Abstract
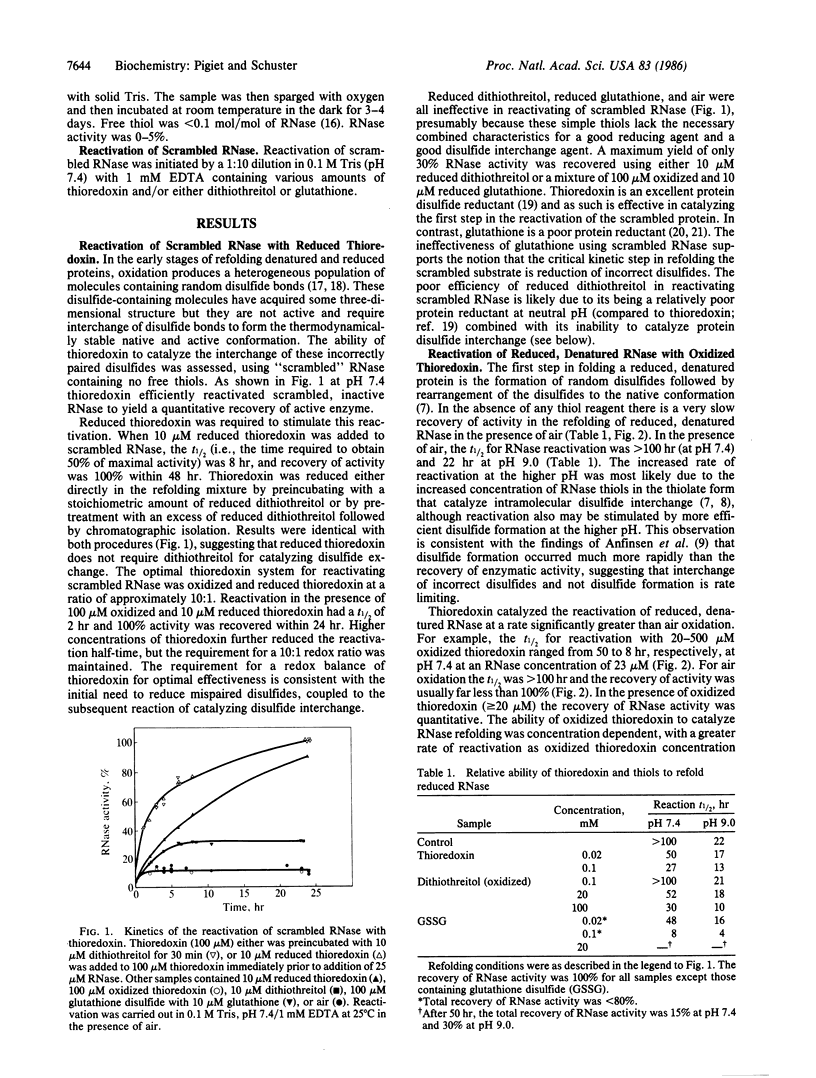
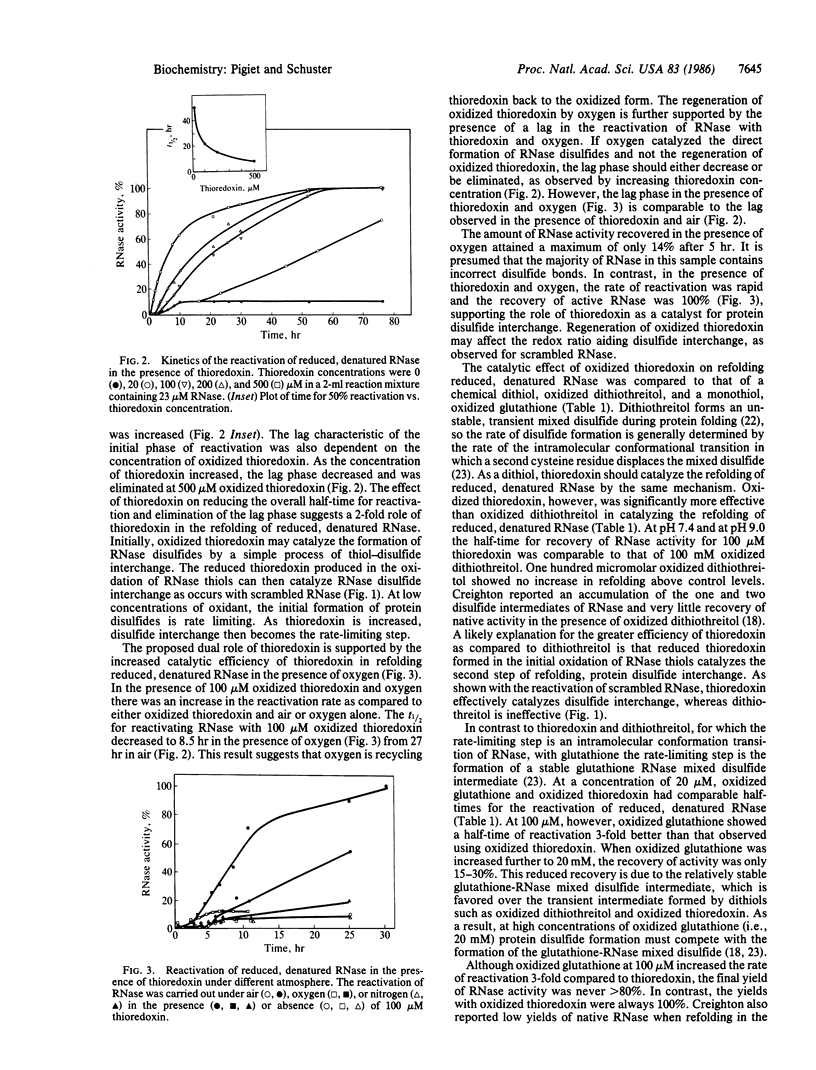
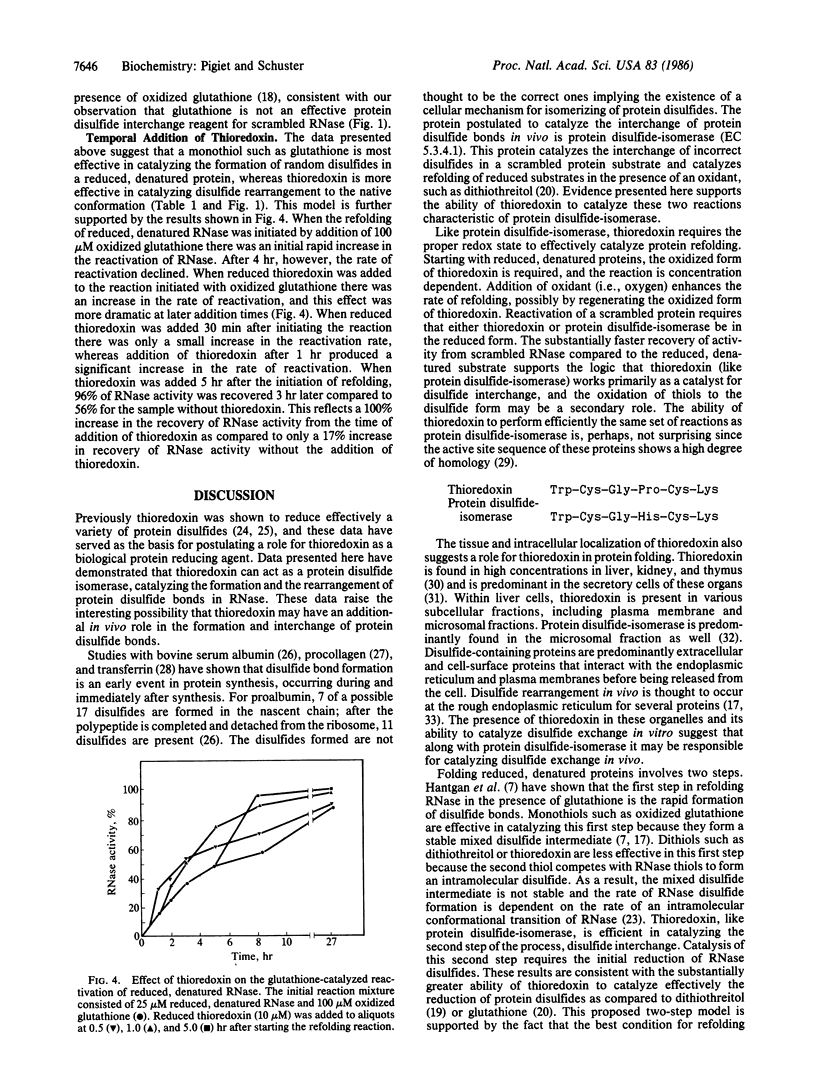
Thioredoxin, a known catalyst for reducing protein disulfides, was shown to catalyze efficiently the refolding of pancreatic RNase either from the reduced, denatured form or from the scrambled form containing oxidized but incorrectly paired disulfides. Thioredoxin was 1000-fold more efficient on a molar basis than the model dithiol, dithiothreitol, in reactivating reduced, denatured RNase, suggesting that thioredoxin acts as an efficient catalyst for disulfide interchange. Starting with reduced, denatured RNase, enzyme activity was recovered quantitatively with a t1/2 of 30 hr with 100 microM thioredoxin compared to only a 10-20% recovery of activity in the control using air oxidation. Oxygen further stimulated the effectiveness of thioredoxin severalfold. Thioredoxin was most effective in reactivating inactive scrambled RNase, which contained mispaired disulfides, showing a t1/2 of 2 hr. Reduced thioredoxin was optimal for catalyzing disulfide interchange in scrambled RNase, whereas oxidized thioredoxin was required for reactivation of the reduced, denatured species. Optimal reactivation of scrambled RNase required a mixture of reduced and oxidized thioredoxin. Addition of reduced thioredoxin after initiating refolding of reduced denatured RNase with oxidized glutathione effected a rapid reactivation of RNase, suggesting a two-step model for protein refolding in which the monothiol catalyzes the rapid initial formation of protein disulfides and thioredoxin catalyzes the second step of disulfide interchange. Arguments are presented suggesting that thioredoxin may serve an in vivo role analogous to the protein disulfide-isomerase (EC 5.3.4.1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., HABER E., SELA M., WHITE F. H., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. K., Schaffer S. W., Wetlaufer D. B. Nonenzymic reactivation of reduced bovine pancreatic ribonuclease by air oxidation and by glutathione oxidoreduction buffers. J Biol Chem. 1975 Nov 10;250(21):8477–8482. [PubMed] [Google Scholar]
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- CROOK E. M., MATHIAS A. P., RABIN B. R. Spectrophotometric assay of bovine pancreatic ribonuclease by the use of cytidine 2':3'-phosphate. Biochem J. 1960 Feb;74:234–238. doi: 10.1042/bj0740234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Hillson D. A., Freedman R. B. Catalysis by protein-disulphide isomerase of the unfolding and refolding of proteins with disulphide bonds. J Mol Biol. 1980 Sep 5;142(1):43–62. doi: 10.1016/0022-2836(80)90205-3. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Intermediates in the refolding of reduced ribonuclease A. J Mol Biol. 1979 Apr 15;129(3):411–431. doi: 10.1016/0022-2836(79)90504-7. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Kinetics of refolding of reduced ribonuclease. J Mol Biol. 1977 Jun 25;113(2):329–341. doi: 10.1016/0022-2836(77)90145-0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Hantgan R. R., Hammes G. G., Scheraga H. A. Pathways of folding of reduced bovine pancreatic ribonuclease. Biochemistry. 1974 Aug 13;13(17):3421–3431. doi: 10.1021/bi00714a001. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978 Sep 19;17(19):4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Holmgren A., Reichard P. Thioredoxin 2: cleavage with cyanogen bromide. Eur J Biochem. 1967 Sep;2(2):187–196. doi: 10.1111/j.1432-1033.1967.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Kallis G. B., Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980 Nov 10;255(21):10261–10265. [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Lunn C. A., Kathju S., Wallace B. J., Kushner S. R., Pigiet V. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10469–10474. [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy M., Lantz C., Lunn C. A., Pigiet V. Isolation and characterization of a protease-nicked thioredoxin. J Biol Chem. 1981 Jul 10;256(13):6646–6650. [PubMed] [Google Scholar]
- Morgan E. H., Peters T., Jr The biosynthesis of rat transferrin. Evidence for rapid glycosylation, disulfide bond formation, and tertiary folding. J Biol Chem. 1985 Nov 25;260(27):14793–14801. [PubMed] [Google Scholar]
- Oohira A., Nogami H., Kusakabe A., Kimata K., Suzuki S. Structural differences among procollagens associated with rough and smooth microsomes from chick embryo cartilage. J Biol Chem. 1979 May 10;254(9):3576–3583. [PubMed] [Google Scholar]
- Peters T., Jr, Davidson L. K. The biosynthesis of rat serum albumin. In vivo studies on the formation of the disulfide bonds. J Biol Chem. 1982 Aug 10;257(15):8847–8853. [PubMed] [Google Scholar]
- Rozell B., Hansson H. A., Luthman M., Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol. 1985 Jul;38(1):79–86. [PubMed] [Google Scholar]
- Russel M., Model P. Thioredoxin is required for filamentous phage assembly. Proc Natl Acad Sci U S A. 1985 Jan;82(1):29–33. doi: 10.1073/pnas.82.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked Z., Szajewski R. P., Whitesides G. M. Rates of thiol-disulfide interchange reactions involving proteins and kinetic measurements of thiol pKa values. Biochemistry. 1980 Sep 2;19(18):4156–4166. doi: 10.1021/bi00559a004. [DOI] [PubMed] [Google Scholar]


