Abstract
HIV-related fatigue remains the most troubling complaint of seropositive people. Researchers often use tools to measure fatigue that were developed for other patient populations; thus, the measurement of fatigue specific to HIV is needed. This article describes results from the HIV-Related Fatigue Scale (HRFS) including: (a) the variability in intensity and chronicity of HIV-related fatigue, (b) the circumstances surrounding changes in fatigue, (c) the impact of fatigue on activities of daily living (ADLs), and (d) the consequences of HIV-related fatigue. We collected data every 3 months over a 3-year period from 128 people. HIV-related fatigue was chronic and did not appear to remit spontaneously; those who were the most fatigued at the beginning of the study remained the most fatigued over 3 years. Fatigue interfered more with instrumental activities of daily living than basic ADLs; it also interfered with work, family, and social life. Stress and depression increased fatigue.
Keywords: HIV, fatigue, measurement
Introduction
Thirty years into the AIDS epidemic, fatigue remains the most frequent and debilitating complaint of HIV-infected people (e.g., Dacosta Dibonaventura, Gupta, Cho, & Mrus, 2012; Holzemer, 2002; Jong et al., 2010; Justice, Rabeneck, Hays, Wu, & Bozzette, 1999; Paddison, Fricchione, Ghandi, & Freudenreich, 2009; Sullivan & Dworkin, 2003). While there have been several studies examining factors related to fatigue in HIV-infected individuals, advancing research in this area has been hampered in part by a lack of consistent measurement of fatigue as the primary variable of interest. We described in 2002 some of the measurement problems: simple dichotomous measurement of the presence or absence of fatigue; measurement of the intensity of fatigue only; or measurement of the effects of fatigue on some other variable, such as employment (Barroso & Lynn, 2002). None of the existing fatigue measures were developed for HIV-infected individuals, and most were developed specifically for other diseases, such as cancer, rheumatoid arthritis, or multiple sclerosis. The features of fatigue that are relevant in these illnesses are very different than those relevant in HIV infection. Finally, most were very brief—four questions or less (Barroso & Lynn, 2002).
Need for a New Instrument to Measure Fatigue in HIV Infection
Extensive review of measurement of fatigue in people who are HIV-infected and completion of a qualitative study that obtained a detailed description of HIV-related fatigue (Barroso, 2001) made it clear that there was a need for an instrument that would measure the relevant features of fatigue as it is experienced by HIV-infected people. Thus, the first author developed the HIV-Related Fatigue Scale (HRFS). Details about its development and initial psychometric testing are reported in Barroso & Lynn (2002). We knew that we had to clarify the important conceptual domains relative to HIV-related fatigue prior to being able to measure it; we decided that we needed to measure the intensity of fatigue, its chronicity, the consequences of fatigue, and the circumstances surrounding its worsening or improvement. Measurement of intensity would help assess the degree to which a person was experiencing fatigue, and would also help us know if an intervention was effective. Measuring the chronicity of fatigue helps us to determine a trajectory that would be useful in knowing when we could intervene most effectively. Measuring the consequences of fatigue helps us to understand which aspects of a person's life are most adversely impacted by fatigue, which helps us know how to direct interventions. Finally, measuring the circumstances of fatigue helps us know the patterns of fatigue, which can be most useful in helping patients plan around such a symptom, and to help us see if there are clusters of patients who have similar aggravating and alleviating factors; this may help in the development of self-care measures (Barroso & Lynn, 2002).
Development of the HIV-Related Fatigue Scale
The 56-item HRFS (Barroso & Lynn, 2002) was developed from five existing scales (with these authors' permission). Several additional items were developed based on our qualitative work. The following three broad areas were measured: (a) intensity of fatigue (8 items), (b) fatigue-related impairment of functioning (22 items), and (c) responsiveness of fatigue to circumstances (15 items). The remaining items are descriptive, and will be described in detail in this article. The fatigue-related impairment of functioning items can be further divided into three subcategories: impairment of activities of daily living (12 items), impairment of socialization (6 items), and impairment of mental functioning (4 items, Pence, Barroso, Leserman, Harmon, & Salahuddin, 2008). All scales are coded from 1 to 10, with higher numbers indicating more fatigue, more impairment, or more responsiveness. Regarding reliability of the HRFS, all scales and subscales demonstrated acceptable internal consistency. Values for Cronbach's α were high for the main scales of fatigue intensity (0.93), fatigue-related impairment of functioning (0.97), and responsiveness of fatigue to circumstances (0.91), and for the subscales of the fatigue-related impairment of functioning scale: impairment of ADL (0.95), impairment of socialization (0.93), and impairment of mental functioning (0.92). The HRFS scales also demonstrated satisfactory convergent validity when compared to other fatigue instruments measuring similar constructs. The HRFS was moderately correlated with quality of nighttime sleep but showed only weak correlations with daytime sleepiness, which was expected, as sleepiness is conceptually distinct from fatigue. The scales were also moderately correlated with general mental and physical health as measured by the SF-36 Health Survey (for a detailed examination of the psychometric properties of the HRFS, see Pence et al., 2008).
While this article is reporting primarily the detailed results of the HRFS data, we have reported other findings from this study. Harmon et al. (2008) reported the demographic and illness-related characteristics of the sample; Pence et al. (2008) reported the psychometric properties of the HRFS; Barroso et al. (2008) reported the physiological correlates of fatigue; Leserman et al. (2008) reported the psychosocial correlates of fatigue; Salahuddin et al. (2009) examined HIV-related fatigue, daytime sleepiness, and impaired nighttime sleep; Pence et al. (2009) reported data about chro-nicity and remission of fatigue in this sample; and Barroso et al. (2010) reported the effects of examining both physiological and psychosocial data together in determining factors associated with increases in fatigue.
Method
Participants were recruited by posting flyers advertising the study at clinics serving persons with HIV infection in a southern U.S. state, as well as the primary AIDS service organization in this community. Interested individuals were invited to participate if they met the following inclusion criteria: (a) HIV-positive, (b) ≥ 21 years old, (c) able to read and speak English competently, and (d) mentally competent to provide reliable data. Both fatigued and nonfatigued individuals were eligible to enroll. Individuals were excluded if they had a comorbid condition marked by fatigue such as renal disease, cancer, or multiple sclerosis. Pregnant women and women less than 12 months postpartum were also excluded, as fatigue is a prominent feature of both pregnancy and the postpartum period. The PI screened all potential participants via telephone. All study procedures were approved by a university Institutional Review Board and all study participants provided written informed consent. The sample is described in Table 1.
Table 1.
Description of Sample.
| Characteristic | N (%) or Median (IQR)* |
|---|---|
| Demographic | |
| Age, years (range: 26-66) | 44 (38-48) |
| Male gender | 84 (65.6%) |
| Race: | |
| African American | 84 (65.6%) |
| Caucasian | 39 (30.5%) |
| Other | 5 (3.9%) |
| Years of schooling (range: 4-20) | 12 (12-14) |
| Employed part/full time | 42 (32.8%) |
| Monthly income, $ (range: 0-6,000) | 685 (501-1,300) |
| Clinical | |
| Years since HIV diagnosis (range: 0-25) | 10 (6-15) |
| On any antiretroviral therapy | 105 (82.0%) |
| CD4 count, cells/mm3 (range: 29-1755) | 457 (268-670) |
| HIV RNA viral load < 400 copies/mL | 87 (68.0%) |
| Fatigue course | |
| Fatigued at baseline | 111 (88.1%) |
| Experienced remission of fatigue during follow-up** | 11 (10.7%) |
Note:
IQR = interquartile range (25th-75th percentile).
Of 103 participants fatigued at baseline and with at least one follow-up visit.
The sample (n = 128) was predominantly comprised of persons who had lived with HIV infection for many years, with a median time since diagnosis of 10 years. Most participants (82%) were on antiretroviral therapy at baseline. Many (65%) reported at least one other comorbid chronic illness. The majority of the 128 participants were African American (66%), and 66% of the subjects were male. Most participants were in their late 30s or 40s (median age = 44 years). A majority of the participants were unemployed at baseline (67%, Harmon et al., 2008).
These participants had seven study visits over a 3-year period; there was a baseline visit and then visits every 6 months. At each study visit, they completed a large number of questionnaires for measurement of psychoso-cial variables, and had blood drawn for measurement of physiological variables; they completed the HRFS at each study visit. Then, at 3-month intervals in between study visits, the HRFS was mailed to them to complete and return to us in order for us to more closely track changes in fatigue over the 3-year study period.
Results
We report here the results of a detailed, item by item analysis of the items of the HRFS, completed by participants every 3 months for 3 years. Descriptive information is presented on the proportion of responses in the most severe range on each question (≥ 7 for questions scored on a 1-10 scale). To draw on the full richness of the data, we report the proportion of responses in the most severe range out of all surveys collected from all participants (1,379 surveys from 128 participants over 13 rounds of data collection). Thus, the figures illustrate the responses that reveal the greatest adverse impact of fatigue, and each item represents 1,379 responses to that item over a 3-year period.
The first set of questions refers to fatigue intensity. Overall, 39.5% of surveys indicated that fatigue had caused the respondent distress, 53.8% indicated that the respondent was easily fatigued, and 55.7% indicated that that fatigue caused frequent problems for the respondent. Of all surveys, 61.6% indicated that the respondent had often been fatigued in the week prior to data collection.
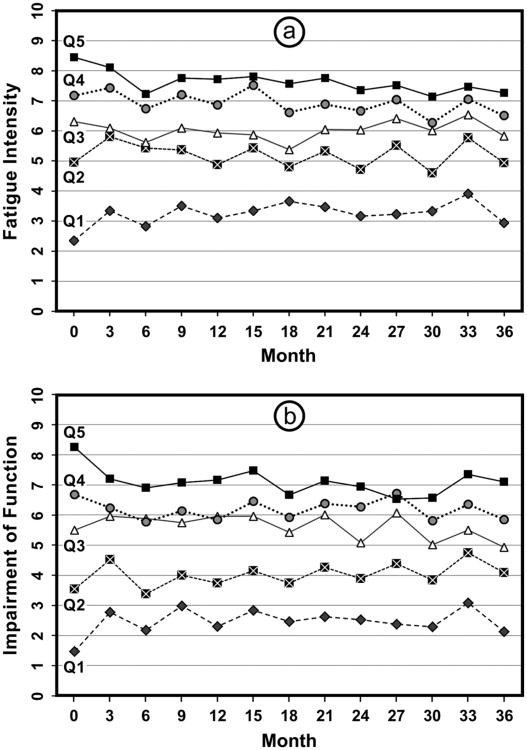
We were particularly intrigued by the data about chronicity of fatigue. We hypothesized going into the study that participants would exhibit quite a bit of variability in their fatigue, being more fatigued at some visits than others. This was based on findings—our own and others—that fatigue was unpredictable and that it was difficult to determine patterns. However, there was remarkably little convergence between the most and least fatigued over the 36 months of the study (see Figures 1a and b). The participants with the highest quintile of fatigue at study entry remained the most fatigued over time, with a slight improvement in the first 6 months but virtually no change thereafter. This is important to know with regard to targeting interventions. The same pattern was discovered for fatigue-related impairment of functioning: the participants in the highest quintile of impairment at baseline remained the most impaired, with little convergence over time between the most and least impaired.
Figure 1.
A: Fatigue intensity by quartile over 36 months; B: Fatigue-related impairment of functioning by quartile over 36 months.
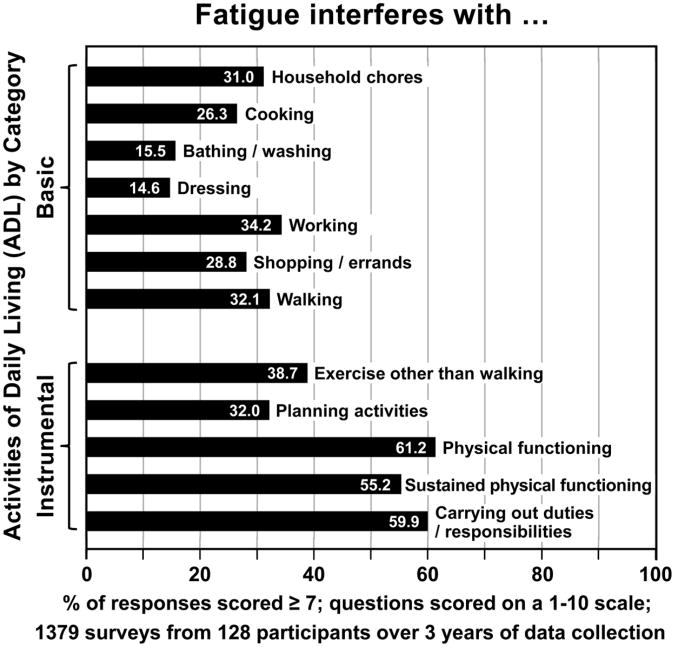
For all of the data described below, the number in parentheses refers to the percentage of surveys with scores in the most severe range. With regard to impairment of functioning, fatigue interfered with the participants' abilities to complete the following activities of daily living: dressing (14.6%), bathing/washing (15.5%), cooking (26.3%), shopping/errands (28.8%), household chores (31.0%), planning activities (32.0%), walking (32.1%), working (34.2%), exercise other than walking (38.7%), sustained physical functioning (61.2%), carrying out duties/responsibilities (59.9%), and physical functioning (61.2%, see Figure 2).
Figure 2.
The impact of fatigue on basic and instrumental activities of daily living.
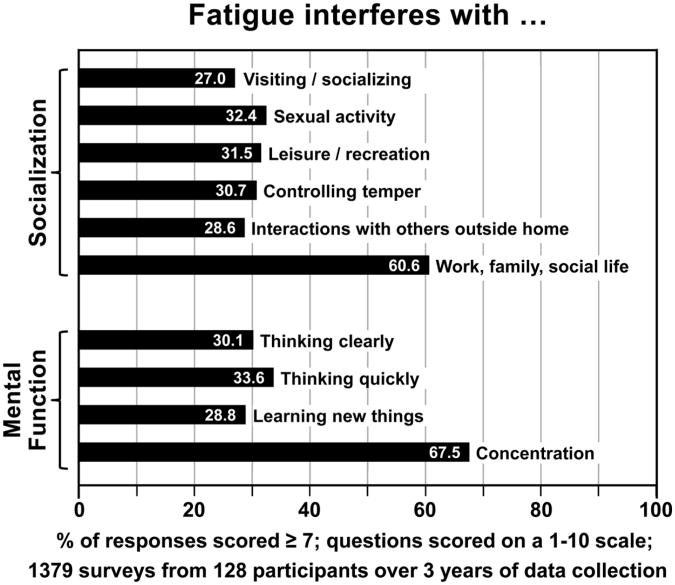
With regard to impairment of socialization, fatigue interfered with visiting/ socializing (27.0%), interactions with others outside the home (28.6%), controlling their temper (30.7%), leisure/recreation (31.5%), sexual activity (32.4%), and work, family, and social life (60.6%). Impaired mental functioning is another outcome of HIV-related fatigue; those who are most fatigued reported problems with learning new things (28.8%), thinking clearly (30.1%), thinking quickly (33.6%), and concentration (67.5%, see Figure 3).
Figure 3.
The impact of fatigue on socialization and mental functioning.
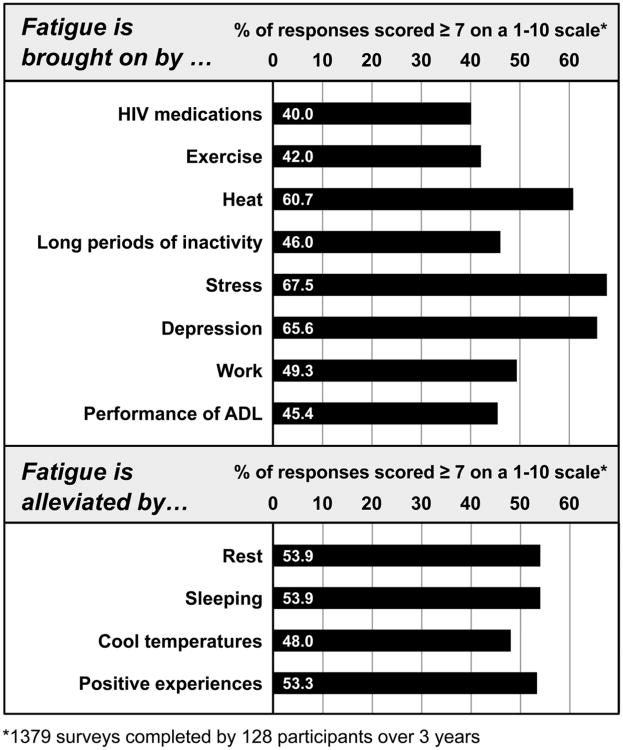
We asked about things that participants thought brought on/increased their fatigue: HIV medications (40.0% of the surveys with scores in the most severe range); exercise (42.0%), with 45.6% reporting prolonged fatigue after exercise; performance of activities of daily living (45.4%); long periods of inactivity (46.0%); work (49.3%); heat (60.7%); depression (65.6%); and stress (67.5%). Participants reported that fatigue was alleviated by the following: cool temperatures (48.0% of the surveys with scores in the most severe range), positive experiences (53.3%), rest (53.9%), and sleeping (53.9%, see Figure 4).
Figure 4.
HIV-related fatigue: Precipitating and alleviating factors.
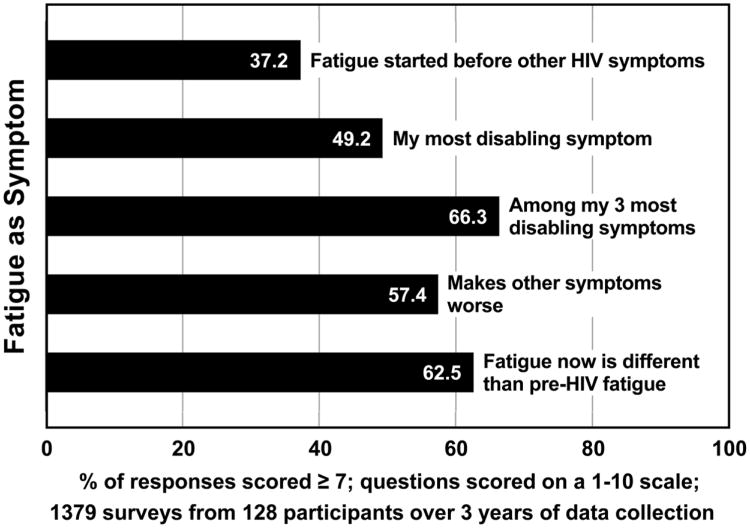
Other items asked for descriptions surrounding the experience of fatigue. Nearly half of the surveys with scores in the most severe range indicated that fatigue was worse in the afternoon (49.2%), while 30.5% indicated that it was worse in the morning. About a third of the surveys with scores in the most severe range (37.2%) indicated that fatigue started before other HIV-related symptoms. Fatigue caused them to eat less (40.8% of the surveys). Nearly half (49.2%) indicated that fatigue was their most disabling symptom; 66.3% indicated that fatigue was among their 3 most disabling symptoms. Fatigue since becoming HIV-infected was different than pre-HIV fatigue (62.5% of the surveys with scores in the most severe range). Many of those who were most fatigued said that their fatigue had changed during past week (54.8% of the surveys), and 54.8% indicated that their fatigue was unpredictable. Over half (57.4% of the surveys) indicated that fatigue made other symptoms worse. Fatigue caused them to lose their patience (59.3% of the surveys). Nearly two thirds (64.6% of the surveys) indicated that they feel drowsy when fatigued, and 72.8% of the surveys reported that fatigue lessened their motivation (see Figure 5).
Figure 5.
Description of fatigue as a symptom.
Discussion
This study is significant because it is the first to report prospective, longitudinal data on fatigue in HIV-infected study participants with a tool developed specifically to measure fatigue in this population. The data reported here represent items endorsed by those most adversely impacted by HIV-related fatigue (scores ≥ 7 on each 1-10 scale), and each item represents nearly 1,400 responses to that item. Thus, this report represents the largest, most comprehensive analysis of HIV-related fatigue to date. As such, it extends our understanding of all of the domains that are associated with this symptom, and goes far beyond the simple measurement of presence and intensity that is commonly reported.
We chose to select items for inclusion in the HRFS and to group the items into scales based on the conceptual framework and dimensions identified through formative qualitative research rather than employing a data-driven procedure such as exploratory factor analysis. While there is some conceptual overlap, as evidenced by the strong correlations of the three HRFS sub-scales, this overlap is critical to helping us gain a deep understanding of phenomenon (Pence et al., 2008). It is important to know about both fatigue intensity and fatigue-related impairment of functioning, as a person can have intense fatigue but have no impairment of functioning, or they can have impairment of functioning from something other than fatigue. In either case, these are not the people most in need of interventions. Those that report intense fatigue AND impairment of functioning because of the fatigue should be the group to whom interventions would be targeted.
There are limitations to the study. We admitted participants based on self-referral, and those who responded to the study flyers may have been more severely fatigued. However, the participants in the study closely matched the demographic profile of people infected with HIV in our geographical area. Finally, because we had so little change in the profile of those who were most fatigued, we do not know if the tool will be sensitive enough to measure change in an intervention study. However, we also gained important knowledge from this examination of the HRFS. Most surprising was the finding that those who entered the study with the most fatigue remained the most fatigued over three years; we expected to observe more variability in fatigue over a 3-year period. HIV-related fatigue appears to be chronic and generally does not remit, making the search for interventions more pressing. While there was little impairment with basic activities of daily living, more advanced (or instrumental) activities of daily living were more of a problem. This may help direct resources for those who are fatigued; instrumental ADLs are those that allow people to remain independent in the community and able to avoid more intense, expensive services.
HIV-related fatigue clearly impacts socialization and mental functioning; many of the activities listed in these two areas are those that keep people in the workforce, which again has economic implications. The ability to socialize involves something more important, however, and that is the person's ability to be with friends and family. When people have to withdraw from interacting with others because they are too tired to do so, the risk of depression increases, and there is a strong relationship between fatigue and depression in HIV infection (e.g., Barroso, 2001; Barroso et al., 2003; Barroso et al., 2010; Barroso & Lynn, 2002; Breitbart et al.1998; Henderson et al., 2005; Millikin, Rourke, Halman, & Power, 2003; Phillips et al., 2004; Schifitto et al., 2011; Voss, 2005). Fatigue can cause depression, and depression has fatigue as one of its cardinal symptoms. Thus, social withdrawal by a fatigued HIV-infected individual may signal the beginning of a cascade of negative events.
With regard to recommendations for the future, knowing the aggravating and alleviating factors helps us provide anticipatory guidance to patients with HIV-related fatigue; we can let them know what things may make their fatigue worse and what may make it better. The relationship among stress, depression, and fatigue was in fact borne out by more sophisticated analyses of our data with separate measures for stress and depression (Barroso et al., 2010; Leserman et al., 2008). Helping people learn better coping strategies when they face stressful events may help them avoid depression and fatigue. It is conceivable as well that fatigue, depression, and stress form a symptom cluster, and therefore we would need to deal with all three symptoms to impact any of them. Finally, the notion of a symptom cluster is also supported by the fact that this is a different type of fatigue from that experienced prior to becoming HIV-infected, that it is perceived as disabling, and that it amplifies other symptoms. Thus, further investigation of fatigue, depression, and anxiety as a symptom cluster appears to be merited. The development of interventions that take all three symptoms into account is likely needed. As we enter the fourth decade of the epidemic, important new scientific developments such as treatment as prevention, have led to a global discourse about the beginning of the end of AIDS, yet, it is important to remember that the people who are already infected continue to suffer from a fatigue that is for many a true impediment to a better quality of life. Thus, the search for interventions must continue.
Acknowledgments
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study featured here, entitled Fatigue in HIV-Positive People, was funded by the National Institute of Nursing Research, National Institutes of Health (NIH), 5R01NR008681, Sept. 1, 2004–August 31, 2010 (Julie Barroso, Principal Investigator), and grant number 1UL1RR024128-01, National Center for Research Resources, Duke CTSI, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NINR, or NIH.
Biographies
Julie Barroso, PhD, ANP-BC, ARNP, FAAN, is a professor at the Duke University School of Nursing, Durham, NC.
James L. Harmon, DNP, ANP-BC, ARNP, AAHIVS, is an assistant professor at the Duke University School of Nursing, Durham, NC.
Jane Leserman Madison, PhD, is a professor in the Department of Psychiatry, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC
Brian Wells Pence, PhD, MPH, is an associate professor, Community and Family Medicine and Global Health, Duke University, Durham, NC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Barroso J. “Just worn out:” A qualitative study of HIV-related fatigue. In: Funk SG, Tornquist EM, Leeman J, Miles MS, Harrell JS, editors. Key aspects of preventing and managing chronic illness. New York, NY: Springer; 2001. pp. 183–194. [Google Scholar]
- Barroso J, Carlson JR, Meynell J. Physiological and psychological markers associated with HIV-related fatigue. Clinical Nursing Research. 2003;12:49–68. doi: 10.1177/1054773803238740. [DOI] [PubMed] [Google Scholar]
- Barroso J, Hammill BG, Leserman J, Salahuddin N, Harmon JL, Pence BW. Physiological and psychosocial factors that predict HIV-related fatigue. AIDS and Behavior. 2010;14:1415–1427. doi: 10.1007/s10461-010-9691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Lynn MR. Psychometric properties of the HIV-related fatigue scale. Journal of the Association of Nurses in AIDS Care. 2002;13:66–75. doi: 10.1016/S1055-3290(06)60242-2. [DOI] [PubMed] [Google Scholar]
- Barroso J, Pence BW, Salahuddin N, Harmon JL, Leserman J. Physiological correlates of HIV-related fatigue. Clinical Nursing Research. 2008;17:5–19. doi: 10.1177/1054773807311382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, McDonald MV, Rosenfeld B, Monkman ND, Passik S. Fatigue in ambulatory AIDS patients. Journal of Pain and Symptom Management. 1998;15:159–167. doi: 10.1016/S0885-3924(97)00260-1. [DOI] [PubMed] [Google Scholar]
- Dacosta Dibonaventura M, Gupta S, Cho M, Mrus J. The association of HIV/AIDS treatment side effects with health status, work productivity, and resource use. AIDS Care. 2012;24:744–755. doi: 10.1080/09540121.2011.630363. [DOI] [PubMed] [Google Scholar]
- Harmon J, Barroso J, Pence BW, Leserman J, Salahuddin N. Demographic and illness-related variables associated with HIV-related fatigue. Journal of the Association of Nurses in AIDS Care. 2008;19:90–97. doi: 10.1016/j.jana.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M, Safa F, Easterbrook P, Hotopf M. Fatigue among HIV-infected patients in the era of highly active antiretroviral therapy. HIV Medicine. 2005;6:347–352. doi: 10.1111/j.1468-1293.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- Holzemer WL. HIV and AIDS: The symptom experience. American Journal of Nursing. 2002;102(4):48–52. doi: 10.1097/00000446-200204000-00023. [DOI] [PubMed] [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, Wagener MN, van Duijn M, Fischer S, van Gorp EC. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24:1387–1405. doi: 10.1097/QAD.0b013e328339d004. [DOI] [PubMed] [Google Scholar]
- Justice AC, Rabeneck L, Hays RD, Wu AW, Bozzette SA. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: A comparison with self-reported symptoms. Journal of Acquired Immune Deficiency Syndromes. 1999;21:126–133. [PubMed] [Google Scholar]
- Leserman J, Barroso J, Pence BW, Salahuddin N, Harmon JL. Trauma, stressful life events, and depression predict HIV-related fatigue. AIDS Care. 2008;20:1258–1265. doi: 10.1080/09540120801919410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millikin CP, Rourke SB, Halman MH, Power C. Fatigue in HIV/ AIDS is associated with depression and subjective neurocognitive complaints but not neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 2003;25:201–215. doi: 10.1076/jcen.25.2.201.13644. [DOI] [PubMed] [Google Scholar]
- Paddison J, Fricchione G, Ghandi RT, Freudenreich O. Fatigue in psychiatric HIV patients: A pilot study of psychological correlates. Psychosomatics. 2009;50:455–460. doi: 10.1016/S0033-3182(09)70837-0. [DOI] [PubMed] [Google Scholar]
- Pence BW, Barroso J, Harmon JL, Leserman J, Salahuddin N, Hammill BG. Chronicity and remission of fatigue in patients with established HIV infection. AIDS Patient Care and STDs. 2009;23:239–244. doi: 10.1089/apc.2008.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Barroso J, Leserman J, Harmon JL, Salahuddin N. Measuring fatigue in people living with HIV/AIDS: Psychometric characteristics of the HIV-related fatigue scale. AIDS Care. 2008;20:829–837. doi: 10.1080/09540120701694063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KD, Sowell RL, Rojas M, Tavakoli A, Fulk LJ, Hand GA. Physiological and psychological correlates of fatigue in HIV disease. Biological Research for Nursing. 2004;6:59–74. doi: 10.1177/1099800404264846. [DOI] [PubMed] [Google Scholar]
- Salahuddin N, Barroso J, Leserman J, Harmon JL, Pence BW. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. Journal of the Association of Nurses in AIDS Care. 2009;20:6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Deng L, Yeh TM, Evans SR, Ernst T, Zhong J, Clifford D. Clinical, laboratory, and neuroimaging characteristics of fatigue in HIV-infected individuals. Journal of Neurovirology. 2011;17:17–25. doi: 10.1007/s13365-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P, Dworkin MS. Prevalence and correlates of fatigue among persons with HIV infection. Journal of Pain and Symptom Management. 2003;25:329–333. doi: 10.1016/S0885-3924(02)00676-0. [DOI] [PubMed] [Google Scholar]
- Voss JG. Predictors and correlates of fatigue in HIV/AIDS. Journal of Pain and Symptom Management. 2005;29:173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]