Abstract
The prelimbic cortex has been implicated in the consolidation of previously learned fear. Herein, we report that temporarily inactivating this medial prefrontal cortex subregion with the GABAA agonist muscimol (4.0 nmol in 0.2 μL per hemisphere) was able to equally disrupt 1-, 7-, and 21-d-old contextual fear memories after their brief retrieval in rats. In all cases, this effect was prevented when memory reactivation was omitted. These results indicate that recent and remote fear memories are susceptible to reconsolidation blockade induced by prelimbic cortex inactivation. It was also demonstrated that the disrupting effect of prelimbic cortex inactivation on fear memory persisted over 11 d, and did not show extinction-related features, such as reinstatement. Infusing the same dose and volume of muscimol bilaterally into the infralimbic cortex after brief retrieval/reactivation of the fear memory did not disrupt it, as seen in prelimbic cortex-inactivated animals. The expression of Zif268/Egr1, the product of an immediate early gene related to memory reconsolidation, was also less pronounced in the infralimbic cortex than in prelimbic cortex following memory retrieval/reactivation. Altogether, the present findings highlight that activity in the prelimbic cortex may reestablish reactivated aversive memories and, therefore, contribute to their maintenance over time.
Similar to other types of memory, an emotional memory charged with negative valence has to be consolidated to allow its later retrieval (Dudai and Eisenberg 2004). On such an occasion, an established contextual fear memory, for instance, is rendered labile again after its reactivation, through briefly reexposing the experimental subjects to the conditioning context (Bustos et al. 2009). Memory reactivation can be followed by a new phase of stabilization, referred to as reconsolidation, that, in turn, is thought to reestablish and maintain it over time (Nader et al. 2000; Sara 2000; Dudai 2012). Accumulating evidence has indicated that the search for interventions that block the reconsolidation of aberrant and enduring memories could have clinical relevance to the treatment of psychiatric conditions, such as post-traumatic stress disorder (Debiec and LeDoux 2004; Diergaarde et al. 2008; Taubenfeld et al. 2009; Stern et al. 2012).
The medial prefrontal cortex has long been implicated in fear memory processing and its extinction in rats (Santos-Anderson and Routtenberg 1976; Morgan et al. 1993; Morgan and LeDoux 1995; Joel et al. 1997; Milad and Quirk 2002; Thomas et al. 2002; Quinn et al. 2008; Roozendaal et al. 2009; Do Monte et al. 2013; Gonzalez et al. 2013). However, whether and how its anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) subregions (Fig. 1) contribute to the acquisition, consolidation, retrieval, reconsolidation, and extinction of aversive memories has been revisited. In part, this is because several of the early studies adopted permanent lesion approaches and/or aimed, for example, at the ventromedial prefrontal cortex that includes the ventral PL and the IL cortices (Morgan et al. 1993; Sierra-Mercado et al. 2006; Fuster 2008). As these medial prefrontal cortex subregions diverge in terms of cytoarchitectonic characteristics and neural connectivity (Heidbreder and Groenewegen 2003; Hoover and Vertes 2007), one would anticipate their functional heterogeneity in each one of the above-mentioned cases. Indeed, convergent evidence now indicates that activity in AC, PL, and/or IL cortices is necessary for various memory stages to occur properly but, occasionally, their function has been shown to contrast this. A representative example of a differential role is provided by PL and IL cortices in fear memory extinction (Burgos-Robles et al. 2009; Laurent and Westbrook 2009). In other cases, however, two medial prefrontal cortex subdivisions work equally, such as in the consolidation of learned fear in which the PL and AC cortices have corresponding roles (Choi et al. 2010; Zhang et al. 2011; Einarsson and Nader 2012). The AC cortex is also recruited to reconsolidate recent and remote contextual fear memories (Einarsson and Nader 2012). Involvement of the PL cortex in recent olfactory fear memory reconsolidation has been reported (Do Monte et al. 2013). It is still unknown, however, whether the PL cortex works correspondingly with the AC cortex to reconsolidate learned fear over time. The PL cortex sends excitatory projections to the basolateral nucleus of the amygdala and the hippocampus (McDonald 1998; Vertes 2006; Hoover and Vertes 2007), which are also implicated in the reconsolidation of fear memories (Nader et al. 2000; Debiec et al. 2002; Lee et al. 2004, 2006; Parsons et al. 2006; Tronson et al. 2006; de Oliveira Alvares et al. 2008; Rehberg et al. 2010; Giachero et al. 2013). The working hypothesis of the present study was that the PL cortex subserved recent and remote fear memory reconsolidation, likely owing to its modulating role in these limbic brain areas.
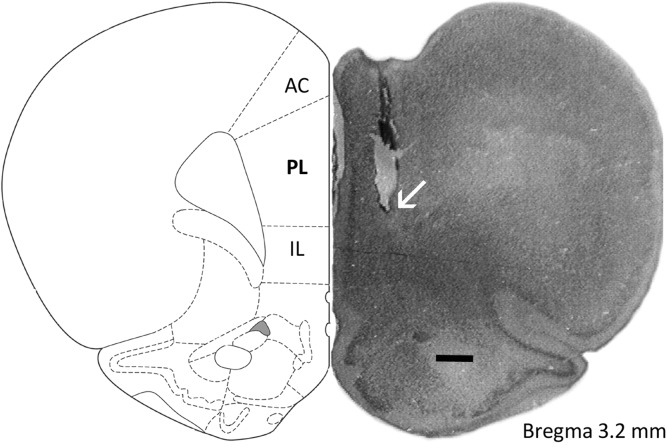
Figure 1.
Schematic drawing (scale bar, 500 µm) of the rat medial prefrontal cortex showing its main subregions, namely the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) cortices, and a representative infusion site placement (indicated by an arrow) in the PL cortex.
To address this issue, we investigated the potential disrupting effect of temporary and reversible PL cortex inactivation induced by the GABAA agonist muscimol on fear memory of different ages in rats. We demonstrate that: (1) such an experimental intervention is able to affect the reconsolidation of contextual fear memories aged 1, 7, and 21 d when conducted immediately after their brief retrieval; (2) the disrupting effect of PL cortex inactivation on fear memory is long-lasting and does not show extinction-related features, such as reinstatement, but depends on its reactivation; (3) infusing muscimol bilaterally into the IL cortex during fear memory reconsolidation does not disrupt it, as seen in PL cortex-inactivated animals; and (4) the expression of Zif268/Egr1, the product of an immediate early gene related to memory reconsolidation (Lee et al. 2004), increases in both the PL and AC cortices, but not in the IL cortex, following a brief fear memory reactivation.
Results
Experiment 1: Activity in PL cortex is necessary for recent contextual fear memory reconsolidation
To investigate whether PL cortex activity contributes to the reconsolidation of a 1-d-old fear memory, 15 contextually conditioned rats were randomly allocated to two groups based on treatment, vehicle (n = 7) or muscimol (4.0 nmol/0.2 μL, n = 8), infused bilaterally into this brain region immediately after memory retrieval/reactivation.
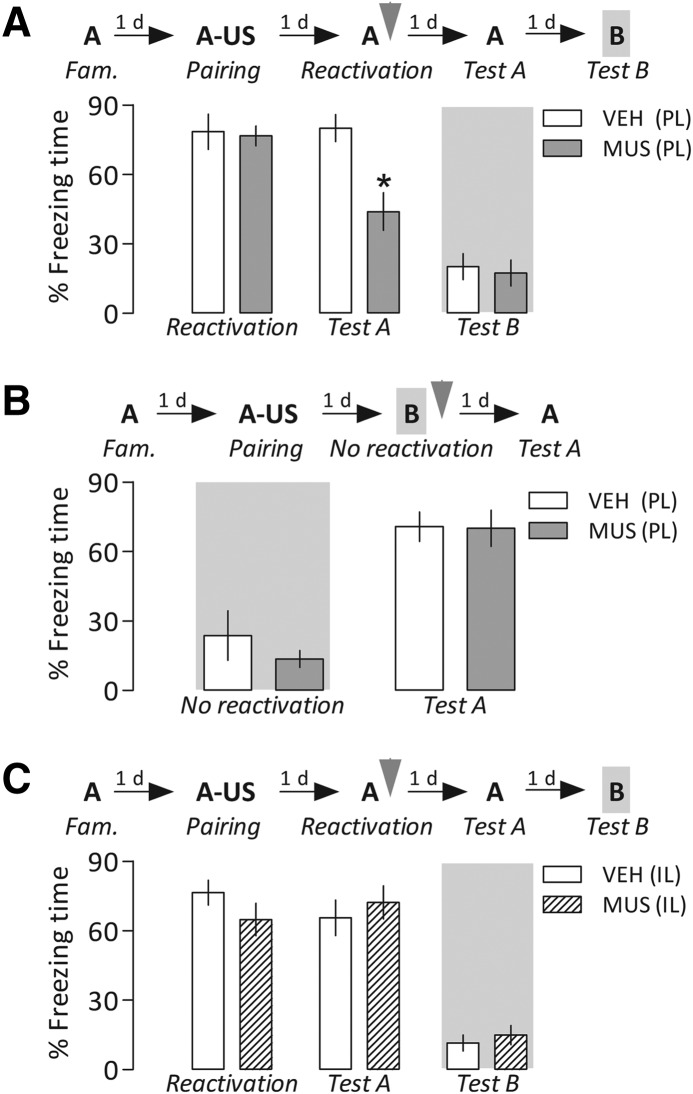
Repeated-measures analysis of variance (ANOVA) showed a significant interaction between drug treatment and Context A reexposure for freezing time (F(1,13) = 21.1, P = 0.0005). As shown in Figure 2A, both groups presented a similarly long freezing time in the retrieval/reactivation session, but during reexposure to the paired context (Test A), animals infused with muscimol expressed significantly less freezing than controls. This latter result suggests a blockade in memory reconsolidation following the PL cortex inactivation. Moreover, one-way ANOVA did not show significant drug effects for exposure to an unpaired Context B (Test B) performed 24 h later (F(1,13) = 0.05, P = 0.82), suggesting that the attenuation of freezing was restricted to the paired Context A.
Figure 2.
(A) Disruptive effect of temporary prelimbic (PL) cortex inactivation induced by muscimol (MUS) on fear memory reconsolidation. After a familiarization (Fam.) session, animals had Context A paired with foot shocks, the unconditioned stimulus (US). On the next day, they were reexposed to Context A to retrieve/reactivate the established memory, and then received bilaterally into the PL cortex MUS (4.0 nmol in 0.2 µL per side) or vehicle (VEH). Relative to controls, MUS-treated animals froze less when reexposed to the paired context 1 d later (Test A). No differences between groups were seen in an unpaired context (Test B). (B) Fear memory reactivation was necessary for reconsolidation blockade induced by PL-cortex inactivation to occur. On the day following the contextual conditioning session described above, the animals were infused with MUS or VEH after being exposed to Context B (unpaired context) for 3 min. No differences were found when they were reexposed to the paired Context 24 h later (Test A). (C) Bilateral infusion of MUS (4.0 nmol in 0.2 µL per side) into the infralimbic (IL) cortex after memory retrieval/reactivation did not change freezing time relative to the respective controls during both Tests A and B. Gray arrowheads indicate the moment of drug treatment. Bars represent the percentage of total time spent freezing. Values are expressed as mean ± SEM. (*) Significant difference (P < 0.05) compared with the respective controls (repeated-measures ANOVA followed by Newman–Keuls test).
To further examine the disrupting effect of PL cortex inactivation on fear memory, independent groups of contextually conditioned rats were administered with vehicle (n = 6) or muscimol (n = 8) after exposure to Context B, a neutral context different from that used for conditioning (“no reactivation” session). One-way ANOVA showed no significant drug effects on this session (F(1,12) = 0.005, P = 0.94) or on reexposure to Context A (Test A) performed 24 h later (F(1,12) = 0.62, P = 0.45). In both cases, the groups froze equally (Fig. 2B), suggesting that the reconsolidation blockade induced by muscimol depended on prior memory retrieval/reactivation, and that the PL cortex was functional during reexposure to the paired context on the next day.
To investigate whether activity in the IL cortex, which is located ventrally to the PL cortex, is also associated with memory reconsolidation, 15 contextually conditioned rats were randomly allocated to two groups based on treatment, vehicle (n = 7) or muscimol (n = 8). The treatment was infused bilaterally into the IL cortex just after brief retrieval/reactivation of the fear memory.
Repeated-measures ANOVA showed neither a drug treatment × Context A reexposure interaction (F(1,13) = 3.5, P = 0.08) nor significant main effects of these factors (F(1,13) = 0.09, P = 0.76, and F(1,13) = 0.87, P = 0.36, respectively). As shown in Figure 2C, the groups presented a similarly long freezing time not only in the retrieval/reactivation session, but also in reexposure to the paired context (Test A). These results indicate that after being infused into the IL cortex, the same dose and volume of muscimol that was able to induce a reconsolidation blockade when infused into the PL cortex, no longer affected this memory phase. Moreover, one-way ANOVA did not show significant drug effects for Test B performed 24 h later (F(1,13) = 0.31, P = 0.58).
Experiment 2: Memory reconsolidation is associated with elevated expression of Zif268/Egr1 in PL cortex
The occurrence of an up-regulation of Zif268/Egr1 has been demonstrated in brain regions recruited to contextual fear memory reconsolidation, such as the hippocampus, amygdala, and AC cortex (Hall et al. 2001; Thomas et al. 2002). If the PL cortex is more important than the IL cortex to this process, one could expect a differential level of Zif268/Egr1 expression after memory retrieval/reactivation. To investigate this issue, 1 h after a 3-min reexposure to Context A (memory reactivated group, n = 4) or a 3-min exposure to Context B (nonmemory reactivated group, n = 3), the PL, AC, and IL cortices of contextually conditioned animals were subjected to immunohistochemical analysis.
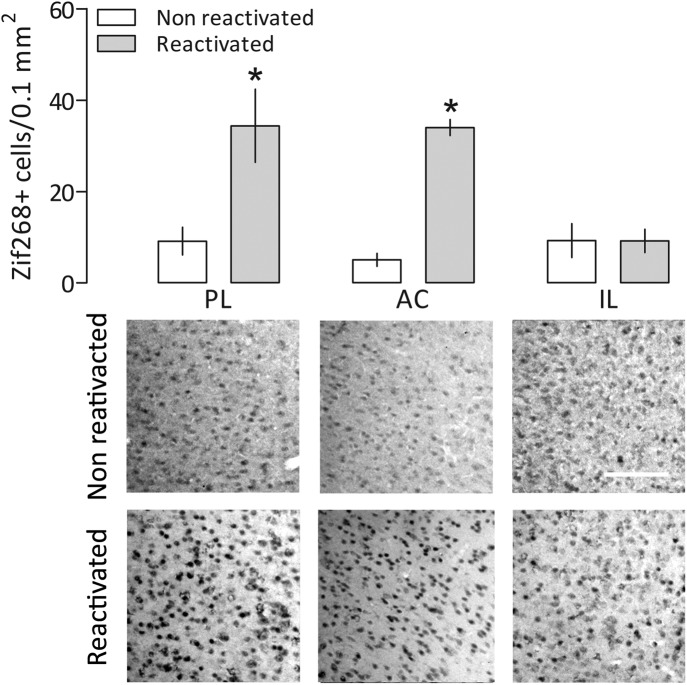
There was a significant interaction between memory reactivation and medial prefrontal cortex subregion factors (F(2,15) = 6.18, P = 0.01). As shown in Figure 3, the reactivated group expressed significantly more Zif268/Egr1 in the PL and AC cortices than in the nonreactivated group. No statistically significant differences between the groups were observed in the IL cortex.
Figure 3.
Comparative expression of Zif268/Egr1 in the prelimbic (PL), anterior cingulate (AC), and infralimbic (IL) cortices of contextually conditioned rats briefly exposed to the unpaired Context B (nonreactivated group) or reexposed to the paired Context A (reactivated group). Values are expressed as mean ± SEM. White bar, 100 µm. (*) Significant difference (P < 0.05) compared with the respective nonreactivated group (two-way ANOVA followed by Newman–Keuls test). The experimental design was similar to that used in Experiment 1.
Experiment 3: Disruption of fear memory induced by PL cortex inactivation is long-lasting and does not show reinstatement
To investigate whether muscimol-induced PL cortex inactivation could induce a persistent disruption of fear memory through reconsolidation blockade, 16 contextually conditioned rats received vehicle (n = 6) or muscimol (n = 10) in this brain region immediately after memory retrieval/reactivation, and were reexposed to Context A both 1 and 10 d later.
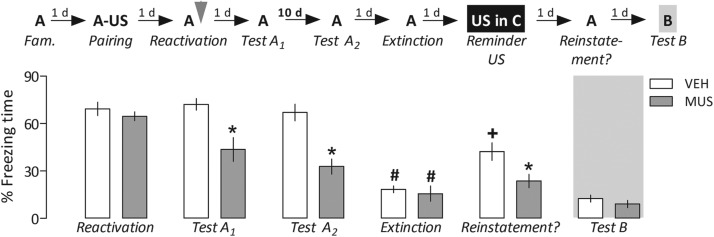
Repeated-measures ANOVA showed significant drug treatment effects at these time-points (F(2,26) = 6.5, P = 0.005). As shown in Figure 4, in both cases, Tests A1 and A2, animals in which the PL cortex was inactivated early expressed significantly less freezing than controls, which maintained a similarly high freezing time during these reexposures to the paired context as the level expressed in the reactivation session.
Figure 4.
Lack of evidence for reinstatement of the fear memory after prelimbic (PL) cortex inactivation-induced reconsolidation blockade. After a familiarization (Fam.) session, animals had Context A paired with foot shocks, the unconditioned stimulus (US). On the next day, they were reexposed to Context A to retrieve/reactivate the established fear memory, and then received vehicle (VEH) or muscimol (MUS, 4.0 nmol in 0.2 µL), bilaterally infused into the PL cortex. In comparison with controls, MUS-treated animals froze less when reexposed to the paired Context A either 1 d (Test A1) or 10 d (Test A2) later, indicating that the disrupting effect of the drug was long-lasting. Both groups were subjected, 24 h later, to extinction of fear memory in the paired Context A for 15 min. On the next day, animals were exposed to Context C for 1 min where they received a single lower-intensity reminder foot shock (US). Twenty-four hours later, they were tested for memory reinstatement by being reexposed to the conditioned Context A for 5 min. During this session, controls showed a higher freezing time than PL cortex-inactivated animals. Both groups, however, behaved similarly in the neutral Context B (Test B). The gray arrowhead indicates the moment of drug treatment. Bars represent the percentage of total freezing time. Values are expressed as mean ± SEM. (*) Significant difference (P < 0.05) compared with the respective controls, (#) significant difference from the same group during Test A2, (+) significant difference from the same group during extinction (repeated-measures ANOVA followed by Newman–Keuls test).
Long and/or repeated reexposures to the paired context without unconditioned stimulus presentation may lead to fear extinction. Thus, one could assume that the attenuated freezing observed in Experiment 1 involved facilitated extinction and not reconsolidation blockade. As reinstatement is a memory extinction feature (Myers and Davis 2002), we attempted to rule out this possibility by investigating whether exposure to a mild foot shock in a context different from that used for conditioning would reinstate fear memory. Thus, 1 d after testing the persistence of the disrupting effect of PL cortex inactivation on fear memory, vehicle- and muscimol-treated animals underwent fear extinction for 15 min in Context A. On the next day, both groups were exposed to a new context, Context C, for 30 sec (pre-shock period), then received a single foot shock of 0.3 mA for 3 sec, and continued to be in this chamber for an additional 30 sec (post-shock period). Twenty-four hours after this reminder foot-shock, they were subjected to a test of memory reinstatement that consisted of a 5-min reexposure to Context A.
Repeated-measures ANOVA showed a significant interaction between drug treatment and Context A reexposure factors (F(4,48) = 4.3, P = 0.004). As shown in Figure 4, during the session of fear extinction, the groups presented low and comparable levels of freezing. However, when they were reexposed to the paired Context A following a reminder foot shock in Context C, controls, but not PL cortex-inactivated animals, reinstated the extinguished conditioned freezing response. Overall, these results confirm that muscimol-induced temporary PL cortex inactivation after fear memory retrieval/reactivation blocked reconsolidation, rather than facilitated memory extinction. Moreover, one-way ANOVA did not show significant drug treatment effects during Test B performed 24 h later (F(1,10) = 1.1, P = 0.31). Both groups expressed a low level of freezing when exposed to the neutral Context B (Fig. 4).
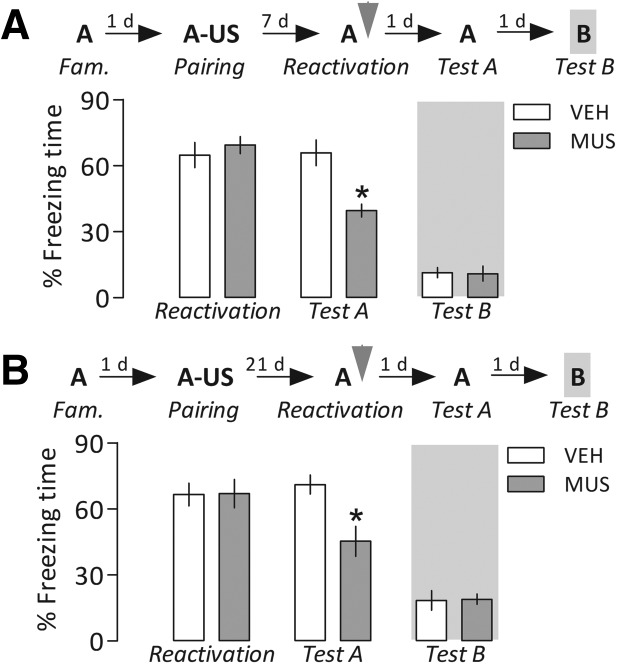
Experiment 4: Reconsolidation of more remote fear memories is equally disrupted by PL cortex inactivation
To investigate whether PL cortex activity is also critical for the reconsolidation of older fear memories, 33 contextually conditioned rats were randomly allocated to four groups (n = 8–9 per group) and treated with vehicle or muscimol immediately after retrieval/reactivation of a fear memory acquired 7 or 21 d earlier.
Repeated-measures ANOVA showed, in both cases, a significant interaction between drug treatment and Context A reexposure factors (F(1,14) = 34.7, P = 0.00004, and F(1,15) = 27.9, P = 0.00009, respectively). As shown in Figure 5, all groups presented a similar high freezing time in the reactivation session, but when reexposed to Context A (Test A), the PL cortex-inactivated groups froze significantly less than the respective controls. These results suggest that the PL cortex was equally (Table 1) recruited to reconsolidate more remote fear memories. Moreover, one-way ANOVA showed no significant drug treatment effects when these groups were exposed to the neutral Context B (7 d, F(1,14) = 0.02, P = 0.91; 21 d, F(1,15) = 0.03, P = 0.86).
Figure 5.
More remote fear memories were also susceptible to reconsolidation blockade induced by prelimbic (PL) cortex inactivation. One (A) or 3 (B) weeks after the contextual conditioning session in Context A (described in Figure 2), the animals were briefly reexposed to this chamber to reactivate the established fear memory, and immediately after they received vehicle (VEH) or muscimol (MUS, 4.0 nmol/side). In either case, MUS-treated animals froze less than controls when reexposed to the paired context 1 d later (Test A). No differences between groups were observed in an unpaired context (Test B). Gray arrowheads indicate the moment of drug treatment. Bars represent the percentage of total time spent freezing. Values are expressed as mean ± SEM. (*) Significant difference (P < 0.05) compared with the respective controls (repeated-measures ANOVA followed by Newman–Keuls test).
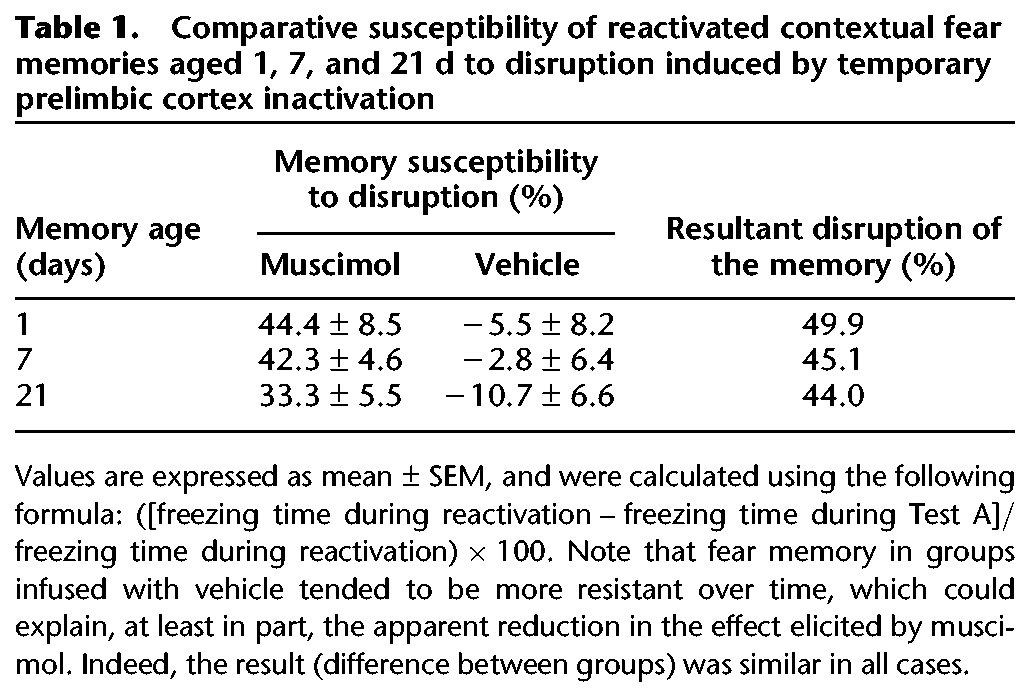
Table 1.
Comparative susceptibility of reactivated contextual fear memories aged 1, 7, and 21 d to disruption induced by temporary prelimbic cortex inactivation
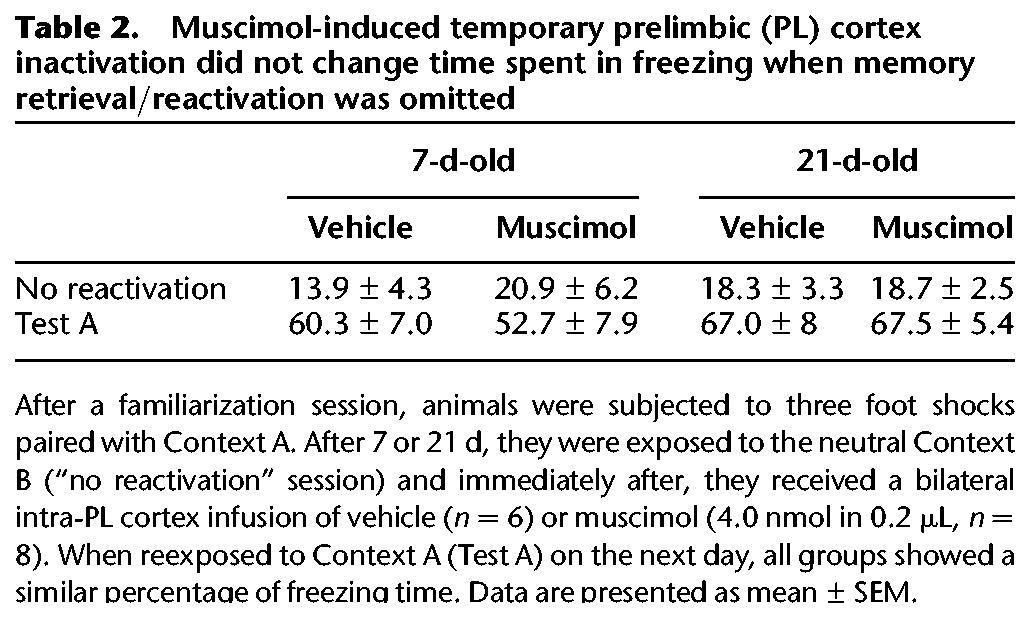
Importantly, the muscimol-induced temporary PL cortex inactivation no longer blocked the reconsolidation of either 7- or 21-d-old fear memories when their retrieval/reactivation was omitted (Table 2).
Table 2.
Muscimol-induced temporary prelimbic (PL) cortex inactivation did not change time spent in freezing when memory retrieval/reactivation was omitted
Discussion
Inactivating the PL cortex during the reconsolidation of a 1-d-old fear memory reduced the freezing exhibited by rats when subsequently reexposed to the paired context. As the disrupting property of such an experimental intervention was shown 24 h after the infusion of muscimol, and its effect lasts for a shorter period than the time elapsed between drug administration and behavioral testing (Martin 1991; Allen et al. 2008), it is thought that this medial prefrontal cortex subregion was functional during Test A. As a result, the above-mentioned outcome is not directly attributable to a deficit in other aspects in which the PL cortex has been implicated, such as fear memory retrieval (Corcoran and Quirk 2007), behavioral expression of conditioned fear (Stevenson 2011), and attention (Sharpe and Killcross 2013). If the bilateral infusion of muscimol into the PL cortex is able to block the reconsolidation process, one could expect no changes in freezing time without briefly retrieving/reactivating the fear memory. Indeed, when administered after a brief exposure to Context B, muscimol did not reduce this conditioned fear response in the subsequent reexposure to Context A. This finding supports the premise that temporary PL cortex inactivation has disrupted fear memory by reconsolidation blockade.
Alternatively, one could associate the results from Experiment 1 with facilitated memory extinction. This process can be induced by repeated and/or longer presentations of the conditioned stimulus in the absence of the unconditioned one, and leads to the formation of a new memory that ultimately suppresses the original fear memory (Bouton et al. 2006; Bustos et al. 2009). It also causes a reduction in freezing behavior indistinguishable from that produced when the reconsolidation of the original fear memory is blocked. Nevertheless, once the original fear memory is preserved in extinction, it may reemerge under certain conditions, such as the reinstatement induced by a mild reminder of the unconditioned stimulus (Myers and Davis 2002). In the present study, no evidence for reinstatement was found after post-reactivation PL cortex inactivation. This result agrees with those showing the absence of this memory extinction-related feature after impairing the reconsolidation process in other aversive memory paradigms, such as fear-potentiated startle (Lin et al. 2006), inhibitory avoidance (Taubenfeld et al. 2009), and auditory fear conditioning (Maddox and Schafe 2011). It also confirms that interference with memory reconsolidation, not extinction, accounts for the enduring attenuation of freezing seen in PL cortex-inactivated animals reexposed to the paired context.
Infusing muscimol bilaterally into the IL cortex after brief retrieval/reactivation of the fear memory does not disrupt it, as seen in PL cortex-inactivated animals. The expression of Zif268/Egr1 was also less pronounced in the IL than PL cortex during memory reconsolidation. Altogether, these findings suggest a differential contribution of these medial prefrontal cortex subregions to the reconsolidation of a fear memory.
A similar brain network involving the hippocampus, amygdala, and neocortex has been shown to be active during the acquisition and retrieval of a recent contextual fear memory (Tayler et al. 2013). When it is recalled more remotely, however, only the cortical network seems to remain stable (Quinn et al. 2008; Tayler et al. 2013). Herein, PL cortex inactivation-induced reconsolidation blockade was also demonstrated for fear memories acquired 7 and 21 d earlier. This indicates that activity in this area is still important for the restabilization and maintenance of more remote aversive memories after their retrieval and reactivation. Of note, the AC cortex has also been shown to have a corresponding role with the PL cortex in contextual memory reconsolidation over time (Einarsson and Nader 2012). Moreover, both the PL and AC cortices are alternate structures required for compensation during aversive memory processing following prolonged inhibition or damage to the hippocampus (Goshen et al. 2011; Zelikowsky et al. 2013).
The PL cortex sends excitatory projections to the basolateral nucleus of the amygdala that gives the valence of a stimulus (McDonald 1998), and to the hippocampus that is important for contextual and temporal information (Vertes 2006; Hoover and Vertes 2007). Based on this neuronal circuitry, one speculation would be that inactivating the PL cortex during reconsolidation attenuates the input to the basolateral nucleus of the amygdala and/or hippocampus, brain hubs that modulate the maintenance of emotional memories charged with negative valence (Nader et al. 2000; Debiec et al. 2002; Lee et al. 2004, 2006; Parsons et al. 2006; Tronson et al. 2006; de Oliveira Alvares et al. 2008; Rehberg et al. 2010; Giachero et al. 2013). Of note, the rat PL cortex has a similar anatomy and connectivity to that of the primate dorsal anterior cingulate cortex (McDonald 1998; Stefanacci and Amaral 2002), which has a similar functional role in maintaining the original aversive memory (Klavir et al. 2012). In humans, this area is suggested to be dysfunctional (commonly hyperactive) in anxiety disorders, such as post-traumatic stress (Fani et al. 2012; Pitman et al. 2012; Admon et al. 2013).
In summary, PL cortex activity is necessary for the reconsolidation of recent and remote contextual fear memories. Our results might help provide insight into the involvement of the human homolog of the PL cortex in conditions detrimental to mental health, in which interventions aimed at mitigating the maintenance of aberrant and long-lasting memories could have therapeutic potential.
Materials and Methods
Animals
Experiments were performed in male Wistar rats (Federal University of Santa Catarina vivarium, Florianopolis, Brazil) weighing 320–350 g and aged 14–16 wk. The animals were housed in groups of four per cage (50 × 30 × 15 cm), kept on a 12-h light–dark cycle (lights on at 7:00 a.m.), and received food and water ad libitum. All procedures were approved by the Institutional Ethical Committee for the Care and Use of Laboratory Animals of the Federal University of Santa Catarina (23080.016341/2010-30) in compliance with Brazilian Society of Neuroscience and Behavior guidelines.
Drugs
Temporary and reversible PL cortex inactivation was made with muscimol, which activates GABAA receptors and suppresses the neurophysiological activity within 0.5–1.0 mm of the infusion site (Martin 1991; Allen et al. 2008). Muscimol (Sigma) was dissolved in 0.9% phosphate-buffered saline (PBS). The dose and volume selected herein were based on pilot and previously published (Shah et al. 2004) studies.
Stereotaxic surgery and drug infusion
Each animal was anesthetized using 1.0 mL/kg of a solution containing xylazine (10 mg/mL, Carlier) and ketamine (100 mg/mL, Sespo), in association with local anesthesia (3.0% lidocaine with 1:50000 norepinephrine, Dentsply). After that, the animal was positioned in a stereotaxic frame. Two stainless-steel guide cannulas (length = 11 mm, and outer diameter = 0.6 mm) were implanted bilaterally and aimed at the PL cortex or the IL cortex, following the coordinates from the rat brain atlas by Paxinos and Watson (2009), and fixed to the skull with acrylic resin and two stainless-steel screws. The cannula tips were 2.2 mm (PL cortex) or 3.6 mm (IL cortex) above the site of drug infusion. A stylet was introduced inside each guide cannula to reduce possible occlusion. For post-surgery analgesia, each animal received subcutaneous flunixin meglumine (2.5 mg/kg, Schering-Plough), an analgesic, antipyretic, and anti-inflammatory drug. Moreover, an antibiotic formulation containing benzylpenicillin and streptomycin (1.0 mL/kg, Fort Dodge) was administered intramuscularly to prevent possible infection.
From 7 to 10 d after the stereotaxic surgery, each animal received a bilateral infusion with dental needles (outer diameter = 0.3 mm) introduced through the guide cannulas until their tips were 2.2 mm (PL cortex) or 3.6 mm (IL cortex) below the end of the cannula. Using two microsyringes connected to an infusion pump, a 0.2 µL/hemisphere of either vehicle or muscimol was injected during 1 min. A polyethylene catheter was interposed between the upper end of the dental needles and the microsyringes. To monitor drug flow, the displacement of an air bubble inside the polyethylene catheter was used. To prevent backflow, the needles were removed 40 sec after the end of injections.
Apparatus
Fear conditioning was assessed in a rectangular chamber (35 × 20 × 30 cm) with aluminum sidewalls and a front wall and ceiling door made of Plexiglas, designated as Context A. Its grid floor, made of stainless-steel bars (3.0 mm diameter, spaced 9.0 mm center-to-center), was connected to a circuit board and a shock generator (Insight) that could deliver electrical foot shocks as detailed in the general procedures section below. A second rectangular chamber (33 × 25 × 33 cm), designated as Context B, was made of glass and had a grid lid and transparent walls and floor, providing contextual cues as different as possible from the context used for conditioning. It was used as a neutral context unable to induce fear memory reactivation. A third chamber (40 × 25 × 30 cm), different from those previously described, was designated as Context C, and used in Experiment 3.
General procedures and data collection
Behavioral testing was carried out during the diurnal phase (1–5 p.m.) under low illumination (70 lux). In all cases, familiarization refers to the session in which the animal was placed in Context A and allowed to explore freely for 3 min. In the conditioning session, in the pre-shock period, the animal was placed again in Context A for 30 sec before receiving the unconditioned stimulus (three electrical foot shocks of 0.8 mA for 3 sec, with a 30-sec inter-trial period). The animal remained in this chamber for an additional 30 sec (post-shock period). To induce the retrieval/reactivation of the established fear memory, the animal was reexposed to the conditioning chamber (Context A) for 3 min, without the unconditioned stimulus being presented. In Test A, the animal was reexposed to Context A for 3 min, in the absence of unconditioned stimulus. Test B refers to the session where the animal was exposed to Context B, a neutral and unpaired chamber, also for 3 min. Both chambers were cleaned with a 10% ethanol–water solution after each session. The experimenter was unaware of the treatment condition in all studies.
Freezing behavior, a commonly used index of fear in rats (Blanchard and Blanchard 1969; Gazarini et al. 2013), and defined as a total absence of body and head movements except those associated with breathing, was continuously recorded during the experimental sessions by a video camera. The freezing time in each period was quantified (in seconds) by a trained observer (inter- and intra-rater reliabilities ≥90%) blind to the experimental groups using a stopwatch and was expressed as a percentage of total session time.
Histology
After the conclusion of each experiment, each rat was intraperitoneally (i.p.) anesthetized using 1.0 mL/kg of a solution containing xylazine (10 mg/mL, Carlier) and chloral hydrate (2.3 mg/mL, Vetec). Evans Blue (0.2 µL/side) was then injected through guide cannulas to mark the sites where vehicle or muscimol was previously infused. After that, each animal was transcardially perfused with 0.9% NaCl followed by a 10% formalin solution, and its brain was removed and immersed in a 10% formalin solution. Brain slices (50 µm thick) were obtained in a cryostat (Leica), mounted on glass microscope slides, and stained with Giemsa to localize anatomically the Evans Blue marks following diagrams from Paxinos and Watson's (2009) rat brain atlas. Their location was in the PL cortex or the IL cortex, and ranged from 3.7 to 2.7 mm anterior to the bregma. Animals receiving bilateral drug infusion outside these regions were excluded from the analysis. Figure 1 shows a diagram of a rat brain slice showing the placement of a representative infusion site in the PL cortex.
Immunohistochemistry analysis
Tissue preparation
One hour after being briefly reexposed to Context A or exposed to Context B, seven contextually conditioned animals were anesthetized with 15% chloral hydrate (2.5 mL/kg of body weight, i.p.) and xylazine (10 mg/mL/kg, i.p.), and perfused transcardially with sucrose followed by freshly prepared 4% paraformaldehyde in 0.1 M PBS, pH 7.4. After excision of the skull, brains were removed and post-fixed over 24 h in 4% paraformaldehyde and stored in a 20% sucrose solution for cryoprotection. Coronal brain sections (40 μm thick) were serially cut in a cryostat (Leica), and divided into five interleaved sets collected into an antifreeze solution (32.6% propylene glycol and 18.7% sucrose in PBS) and maintained at −20°C for later processing.
Zif268/Egr1 labeling
Sections were rinsed (1 × 5 min) with 0.01 M PBS to remove antifreeze solution. They were then treated for 30 min with 0.3% H2O2 to inactivate endogenous peroxidase activity. After washing (3 × 5 min) with 0.1 M PBS containing 0.15% Triton X-100 (PBS-X, Sigma-Aldrich), sections were incubated for 60 min at room temperature in 1% bovine serum albumin (BSA, Sigma Aldrich) in PBS-X. The sections were then incubated overnight at 4°C with rabbit anti-Zif268/Egr1 polyclonal antibody (1:800, dilution in BSA, Santa Cruz Biotechnology). The sections were washed with BSA-X (3 × 5 min) and incubated at room temperature for 90 min in biotinylated goat-anti rabbit IgG (1:200, dilution in PBS-X, Santa Cruz Biotechnology). They were then washed (3 × 5 min) with PBS-X and subsequently incubated for 2 h in avidin–biotin complex (1:500, dilution in PBS-X, Vector Laboratories). Sections were rinsed (3 × 5 min) in PBS-X and then stained with 3,3′-diaminobenzidine (DAB, Sigma-Aldrich). The peroxidase complex was visualized by a 5- to 10-min exposure to a chromogen solution containing 0.02% DAB with 0.3% nickel ammonium sulfate in 0.1 M PBS. The reaction was stopped by washing in PBS. The sections were mounted on gelatin-coated slides and then dehydrated and cover slipped with DPX (Sigma-Aldrich).
Regions of interest and Zif268/Egr1 counts
Three brain sections from 3.2 to 2.2 mm anterior from the bregma were selected by using the 10× objective of an Eclipse 50i light microscope (Nikon) equipped with a digital camera. Images of 0.46 mm2 were taken from the AC, PL, and IL cortices, and only the black oval nuclei were counted by using the software ImageJ. Each analyzed brain was counted in triplicate and the mean was calculated. The results are expressed as the number of Zif268/Egr1 positive cells per 0.1 mm2. The number of Zif268/Egr1-positive neurons was evaluated by a trained observer (inter-rater reliabilities ≥90%) blind to the experimental groups.
Statistical analysis
Results are expressed as mean ± SEM. After ensuring the assumptions of normality and homoscedasticity, the percentages of freezing time observed in Context A (reactivation session, Test A, Test A1, Test A2, extinction session, and/or reinstatement session) and Context B (no reactivation session and/or Test B) were subjected to separate repeated-measures or one-way ANOVA. After ensuring the assumptions of normality and homoscedasticity, the number of Zif268/Egr1-positive cells was subjected to two-way ANOVA. When ANOVA revealed a significant interaction between independent variables under study, the F values from their main effects were omitted. The Newman–Keuls test was used for post-hoc comparisons. The statistical significance level was set at P < 0.05.
Acknowledgments
This work was supported by Brazilian grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We thank Dr. C. Lino-de-Oliveira and Dr. E. Pavesi for helping us to conduct the immunohistochemical study.
Authors’ contributions: C.A.J.S. and L.J.B. designed the study; C.A.J.S., L.G., A.C.V and M.S.H. performed the experiments; and C.A.J.S. and L.J.B. analyzed the data. All authors participated in the discussion and writing of the manuscript.
References
- Admon R, Milad MR, Hendler T 2013. A causal model of post-traumatic stress disorder: Disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci 17: 337–347 [DOI] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH 2008. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Meth 171: 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC 1969. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol 68: 129–135 [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S 2006. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 60: 352–360 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ 2009. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos SG, Maldonado H, Molina VA 2009. Disruptive effect of midazolam on fear memory reconsolidation: Decisive influence of reactivation time span and memory age. Neuropsychopharmacology 34: 446–457 [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ 2010. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci 107: 2675–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ 2007. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27: 840–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA 2008. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience 154: 1648–1655 [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE 2004. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129: 267–272 [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K 2002. Cellular and systems reconsolidation in the hippocampus. Neuron 36: 527–538 [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ 2008. Pharmacological manipulation of memory reconsolidation: Towards a novel treatment of pathogenic memories. Eur J Pharmacol 585: 453–457 [DOI] [PubMed] [Google Scholar]
- Do Monte FH, Souza RR, Wong TT, Carobrez Ade P 2013. Systemic or intra-prelimbic cortex infusion of prazosin impairs fear memory reconsolidation. Behav Brain Res 244: 137–141 [DOI] [PubMed] [Google Scholar]
- Dudai Y 2012. The restless engram: Consolidations never end. Annu Rev Neurosci 35: 227–247 [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M 2004. Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron 44: 93–100 [DOI] [PubMed] [Google Scholar]
- Einarsson EÖ, Nader K 2012. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem 19: 449–452 [DOI] [PubMed] [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, Ressler KJ 2012. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 90: 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM 2008. The prefrontal cortex, 4th ed Elsevier, London [Google Scholar]
- Gazarini L, Stern CA, Carobrez AP, Bertoglio LJ 2013. Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting α1- and β-adrenergic receptors. Learn Mem 20: 210–219 [DOI] [PubMed] [Google Scholar]
- Giachero M, Bustos SG, Calfa G, Molina VA 2013. A BDNF sensitive mechanism is involved in the fear memory resulting from the interaction between stress and the retrieval of an established trace. Learn Mem 20: 245–255 [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Kramar C, Garagoli F, Rossato JI, Weisstaub N, Cammarota M, Medina JH 2013. Medial prefrontal cortex is a crucial node of a rapid learning system that retrieves recent and remote memories. Neurobiol Learn Mem 103: 19–25 [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K 2011. Dynamics of retrieval strategies for remote memories. Cell 147: 678–689 [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ 2001. Cellular imaging of Zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: Selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 21: 2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ 2003. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27: 555–579 [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212: 149–179 [DOI] [PubMed] [Google Scholar]
- Joel D, Tarrasch R, Feldon J, Weiner I 1997. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited, 8-arm radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain Res 765: 37–50 [DOI] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R 2012. Low-frequency stimulation depresses the primate anterior-cingulate-cortex and prevents spontaneous recovery of aversive memories. J Neurosci 32: 8589–8597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF 2009. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem 16: 520–529 [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304: 839–843 [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ 2006. Reconsolidation and extinction of conditioned fear: Inhibition and potentiation. J Neurosci 26: 10051–10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW 2006. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem 13: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE 2011. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem 18: 579–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH 1991. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127: 160–164 [DOI] [PubMed] [Google Scholar]
- McDonald AJ 1998. Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332 [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74 [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE 1995. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681–688 [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE 1993. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113 [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M 2002. Behavioral and neural analysis of extinction. Neuron 36: 567–584 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE 2000. The labile nature of consolidation theory. Nat Rev Neurosci 1: 216–219 [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ 2006. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci 23: 1853–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 2009. The rat brain in stereotaxic coordinates, Compact 6th ed. Academic Press, San Diego, CA [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I 2012. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: 769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS 2008. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem 15: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg K, Bergado-Acosta JR, Koch JC, Stork O 2010. Disruption of fear memory consolidation and reconsolidation by actin filament arrest in the basolateral amygdala. Neurobiol Learn Mem 94: 117–126 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK 2009. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci 29: 14299–14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Anderson RM, Routtenberg A 1976. Stimulation of rat medial or sulcal prefrontal cortex during passive avoidance learning selectively influences retention performance. Brain Res 103: 243–259 [DOI] [PubMed] [Google Scholar]
- Sara SJ 2000. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn Mem 7: 73–84 [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D 2004. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res 1028: 112–115 [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Killcross S 2013. The prelimbic cortex contributes to the down-regulation of attention toward redundant cues. Cereb Cortex (in press) [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D Jr., Corcoran KA, Lebrón-Milad K, Quirk GJ 2006. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci 24: 1751–1758 [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG 2002. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. J Comp Neurol 451: 301–323 [DOI] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ 2012. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology 37: 2132–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CW 2011. Role of amygdala-prefrontal cortex circuitry in regulating the expression of contextual fear memory. Neurobiol Learn Mem 96: 315–323 [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM 2009. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biol Psychiatry 65: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ 2013. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr Biol 23: 99–106 [DOI] [PubMed] [Google Scholar]
- Thomas KL, Hall J, Everitt BJ 2002. Cellular imaging with Zif268 expression in the rat nucleus accumbens and frontal cortex further dissociates the neural pathways activated following the retrieval of contextual and cued fear memory. Eur J Neurosci 16: 1789–1796 [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR 2006. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9: 167–169 [DOI] [PubMed] [Google Scholar]
- Vertes RP 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142: 1–20 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS 2013. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci 110: 9938–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fukushima H, Kida S 2011. Induction and requirement of gene expression in the anterior cingulate cortex and medial prefrontal cortex for the consolidation of inhibitory avoidance memory. Mol Brain 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]