Abstract
The 5-HT3 receptor, the only ionotropic 5-HT receptor, is expressed in limbic regions, including the hippocampus, amygdala, and cortex. However, it is not known whether it has a role in fear memory processes. Analysis of 5-HT3A receptor knockout mice in fear conditioning paradigms revealed that the 5-HT3A receptor is not required for the acquisition or retention of fear memory but is essential for the extinction of contextual and tone-cued fear. Our data suggest that the 5-HT3A receptor could be a key molecule regulating fear memory processes and a potential therapeutic target for fear disorders.
Fear is an emotion that is central to the organization of defensive behaviors in response to threat and, therefore, has an essential role in survival for animals. Unfortunately, in some cases, dysfunction in the fear system produces inappropriate and exaggerated fears that lead to psychiatric disorders, such as post-traumatic stress disorder (PTSD) (Johansen et al. 2011; Orsini and Maren 2012; Maren et al. 2013). These disorders severely affect the lives of patients and are an increasing burden on our societies. Treatment of such disorders generally involves the modulation of fear memory processes, such as promotion of fear extinction (Parsons and Ressler 2013). Therefore, understanding the molecular mechanisms underlying fear memory processes could help with the development of therapeutic strategies for fear disorders.
The 5-HT3 receptor is the only ionotropic receptor in the family of 5-HT receptors (Derkach et al. 1989). The 5-HT3 receptor comprises two subunits (5-HT3A and 5-HT3B), of which the 5-HT3A subunit is essential for formation of a functional receptor (Maricq et al. 1991; Davies et al. 1999). In the brain, the 5-HT3A receptor is mainly expressed on interneurons in limbic regions, such as hippocampus, amygdala, and cortex (Tecott et al. 1993; Morales et al. 1996b; Morales and Bloom 1997), suggesting its involvement in cognitive and emotional brain functions. Indeed, previous studies have indicated that the 5-HT3 receptor plays roles in spatial learning and memory (Stäubli and Xu 1995; Naghdi and Harooni 2005), anxiety-like behavior (Kelly et al. 2003; Bhatnagar et al. 2004), and social behavior (Smit-Rigter et al. 2010). However, it is not known whether the 5-HT3 receptor regulates fear memory processes. Therefore, to address this question, we used 5-HT3A receptor knockout (5-ht3ar−/−) mice and performed a contextual and tone-cued fear conditioning test. We also examined the fear extinction processes for contextual and tone-cued fear in 5-ht3ar−/− mice.
The generation of 5-ht3ar−/− mice was described previously (Zeitz et al. 2002). We confirmed no expression of 5-HT3A receptor in the brains of 5-ht3ar−/− mice by RT-PCR analysis (Supplemental Fig. 1). The fear conditioning test was performed as described previously (Supplemental Methods; Kondo et al. 2012).
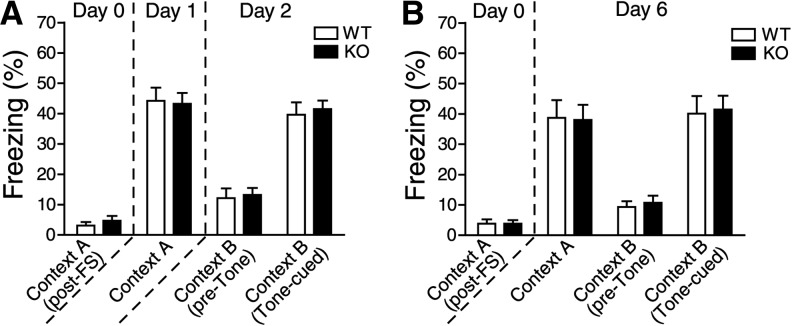
Figure 1.
The 5-HT3A receptor is not required for the acquisition or retention of fear memory. (A,B) Contextual and tone-cued freezing responses after conditioning (WT, n = 18; KO, n = 17 mice) (A), and both groups (n = 14 mice) (B). (FS) Footshock. Means ± SEM are shown in all histograms.
Because changes in pain sensitivity and abnormalities in motor activity affect the conditioning process and freezing assessment, we examined whether the 5-ht3ar−/− mutation altered nociceptive reactions to footshock or altered motor function (Kiyama et al. 1998). There were no significant differences in the current thresholds for common reactions (flinch, vocalization, and jump) to nociceptive shock (WT vs. KO [mA]: flinch, 0.1 vs. 0.1; vocal, 0.132 ± 0.012 vs. 0.138 ± 0.013, P = 0.7595; jump, 0.211 ± 0.014 vs. 0.207 ± 0.011, P = 0.8395). In addition, there were no significant differences in the observed values of spontaneous motor activity measured by means of a Supermex and a photocell beam system (Masuo et al. 1997) (WT vs. KO [counts/20 min], 5618 ± 61.86 vs. 5726 ± 84.04, P = 0.3134), or the latency to fall in the rotarod test (WT vs. KO [sec], 157.4 ± 17.3 vs. 165.0 ± 18.3, P = 0.7695) between wild-type and 5-ht3ar−/− mice. These data indicate that 5-ht3ar−/− mice show normal pain sensitivity, no abnormalities in spontaneous motor activity, and no motor dysfunction during rotarod performance.
We then examined whether the 5-HT3A receptor plays a possible role in the acquisition and retention of fear memory using the fear conditioning test. On the conditioning day (Day 0), the mice showed few freezing responses, with no differences between wild-type and 5-ht3ar−/− mice (WT vs. KO, post-first footshock, 3.15% ± 1.16% vs. 4.71% ± 1.54%, P = 0.4214) (Fig. 1A). After the conditioning day, we performed the contextual fear test on Day 1 and the tone-cued fear test on Day 2. There were no significant differences in contextual freezing responses under context A (Day 1) (WT vs. KO, 44.26% ± 4.30% vs. 43.24% ± 3.58%, P = 0.8566), or in tone-cued freezing responses under context B (Day 2) (WT vs. KO, 39.66% ± 4.07% vs. 41.50% ± 2.81%, P = 0.7151) between wild-type and 5-ht3ar−/− mice (Fig. 1A). To examine the long-term retention of fear memory, we performed the contextual fear test and the tone-cued fear test after an interval of 6 d. To rule out the possibility that the previous fear tests could affect the behavior of mice in the subsequent tests on Day 6, we used another cohort of mice. There were also no significant differences in contextual freezing responses under context A (Day 6) (WT vs. KO, 38.69% ± 5.84% vs. 37.98% ± 5.07%, P = 0.9271), or in tone-cued freezing responses under context B (Day 6) (WT vs. KO, 40.08% ± 5.80% vs. 41.47% ± 4.53%, P = 0.8517) between wild-type and 5-ht3ar−/− mice (Fig. 1B). These data suggest that the 5-ht3ar−/− mutation does not affect the acquisition or retention of fear memory, irrespective of the fear conditioning paradigm.
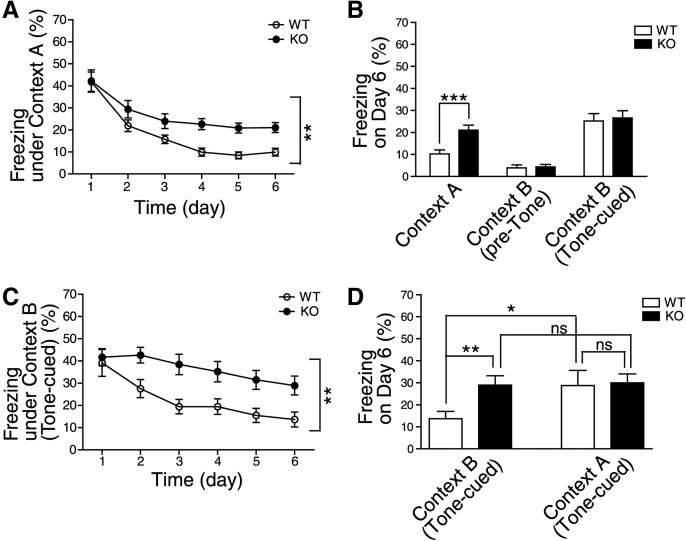
We next investigated the effects of the 5-ht3ar−/− mutation on the processes underlying extinction of learned fear. The fear extinction protocol was performed as described in Supplemental Methods. In the first extinction paradigm, 5-ht3ar−/− mice showed a significantly impaired reduction in freezing behavior, compared with wild-type mice (genotype, F(1,30) = 8.016, P = 0.0082; time, F(5,150) = 43.30, P < 0.0001; genotype × time interaction, F(5,150) = 2.126, P = 0.0653) (Fig. 2A), indicating that the extinction of contextual fear was impaired in 5-ht3ar−/− mice. Meanwhile, in the post-extinction tests on Day 6, both wild-type and 5-ht3ar−/− mice displayed similar freezing responses in a novel chamber (context B), which was different from the conditioned context (context A), following either exposure to the tone presentation or no exposure (WT vs. KO, pre-tone, 3.89% ± 1.40% vs. 4.34% ± 1.10%, P = 0.8009; tone-cued, 25.2% ± 3.38% vs. 26.56% ± 3.27%, P = 0.7716) (Fig. 2B). This suggested that the differential extinction responses between wild-type and 5-ht3ar−/− mice were specific to the context of extinction trials. These data suggest that the 5-HT3A receptor is required for the extinction process for contextual fear.
Figure 2.
The 5-HT3A receptor is required for the extinction of contextual and tone-cued fear. The extinctions of contextual (A,B) and tone-cued (C,D) fear were impaired in 5-ht3ar−/− mice. (A,C) Mean percentage freezing time averaged every day during the fear extinction trials (Days 1–6). (**) P < 0.01 (genotype effect in two-way repeated-measures ANOVA). (B,D) Contextual and tone-cued freezing responses in the fear extinction and post-extinction trials on Day 6. (*) P < 0.05, (**) P < 0.01, (***) P < 0.001, (ns) not significant (two-tailed t-test) (A,B, for extinction trials, both groups, n = 16 mice; for post-extinction test, WT, n = 15; KO, n = 16 mice; C,D, for extinction trials, both groups, n = 12 mice; for post-extinction test, both groups, n = 9 mice). Mean ± SEM shown in all histograms.
Then, in the second extinction paradigm, 5-ht3ar−/− mice showed a significantly impaired reduction in tone-cued freezing behavior, compared with wild-type mice (genotype, F(1,22) = 10.78, P = 0.0034; time, F(5,110) = 10.91, P < 0.0001; genotype × time interaction, F(5,110) = 1.750, P = 0.1293) (Fig. 2C), indicating that the extinction of tone-cued fear was impaired in 5-ht3ar−/− mice. In the post-extinction tests on Day 6, both wild-type and 5-ht3ar−/− mice displayed similar tone-cued freezing responses in context A, which was different from the context of extinction trials (context B) (WT vs. KO, 28.70% ± 6.91% vs. 29.94% ± 4.08%, P = 0.8797) (Fig. 2D), suggesting that the differential extinction responses between wild-type and 5-ht3ar−/− mice were specific to the context of extinction trials. These data suggest that the 5-HT3A receptor is required for the extinction process for tone-cued fear.
It is known that an extinguished conditional fear response reappears when the conditioned stimulus (CS) is presented in a context different from the one in which the extinction trials took place. This phenomenon is known as the renewal effect, one of the properties of context-specific extinction (Ji and Maren 2007; Myers and Davis 2007; Maren 2011; Maren et al. 2013). Wild-type mice showed significantly higher freezing responses in context A (i.e., a context different from the extinction context) than in context B (i.e., the extinction context) (context B vs. context A, 13.66% ± 3.32% vs. 28.70% ± 6.91%, P = 0.0469) (Fig. 2D), indicating the existence of a renewal effect. Interestingly, there was no significant difference in freezing responses between the contexts in 5-ht3ar−/− mice (context B vs. context A, 28.94% ± 4.22% vs. 29.94% ± 4.08%, P = 0.8696) (Fig. 2D). These data support the idea that the 5-HT3A receptor contributes to the context-specificity of extinction processes.
In this study, we found that the 5-HT3A receptor is not required for the acquisition or retention of fear memory, but is essential for the extinction of contextual and tone-cued fear. In contrast to our findings, Park and Williams (2012) reported that systemic injection of a 5-HT3 receptor antagonist (granisetron) facilitated the memory of cued and contextual fear extinction in rats. However, there are several points of difference between our experiments and theirs, which could account for the differences between the results obtained. First, the species studied differed. Park and Williams (2012) used rats in their experiments, whereas we used mice. It is known that differences in the species, strains, gender, and ages of animals used in behavioral tests can influence the results (Whishaw 1995; Frick et al. 2000; Ammassari-Teule and Castellano 2004; Stranahan 2011). Second, the experimental paradigms differed. Park and Williams (2012) used passive avoidance paradigms to measure conditioned contextual fear, whereas we used the fear conditioning paradigms described above. These two behavioral tasks are known to be fear learning tests; however, the requirements vary across learning tasks. Therefore, it is possible that fear learning processes (including extinction processes of learned fear) are different between the two tasks. Furthermore, as an index of conditioned fear, Park and Williams (2012) used the latency to enter the dark compartment in the passive avoidance task, whereas we have used freezing behavior in the fear conditioning test. It has been reported that different types of conditioned fear behavior (i.e., avoidance behavior of the aversive stimulus vs. freezing responses) are mediated by distinct neural systems (Killcross et al. 1997). In addition, the conditions of extinction trials are different. Park and Williams (2012) performed tone-cued extinction trials, which gave 10 presentations of the CS tone with 60-sec intertrial intervals (ITIs) within 1 d. In contrast, we performed the extinction trials over six consecutive days with 24-h ITIs. It has been reported that ITIs in extinction trials can affect the processes of fear extinction (Bouton et al. 2006). Taken together, these differences in experimental paradigms (i.e., behavioral tasks, indices of conditioned fear, and extinction trials) could account for the differences between our results and theirs. Third, to block 5-HT3 receptor function, Park and Williams (2012) administered a 5-HT3 receptor antagonist before the extinction trials. In contrast, we used 5-HT3A receptor knockout mice, which are devoid of 5-HT3A receptors. The methodological differences between pharmacological blockage and gene knockout of 5-HT3 receptor could also have contributed to the differences between our results and theirs.
Previous reports have indicated that the 5-HT3 receptor is involved in anxiety-like behavior (Kelly et al. 2003; Bhatnagar et al. 2004). To examine anxiety in 5-ht3ar−/− mice, we performed the elevated plus maze test as described previously (Walf and Frye 2007). The results of the elevated plus maze test (Supplemental Table 1) suggest that 5-ht3ar−/− mice exhibit indices of decreased anxiety compared with wild-type mice, consistent with previous reports (Kelly et al. 2003; Bhatnagar et al. 2004). This difference in anxiety could have affected the performance of mice in the fear conditioning test. Our results showed that the freezing behavior either before or after the footshock on the day of conditioning of 5-ht3ar−/− mice was comparable to that observed in wild-type mice (Fig. 1A,B). In addition, there were no significant differences in freezing behavior under no exposure to the tone presentation in a novel chamber (context B), which was different from the conditioned context, between wild-type and 5-ht3ar−/− mice (Fig. 1A,B). These data indicate that baseline freezing responses in the fear conditioning test did not differ between wild-type and 5-ht3ar−/− mice. However, the difference in anxiety might potentially influence the acquisition of contextual and cued fear memory and the subsequent extinction of those memories in the fear conditioning paradigms.
It is known that fear extinction is mediated by a distributed network, including the hippocampus, amygdala, and prefrontal cortex (Myers and Davis 2007; Maren 2011; Orsini and Maren 2012; Maren et al. 2013). Previous studies have shown that the 5-HT3A receptor is selectively expressed in GABA neurons in these brain areas (Morales et al. 1996a; Morales and Bloom 1997; Lee et al. 2010). Further, it has been demonstrated that activation of 5-HT3A receptors directly excites GABAergic interneurons (Kawa 1994; McMahon and Kauer 1997; Puig et al. 2004) and regulates GABA neurotransmission (Ropert and Guy 1991; Koyama et al. 2000; Katsurabayashi et al. 2003; Turner et al. 2004). Interestingly, the inhibitory neurotransmitter GABA system has been implicated in fear extinction via modulation of neural circuits in limbic brain regions (Harris and Westbrook 1998; Makkar et al. 2010; Orsini and Maren 2012). Taken together, our results and those findings raise the possibility that the 5-HT3A receptor is involved in the processes of fear extinction through GABA neurotransmission. In addition, it was reported that the 5-HT3 receptor is colocalized with cholecystokinin (CCK) immunoreactivity, and that CCK neurotransmission is highly regulated by the 5-HT3 receptor (Paudice and Raiteri 1991; Férézou et al. 2002). Moreover, it was recently shown that CCK is involved in fear extinction (Joseph et al. 2013). Therefore, CCK transmission may also contribute to the 5-HT3A receptor-mediated extinction of fear memory. It has been suggested that 5-HT3 receptor-containing interneurons are composed of a biochemically heterogeneous subpopulation of neurons that may be involved in different inhibitory circuits (Morales and Bloom 1997; Lee et al. 2010; von Engelhardt et al. 2011); however, the specific function of each subgroup in fear memory processes remains to be established. Therefore, detailed analysis based on the subgroups of 5-HT3 receptor-expressing interneurons will provide further insight into their specific roles in the regulation of fear memory.
The process of extinction involves a complex neuronal circuitry in numerous brain regions (Myers and Davis 2007; Maren 2011; Orsini and Maren 2012; Maren et al. 2013); however, it remains to be determined what roles the 5-HT3A receptor plays in those brain areas during fear extinction, and which regions of 5-HT3A receptor expression are centrally involved in the 5-HT3A receptor-mediated extinction of fear memory. Further studies using conditional 5-HT3A receptor knockout mice should provide answers to these questions.
The processes of fear extinction share several attributes with other steps of fear memory formation (Makkar et al. 2010; Johansen et al. 2011; Maren 2011); however, there is some evidence that several cellular pathways are involved specifically in extinction, but not in the acquisition or consolidation of fear memory (Marsicano et al. 2002; Deng et al. 2009). It has been reported that PTSD is associated with impairment of fear extinction (Guthrie and Bryant 2006; Blechert et al. 2007; Parsons and Ressler 2013). Selective serotonin reuptake inhibitors (SSRIs) are considered to be first-line drug treatments for PTSD; however, it is not fully understood how serotonin neurotransmission is involved in the pathophysiology of PTSD (Sadock and Sadock 2007). This is the first study to demonstrate directly that the 5-HT3A receptor is involved specifically in the processes of fear extinction by behavioral analyses using 5-ht3ar−/− mice. Moreover, recently, Harmer et al. (2006) reported a role for 5-HT3 receptors in some elements of fear processing in humans. The 5-HT3A receptor could be a key molecule for regulation of fear extinction, and a potentially important therapeutic target for disorders of regulation in fear systems, such as PTSD.
Acknowledgments
We thank Toshimitsu Hamasaki (Osaka University) for discussions about statistical analyses. M.K. performed all of the analyses and wrote the manuscript. S.S. supervised the project. M.K., Y.N., Y.I., T.Y., and S.S. contributed to article revising.
Footnotes
[Supplemental material is available for this article.]
References
- Ammassari-Teule M, Castellano C 2004. Strains of rodents and pharmacology of learning and memory. Neural Plast 11: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF 2004. Changes in anxiety-related behaviors and hypothalamic–pituitary–adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav 81: 545–555 [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Markgraf J, Wilhelm FH 2007. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experimental, and behavioural responses. Behav Res Ther 45: 2019–2033 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S 2006. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 60: 352–360 [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF 1999. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397: 359–363 [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH 2009. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 29: 13532–13542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Surprenant A, North RA 1989. 5-HT3 receptors are membrane ion channels. Nature 339: 706–709 [DOI] [PubMed] [Google Scholar]
- Férézou I, Bruno C, Hill EL, Rossier J, Hamel E, Lambolez B 2002. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci 22: 7389–7397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J 2000. Mice are not little rats: Species differences in a one-day water maze task. Neuroreport 11: 3461–3465 [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA 2006. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med 68: 307–311 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ 2006. 5HT3 antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology 186: 18–24 [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF 1998. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology 140: 105–115 [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S 2007. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 17: 749–758 [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE 2011. Molecular mechanisms of fear learning and memory. Cell 147: 509–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Tang M, Mamiya T, Chen Q, Yang LL, Jiao J, Yu N, Tang YP 2013. Temporal association of elevated cholecystokininergic tone and adolescent trauma is critical for posttraumatic stress disorder-like behavior in adult mice. Proc Natl Acad Sci 110: 6589–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsurabayashi S, Kubota H, Tokutomi N, Akaike N 2003. A distinct distribution of functional presynaptic 5-HT receptor subtypes on GABAergic nerve terminals projecting to single hippocampal CA1 pyramidal neurons. Neuropharmacology 44: 1022–1030 [DOI] [PubMed] [Google Scholar]
- Kawa K 1994. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: A patch-clamp study. J Neurophysiol 71: 1935–1947 [DOI] [PubMed] [Google Scholar]
- Kelly SP, Bratt AM, Hodge CW 2003. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol 461: 19–25 [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ 1997. Different types of fear-conditioned behavior mediated by separate nuclei within amygdala. Nature 388: 377–380 [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M 1998. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor є1 subunit. J Neurosci 18: 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Takei Y, Hirokawa N 2012. Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron 73: 743–757 [DOI] [PubMed] [Google Scholar]
- Koyama S, Matsumato N, Kubo C, Akaike N 2000. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdale neurons. J Physiol 529: 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B 2010. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30: 16796–16808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J 2010. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35: 1625–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S 2011. Seeking a spotless mind: Extinction, deconsolidation, and erasure of fear memory. Neuron 70: 830–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I 2013. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D 1991. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 254: 432–437 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, et al. 2002. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534 [DOI] [PubMed] [Google Scholar]
- Masuo Y, Matsumoto Y, Morita S, Noguchi J 1997. A novel method for counting spontaneous motor activity in the rat. Brain Res Brain Res Protoc 1: 321–326 [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA 1997. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol 78: 2493–2502 [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE 1997. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci 17: 3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, de Lecea L, Bloom FE 1996a. The type 3 serotonin receptor is expressed in a subpopulation of GABAergic neurons in the rat neocortex and hippocampus. Brain Res 731: 199–202 [DOI] [PubMed] [Google Scholar]
- Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE 1996b. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Mol Brain Res 36: 251–260 [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M 2007. Mechanisms of fear extinction. Mol Psychiatry 12: 120–150 [DOI] [PubMed] [Google Scholar]
- Naghdi N, Harooni HE 2005. The effect of intrahippocampal injections of ritanserin (5HT2A/2C antagonist) and granisetron (5HT3 antagonist) on learning as assessed in the spatial version of the water maze. Behav Brain Res 157: 205–210 [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S 2012. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36: 1773–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Williams CL 2012. Contribution of serotonin type 3 receptors in the successful extinction of cued or contextual fear conditioned responses: Interactions with GABAergic signaling. Rev Neurosci 23: 555–569 [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ 2013. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 16: 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudice P, Raiteri M 1991. Cholecystokinin release mediated by 5-HT3 receptors in rat cerebral cortex and nucleus accumbens. Br J Pharmacol 103: 1790–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Santana N, Celada P, Mengod G, Artigas F 2004. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex 14: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Ropert N, Guy N 1991. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol 441: 121–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA 2007. Posttraumatic stress disorder and acute stress disorder. In Kaplan & Sadock's synopsis of psychiatry: Behavioral sciences/clinical psychiatry, 10th ed, pp. 612–622 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- Smit-Rigter LA, Wadman WJ, van Hooft JA 2010. Impaired social behavior in 5-HT3A receptor knockout mice. Front Behav Neurosci 4: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli U, Xu FB 1995. Effects of 5-HT3 receptor antagonism on hippocampal θ rhythm, memory, and LTP induction in the freely moving rat. J Neurosci 15: 2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM 2011. Similarities and differences in spatial learning and object recognition between young male C57Bl/6J mice and Sprague-Dawley rats. Behav Neurosci 125: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D 1993. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci 90: 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TJ, Mokler DJ, Luebke JI 2004. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: In vitro slice and synaptosome studies. Neuroscience 129: 703–718 [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Khrulev S, Eliava M, Wahlster S, Monyer H 2011. 5-HT3A receptor-bearing white matter interstitial GABAergic interneurons are functionally integrated into cortical and subcortical networks. J Neurosci 31: 16844–16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ 1995. A comparison of rats and mice in a swimming pool task and matching to place task: Some surprising differences. Physiol Behav 58: 687–693 [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI 2002. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]