. Effects of follicle-stimulating hormone and cyclic AMP on cholesterol side chain cleavage cytochrome P-450 synthesis and activity. J Biol Chem. 1983 Aug 25;258(16):10187–10191. [PubMed] [Google Scholar]
- Funkenstein B., Waterman M. R., Simpson E. R. Induction of synthesis of cholesterol side chain cleavage cytochrome P-450 and adrenodoxin by follicle-stimulating hormone, 8-bromo-cyclic AMP, and low density lipoprotein in cultured bovine granulosa cells. J Biol Chem. 1984 Jul 10;259(13):8572–8577. [PubMed] [Google Scholar]
- Gerendai I., Halasz B. Participation of a pure neuronal mechanism in the control of gonadal functions. Andrologia. 1981 Jul-Aug;13(4):275–282. doi: 10.1111/j.1439-0272.1981.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Kaku K., Kaneko T., Yanaihara N., Kanno T. Vasoactive intestinal peptide binding to specific receptors on rat parotid acinar cells induces amylase secretion accompanied by intracellular accumulation of cyclic adenosine 3'-5'-monophosphate. Endocrinology. 1985 Feb;116(2):686–692. doi: 10.1210/endo-116-2-686. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Hsueh A. J. Pregnenolone biosynthesis by cultured rat granulosa cells: modulation by follicle-stimulating hormone and gonadotropin-releasing hormone. Endocrinology. 1982 Sep;111(3):713–721. doi: 10.1210/endo-111-3-713. [DOI] [PubMed] [Google Scholar]
- Kasson B. G., Meidan R., Davoren J. B., Hsueh A. J. Identification of subpopulations of rat granulosa cells: sedimentation properties and hormonal responsiveness. Endocrinology. 1985 Sep;117(3):1027–1034. doi: 10.1210/endo-117-3-1027. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Kubo K., Uemura T., Nagase M., Hayashi R. Involvement of ovarian innervation in steroid secretion. Endocrinology. 1981 Jul;109(1):136–145. doi: 10.1210/endo-109-1-136. [DOI] [PubMed] [Google Scholar]
- Kramer R. E., Du Bois R. N., Simpson E. R., Anderson C. M., Kashiwagi K., Lambeth J. D., Jefcoate C. R., Waterman M. R. Cell-free synthesis of precursor forms of mitochondrial steroid hydroxylase enzymes of the bovine adrenal cortex. Arch Biochem Biophys. 1982 May;215(2):478–485. doi: 10.1016/0003-9861(82)90106-0. [DOI] [PubMed] [Google Scholar]
- Laburthe M., Prieto J. C., Amiranoff B., Dupont C., Hui Bon Hoa D., Rosselin G. Interaction of vasoactive intestinal peptide with isolated intestinal epithelial cells from rat. 2. Characterization and structural requirements of the stimulatory effect of vasoactive intestinal peptide on production of adenosine 3':5'-monophosphate. Eur J Biochem. 1979 May 15;96(2):239–248. doi: 10.1111/j.1432-1033.1979.tb13034.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky de Muckadell O. B. Vasoactive intestinal polypeptide occurs in nerves of the female genitourinary tract. Science. 1977 Sep 30;197(4311):1374–1375. doi: 10.1126/science.897673. [DOI] [PubMed] [Google Scholar]
- Matocha M. F., Waterman M. R. Discriminatory processing of the precursor forms of cytochrome P-450scc and adrenodoxin by adrenocortical and heart mitochondria. J Biol Chem. 1984 Jul 10;259(13):8672–8678. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Chemical determination of polypeptide hormones. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4115–4119. doi: 10.1073/pnas.75.9.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciak W. H., Waterman M. R., Simpson E. R. Synthesis of the cholesterol side-chain cleavage enzymes in cultured rat ovarian granulosa cells: induction by follicle-stimulating hormone and dibutyryl adenosine 3',5'-monophosphate. Endocrinology. 1986 Jul;119(1):323–330. doi: 10.1210/endo-119-1-323. [DOI] [PubMed] [Google Scholar]
- Welsh T. H., Jr, Zhuang L. Z., Hsueh A. J. Estrogen augmentation of gonadotropin-stimulated progestin biosynthesis in cultured rat granulosa cells. Endocrinology. 1983 Jun;112(6):1916–1924. doi: 10.1210/endo-112-6-1916. [DOI] [PubMed] [Google Scholar]