Abstract
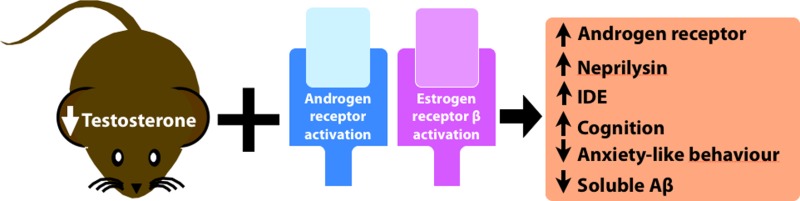
Decreases of the sex steroids, testosterone and estrogen, are associated with increased risk of Alzheimer’s disease. Testosterone and estrogen supplementation improves cognitive deficits in animal models of Alzheimer’s disease. Sex hormones play a role in the regulation of amyloid-β via induction of the amyloid-β degrading enzymes neprilysin and insulin-degrading enzyme. To mimic the effect of dihydrotestosterone (DHT), we administered a selective androgen receptor agonist, ACP-105, alone and in combination with the selective estrogen receptor β (ERβ) agonist AC-186 to male gonadectomized triple transgenic mice. We assessed long-term spatial memory in the Morris water maze, spontaneous locomotion, and anxiety-like behavior in the open field and in the elevated plus maze. We found that ACP-105 given alone decreases anxiety-like behavior. Furthermore, when ACP-105 is administered in combination with AC-186, they increase the amyloid-β degrading enzymes neprilysin and insulin-degrading enzyme and decrease amyloid-β levels in the brain as well as improve cognition. Interestingly, the androgen receptor level in the brain was increased by chronic treatment with the same combination treatment, ACP-105 and AC-186, not seen with DHT or ACP-105 alone. Based on these results, the beneficial effect of the selective ERβ agonist as a potential therapeutic for Alzheimer’s disease warrants further investigation.
Keywords: Alzheimer’s disease, mouse model, SARM, ERβ agonist, cognition, neprilysin, insulin-degrading enzyme, β-amyloid
Alzheimer’s disease (AD) is the predominant cause of dementia in aging populations. Age is the major risk factor for AD, and studies have established a link between depleted sex hormone levels in the brain and the risk for AD in both males and females.1−4 Androgen replacement therapy using testosterone and high levels of free testosterone in men are associated with reduced risk of AD.5−7 Depletion of androgens through gonadectomy in wild-type male rats results in increased levels of soluble amyloid-β (Aβ);8,9 conversely, androgens decrease secretion levels of soluble Aβ in culture.1−4 These studies suggest that sex hormones have an important role in the pathogenesis of AD.
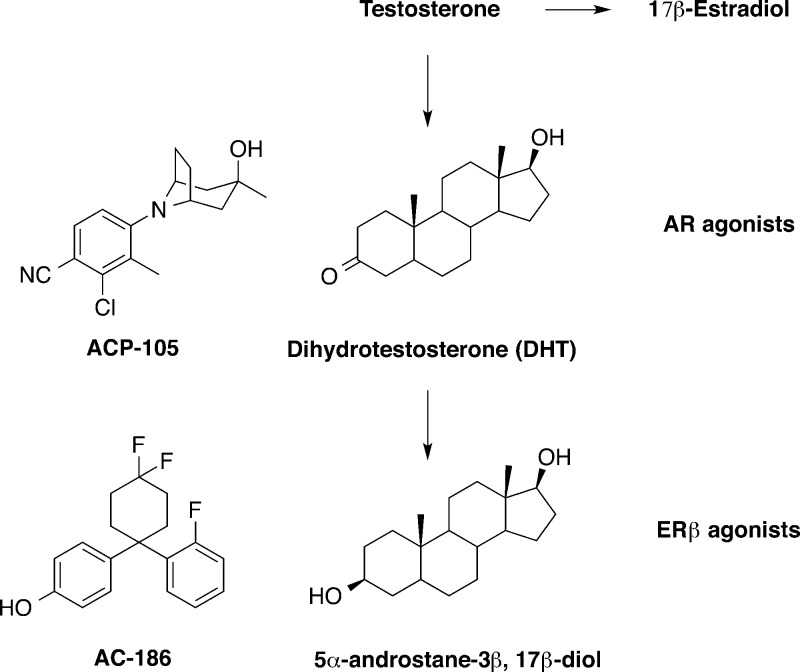
The utility of steroidal androgens as therapies is limited due to the risk of serious side effects, for example, cardiovascular events, hepatotoxicity, and the potential of prostatic hyperplasia or cancer.5,7,10,11 Currently available forms of testosterone exert both anabolic and androgenic effects. Therefore the search for selective androgen receptor modulators (SARMs) that are tissue selective is important. SARMs have been tested in degenerative diseases such as sarcopenia and osteopenia. The SARM called ACP-105 (Figure 1) is well characterized and crosses the blood–brain barrier (brain/plasma ratio 2.7) and functions as a selective androgen receptor (AR) agonist.8,9,12,13 It is beneficial in animal models of dementia, in both male and females.12−16 For example, ACP-105 up-regulates androgen receptor levels in the hippocampus and improves cognitive performance in castrated female mice.8,13
Figure 1.
AR and ERβ agonists: ACP-105 and AC-186.
How androgens and estrogens decrease AD risk is not fully understood. Testosterone possesses both androgenic and estrogenic effects as it can be converted into estrogens. Testosterone is converted to dihydrotestosterone (DHT) and 17β-estradiol (E2); the latter potently activates both nuclear estrogen receptors (ER), α and β. The DHT metabolite 5α-androstane-3β,17β-diol (Figure 1) selectively activates ERβ.8,15,17 Thus, DHT is potentially both androgenic and estrogenic. Interestingly, estrogen and testosterone depletion, induced via chemical castration in men undergoing prostate cancer treatment, is accompanied by a significant increase in plasma Aβ levels.1,18 Estrogen therapy, when initiated at the onset of menopause, is reported to reduce the risk or delay the onset of AD in women.1,19 Additionally, estrogen prevents or slows the development of pathology in animal models of AD.13,20−26 The use of E2 as a treatment is limited due to its feminizing effects and increased cancer risk. These side effects are believed to be due to activation of ERα.25,27 Therefore, compounds selective for ERβ could potentially be used safely in both men and women. Selective ERβ agonists promote cognitive improvement and enhance long-term potentiation via morphological changes in hippocampal neurons.13,28 Such changes are not observed in ERβ knockout mice.13,28 Moreover, ERβ, but not ERα, agonists improve hippocampus-dependent memory tasks in ovariectomized rats.29 Most studies involving estrogens have focused on females, and the effects of ER agonists in males warrants further investigation.
The metabolism of amyloid precursor protein (APP) and tau is critical to the pathophysiology of AD and is partly modulated by sex hormones. The predominant estrogen, E2, up regulates α-secretase expression, promoting non-amyloidogenic cleavage of APP.30 In addition, E2 and testosterone modulate the expression of Aβ degrading enzymes, insulin-degrading enzyme (IDE) and neprilysin (NEP). The genes encoding these two enzymes contain androgen and estrogen response elements.31 Neprilysin appears to be the major Aβ-degrading enzyme, because it accounts for the majority of the inhibitor-sensitive endopeptidase activity in the brain.32 IDE and NEP preferentially degrade various species of Aβ, including oligomers, and lentiviral overexpression of NEP prevents Aβ deposition in the brains of Tg APP mice expressing high levels of Aβ42.33 This suggests that therapies promoting NEP and IDE activities might retard development of AD pathology in vivo.34
In this study, we investigated the effects of AR and ERβ stimulation using the SARM ACP-105 and the selective ERβ agonist AC-186 in gonadectomized male triple transgenic AD (3xTg-AD) mice. The 3xTg-AD mouse model overexpresses human APP, presenilin 1, and tau and exhibits the cardinal features of AD, that is, progressive Aβ deposition, neurofibrillary tangles, and neuronal loss.1,35 In the brains of 3xTg-AD mice, progressive Aβ deposition starts around 6 months of age.1 At 6 months, the mice exhibit impaired retention in the Morris water maze and impaired fear conditioning.5,7 Testosterone-depleted 3xTg-AD mice develop earlier cognitive decline.8 We found that a combination of androgen and estrogen receptor modulating drugs modified long-term spatial memory in the Morris water maze and decreased anxiety-like behavior in gonadectomized 3xTg-AD mice. The treatment increased the levels of NEP and IDE and decreased Aβ levels. The results support the idea of targeting androgen and estrogen receptors in AD.
Results and Discussion
The compounds used in the study are described in Figure 1.
Androgen and Estrogen Receptor Modulating Drugs Improve Cognition and Reduce Anxiety-Like Behavior
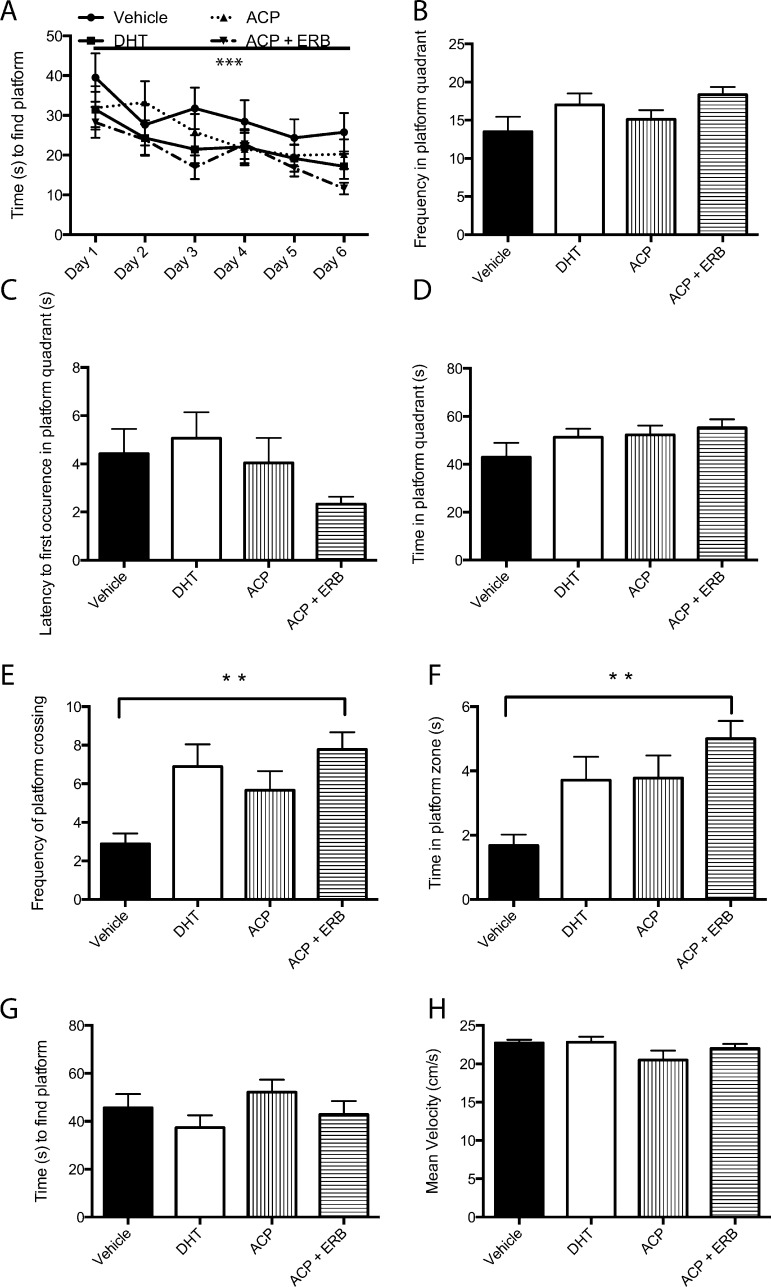
Androgens and estrogens play a role in cognition and emotional behavior.14,16 For example, gonadectomized 3xTg-AD mice exhibit greater cognitive deficits.8 In this study, male gonadectomized 3xTg-AD mice treated with SARM/ERβ agonist for 4 months display improved long-term spatial memory in the Morris water maze (MWM, Figure 2; Supporting Information demonstrates that at the doses used in this study both compounds stimulate AR and ERβ receptors). Mice were trained to find a hidden platform over 6 days. There were no significant differences between the treatment groups and vehicle treated mice in the acquisition of the task; however, there was a significant effect of day, indicating that all mice learned the task by the sixth day (Figure 2A). On the seventh day, the hidden platform was removed, and the probe trial was conducted. Gonadectomized male 3xTg-AD mice treated with the SARM ACP-105 and ERβ agonist displayed increased frequency of platform crossings (Figure 2E) and spent more time in the platform zone (Figure 2F) during the probe trial (p < 0.05). Frequency of entries into the platform quadrant (Figure 2B), latency to first occurrence in the platform quadrant (Figure 2C), and time spent in the platform quadrant (Figure 2D) did not differ significantly from vehicle controls. To test working memory, we applied a reversal learning trial. Twenty-four hours after the probe trial, mice were placed in the MWM, where the hidden platform had a new location. The mice were trained (four trials) to learn the new location of the hidden platform. Mice treated with SARM/ERβ agonist did not differ significantly from control mice in the time to find the hidden platform (Figure 2G). To control for possible confounds in movement scores, the swim speed of the mice was analyzed to reveal no significant difference between the groups (Figure 2H). The cognitive deficit observed in gonadectomized 3xTg-AD mice aged 7 months is modest,8 and the results from our study point toward a moderate effect of androgen and estrogen receptor modulating compounds in male gonadectomized 3xTg-AD mice in the MWM.
Figure 2.
Cognitive behavior in gonadectomized 3xTg-AD mice treated with SARM/ERβ agonists for a 4-month period. (A) Learning curve demonstrating all groups of mice learned to find the hidden platform in the MWM over 6 days (p < 0.0001). (B) Analysis of the probe trial: No significant difference between the groups in the frequency in the platform quadrant. (C) Latency to first occurrence to the platform quadrant during the probe trial did not reveal a significant difference between the groups. (D) Time spent in the platform quadrant did not differ significantly between groups. (E) The frequency of platform crossings indicating when the mouse swam over the area where the hidden platform was placed in the MWM was significantly increased in animals treated with a combination of ACP-105/ERB-186 (p < 0.05). (F) Probe trial analysis revealed a significant increase in the time spent in the hidden platform zone in mice treated with a combination of ACP and ERβ agonist (p < 0.05). (G) A test of working memory, reversal learning, was conducted one day after the probe trial. Mice had to relearn a new location of the hidden platform. There was no significant difference between the groups. (H) Swim speed did not differ between the groups. Data expressed as means ± SEM. Asterisk denotes statistical significance (*p < 0.05; ***p < 0.0001).
Naive 3xTg-AD mice characteristically have memory deficits. They display impaired retention at 4–6 months on the first daily trial in the MWM from the day before.1 In addition, impaired probe trial performance is seen at 6 months of age.1 We also know that gonadectomy in rodents impairs spatial and working memory as demonstrated in the radial arm maze and MWM.13,20,22,25,26 In another model of AD, gonadectomized ApoE4, ApoE3, and ApoE knock out male mice have decreased time in the target quadrant in the probe trial of MWM. (25) Interestingly, a study using a model of AD, ApoE4 Tg female mice, suggested that SARM ACP-105 treatment improved memory, especially the recall in the probe trial of the MWM.13 In our hands, gonadectomized 3xTg-AD mice treated with SARM/ERβ agonist display improved long-term spatial memory, especially in recall in the probe trial of the MWM, compared with vehicle control treated mice over a period of 4 months, mirroring the result described by Acevedo and colleagues.13
Anxiety symptoms are common in AD cases3,4,36−38 and have been modeled in an animal model of AD.6,39 Both the androgen and estrogen receptors play a role in anxiety-like behavior as demonstrated through gonadectomy studies where testosterone decreases anxiety-like behavior in gonadectomized mice.9,16
A pilot study to evaluate the behavioral and biochemical effect of castration on 3xTg-AD mice was conducted prior to this current study. Castration decreased the amount of spontaneous alternations as a measure of working memory (Supplementary Figure 1A, Supporting Information) without affecting locomotor behavior (Supplementary Figure 1B,C, Supporting Information). Anxiety-like behavior was also observed as a decrease in the time spent in the open arms of the elevated plus maze (Supplementary Figure 1D,E, Supporting Information). This data supports previous studies conducted by Rosario et al.8 In addition to behavioral deficits following castration, castrated 3xTg-AD mice have increased levels of APP and decreased neprilysin and levels of the androgen receptor in the brain (Supplementary Figure 2A,B, Supporting Information).
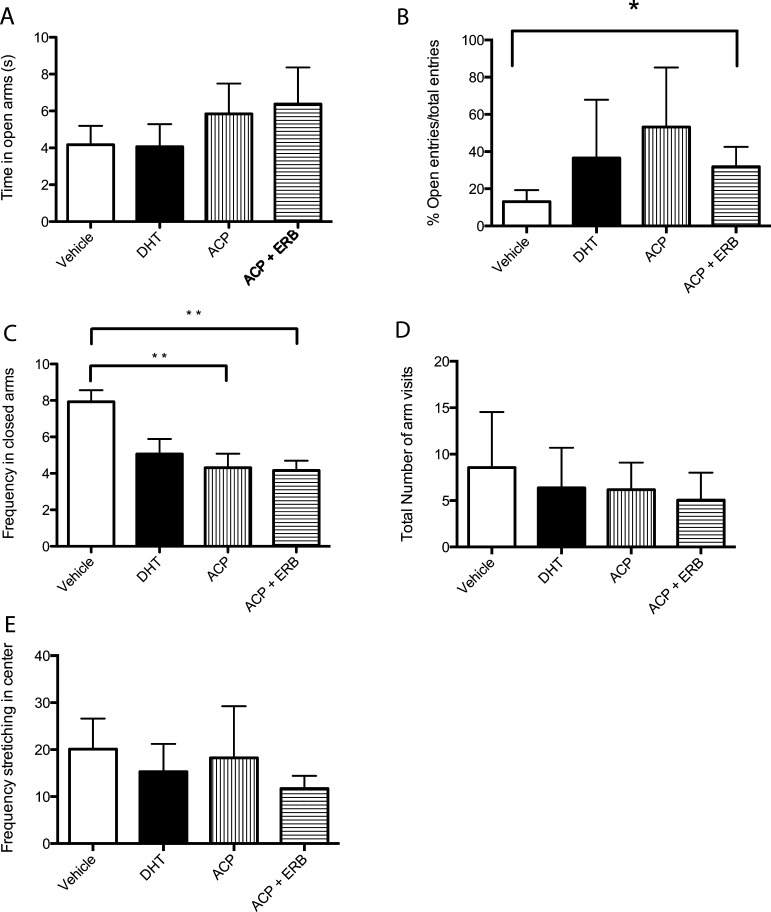
In our study, behavioral tests were employed to interrogate anxiety-like behavior in male gonadectomized 3xTg-AD mice treated with SARM/ERβ agonist. Transgenic mouse groups treated with ACP-105 alone or ACP-105 in combination with AC-186 displayed reduced anxiety-like behaviors on the elevated plus maze (Figure 3). Time in the open arms of the maze (Figure 3A) did not reveal a significant difference between the groups (p > 0.05). The number of open arm entries as a percentage of the total arm entries was significantly increased in mice treated with ACP-105 in combination with AC-186 compared with controls (Figure 3B, p < 0.05). Treated mice showed reduced anxiety-like behavior displayed as a decreased frequency of entries to the closed arms of the elevated plus maze (Figure 3C, p < 0.005). In contrast, there was no difference between treatment groups with respect to the total number of arms visited, indicating similar locomotor behavior (Figure 3D). Stretching-attend postures correlate with stress and anxiety;40 therefore stretching in the center of the elevated plus maze was analyzed to reveal no significant difference between the groups (Figure 3E, p > 0.05).
Figure 3.
Androgen and estrogen receptor modulating drugs reduce anxiety-like behavior on the elevated plus maze in mice treated for 4 months. (A) The time spent in the open arms of the elevated plus maze did not differ between groups. (B) Percentage of open arm entries/total entries differed significantly between groups. (C) A significant reduction in the number of entries to the closed arms in mice treated with ACP-105 alone and ACP-105 in combination with AC-186 compared with the vehicle controls (p < 0.05). (D) The number of total arm entries did not differ significantly between groups. (E) The frequency of stretching in the center of the elevated plus maze was anlayzed to reveal no significant difference between the groups. Data expressed as means ± SEM. Asterisk denotes statistical significance (*p < 0.05).
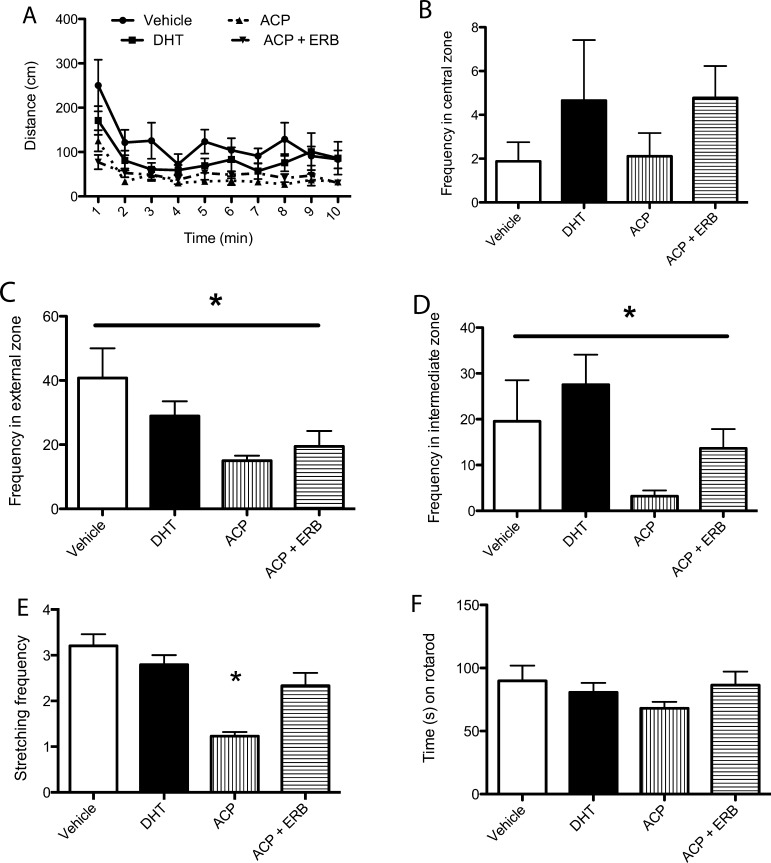
Investigation into the spontaneous locomotor ability and anxiety-like behavior in the open field was assessed in mice treated with SARMs/ERβ agonist. Tg mice were placed into the open field. The analysis in the open field included distinguishing three zones: outer, intermediate, and inner zones. Reduced anxiety-like behavior is expected to exhibit as increases in frequency in the inner zone compared with outer and intermediate zones. Spontaneous locomotor activity was analyzed over 10 min and did not differ between treatment groups (Figure 4A, p > 0.05). There was increased activity in the first 2 min and a subsequent decrease reflecting habituation to the novel environment (Figure 4A). Zone analysis of locomotor activity in the open field revealed a significant effect of treatment in the number of entries (frequency) to the external (Figure 4C, p < 0.05) and intermediate zones (Figure 4D, p < 0.05) but not the central zone (Figure 4B, p > 0.05). To further analyze the anxiety related behavior in the open field, stretching frequency was analyzed to reveal a significant decrease in animals treated with ACP-105 alone (Figure 4E, p < 0.05). A further indication that there was no locomotor impairment in any of the treatment groups was determined by the rotarod with no difference in the amount of time spent on the apparatus (Figure 4F, p > 0.05).
Figure 4.
Androgen and estrogen receptor modulating drugs reduce anxiety-like behavior in the open field in mice treated for 4 months. (A) Tg treated mice did not show changes in spontaneous locomotor activity in the open field as measured by distance moved (p > 0.05). (B) The frequency of entries to the central zone was not significantly different between the groups (p > 0.05). (C) The frequency of entries to the extenal zone was significantly different between the groups (p < 0.05) with a decrease in treated animals compared with vehicle control. (D) The frequency of entries to the intermediate zone was significantly decreased (p < 0.05) in treated animals compared with vehicle control with no significant difference of DHT treatment to controls. (E) Stretching frequency was significantly decreased (p < 0.05) in ACP-105 treated animals compared with vehicle control. (F) Time spent on the rotarod was not significantly different between the groups (p > 0.05). Data expressed as means ± SEM. Asterisk denotes statistical significance (*p < 0.05).
The main finding from the behavioral study performed using the elevated plus maze and open field on gonadectomized 3xTg-AD mice was the reduced anxiety-like phenotype in Tg mice treated with both SARM/ERβ agonist in combination. The decrease in thigmotaxis could be due to the overall decrease in distance traveled in the open field. This is also supported by the significant decrease in stretching frequency in Figure 4E. The observation of a similar number of arm visit scores in all mice confirmed that the reduced anxiety-like phenotype in treated Tg mice is not due to altered locomotor behavior.
Testosterone’s antianxiety effects have been documented.2,16 What is important to note is a study investigating the role of testosterone’s metabolites that bind to ERβ (3α-diol and 3β-diol) mediates testosterone’s antianxiety and cognitive-enhancing effects in male gonadectomized rats.10,11,14 In our study, both ACP-105 alone and ACP-105 in combination with AC-186 had a significant effect on the frequency of entries to the closed arms, and the frequency of entries in both treatment groups were not significantly different from each other. What can be inferred from this data is that stimulation of the AR and ER by SARM/ERβ treatment lead to reduced anxiety-like behavior in the elevated plus maze and open field. Reduced anxiety-like behavior in the open field would be expected to express itself as enhanced initial exploratory activity coupled with faster habituation, as well as a high proportion of activity in the central zone. Treatment groups displayed slightly reduced habituation compared with vehicle control animals and entered the external zone significantly less than the controls. This suggests that the low-anxiety-like phenotype due to treatment with AR and ER modulating compounds was demonstrated on the elevated plus maze and in the open field.
Androgen and Estrogen Receptor Modulating Drugs Decrease Aβ and Increase Amyloid-β Degrading Enzymes Post-treatment
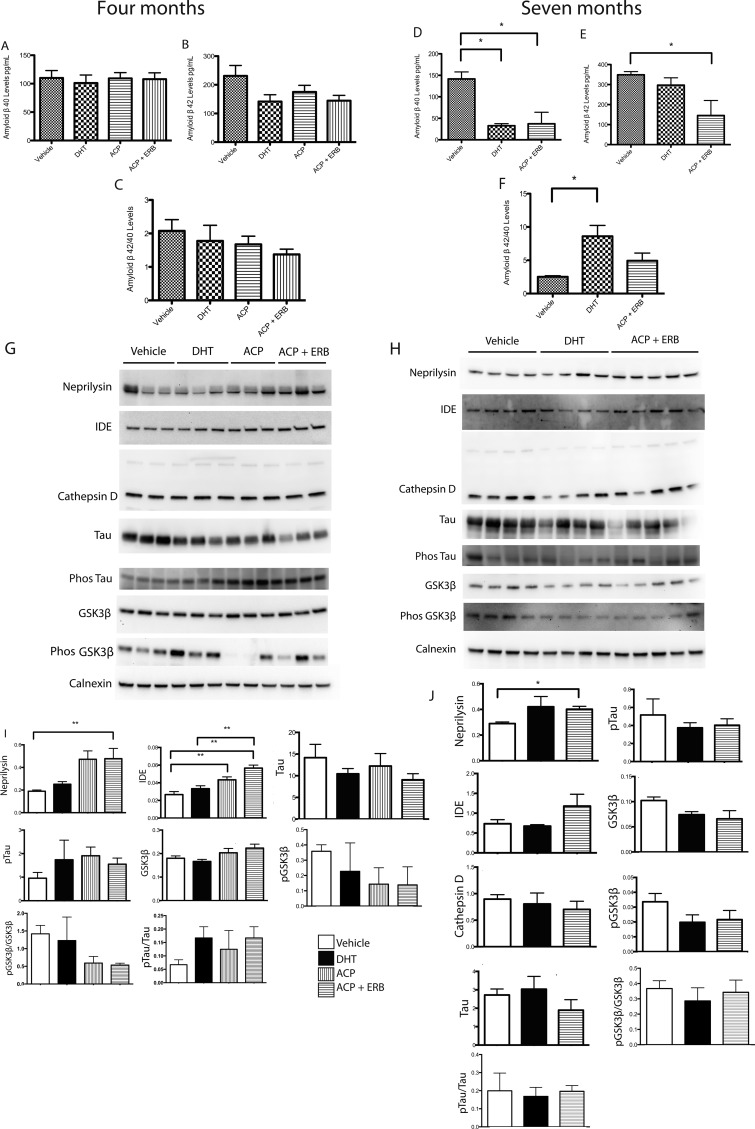
AD is characterized by the deposition of Aβ in the brain. To assess the efficacy of SARM/ERβ agonist to modify this pathological hallmark, the levels of Aβ were assessed using enzyme-linked immunosorbent assay (ELISA, Figure 5). The assay indicates that androgen and estrogen receptor modulating compounds decreased the levels of Aβ40 and Aβ42 and that the ratio of Aβ42/Aβ40 is significantly decreased after drug treatment following 7 but not 4 months of treatment (Figure 5). Aβ levels were also analyzed in the subiculum of mice treated for both 4 and 7 months using immunohistochemistry to reveal a modest decrease in Aβ/C99 immunoreactivity (Supplementary Figure 3, Supporting Information). In addition, to assess changes in several proteins associated with AD pathology, Western blot analysis was used to determine hippocampal protein levels of neprilysin, IDE, cathepsin D, tau, phosphorylated tau, GSK3β and phosphorylated GSK3β.
Figure 5.
The levels of Aβ and related proteins in gonadectomized 3xTg-AD mice treated with SARMs/ERβ agonists for 4 and 7 months. (A) The levels of Aβ40 in the brain of mice treated with SARMs and ERβ agonists for 4 months. There was no significant difference between the groups (p > 0.05). (B) The levels of Aβ42 in the brain of mice treated with SARMs and ERβ agonists for 4 months. There was no significant difference between the groups (p > 0.05). (C) The ratio of Aβ42/Aβ40 in the brains of mice treated with SARMs and ERβ agonists for 4 months. There was no significant difference between the groups, although the data does indicate a trend for a decline in the levels. (D) The levels of Aβ40 in the brains of mice treated with SARMs and ERβ agonists for a period of 7 months are significantly decreased (p < 0.05). (E) The levels of Aβ42 in the brains of mice treated with SARMs and ERβ agonists for a period of 7 months are significantly decreased (p < 0.05). (F) The ratio of Aβ42/Aβ40 in the brains of mice treated with SARMs and ERβ agonists for 7 months. There was a significant difference between the groups (p < 0.05). (G) Representative Western blots from gonadectomized 3xTg-AD mice treated with SARM/ERβ agonist for 4 months compared with controls. Treated mice have increased neprilysin and insulin-degrading enzyme (IDE) levels compared with vehicle control (n = 4 or 5 per group). (H) Representative Western blots from gonadectomized 3xTg-AD mice treated with SARM/ERβ agonist for 7 months and controls. (I) Brain protein levels were quantified using analysis software. Quantification (I, J) of brain protein levels following 4 and 7 months treatment of neprilysin, IDE, tau, phos tau, GSK3β, and phosphorylated GSK3β. Calnexin is an ER marker and was utilized as a loading control as the brain homogenates were prepared in RIPA buffer. Data expressed as means ± SEM. Asterisk denotes statistical significance (**p < 0.01, *p < 0.05).
Two Aβ degrading enzymes, neprilysin and IDE, both had significant increases in the mice treated with both the androgen and estrogen receptor activating compounds for both 4 and 7 month treatment groups (Figure 5I,J). Androgens and estrogens are known to modulate these two enzymes12,13,31,41 and modulate Aβ levels (Figure 5). The total levels and ratio of phosphorylated protein to total protein of tau and its related kinase, GSK3β, were not significantly altered after drug treatment compared with vehicle control mice (Figure 5I,J).
Gonadectomized male 3xTg-AD mice exhibit increased Aβ pathology.8,12,13,15 The lack of a significant effect after 4 months treatment may relate to the fact that Aβ pathology does not occur uniformly in brain, although samples of total brain homogenates are assayed. In addition, a possible effect on neuronal Aβ may be diluted by a lack of effect on Aβ associated with glial cells and blood vessels, which are also present in the assayed brain samples. The reduction in Aβ levels observed in this study could be a result of the increased neprilysin and IDE levels driven by AR stimulation, ERβ, or both, which is a gender dependent effect.13,41Studies indicate that increased neprilysin results in decreased soluble brain Aβ.15,17,42
Additionally, in different mouse models of AD, amyloid reduction also reduces tau pathology and results in neuroprotection.18,43 Thus, a reduction of Aβ potentially reduces tau pathology. In homozygous 3xTg-AD mice, Aβ pathology is observed at 6 months of age and preceeds the appearance of hyperphosphorylated pathological tau at approximately 15 months of age.1,19 Positive effects of drug treatment on the levels of phosphorylated tau or in implicated signaling pathways may therefore only be observed at later time points in 3xTg-AD mice and were not observed in this study.
The treatment of gonadectomized male 3xTg-AD animals using E2, DHT, and testosterone results in amelioration of pathology, both of Aβ and phosphorylated tau.8,21,23,24 However, DHT alone did not alter phosphorylated tau levels.27,44 Because testosterone is converted to E2 and DHT,28,45 activating ARs may not effect phosphorylated tau levels (as observed in this study). E2 activates both ERα and ERβ, and thus, the modulation of phosphorylated tau and Aβ levels may be a result of ERα activation. Female ovariectomized 3xTg-AD mice treated with the ERα agonist propylpyrazole triol display decreased Aβ accumulation in brain. This effect is comparable to that seen following E2 treatment., indicating a role of ERα stimulation in modulating AD pathology.28,46
Androgen and Estrogen Receptor Modulating Drugs Increase Androgen Receptor Levels in the Hippocampus Post-treatment
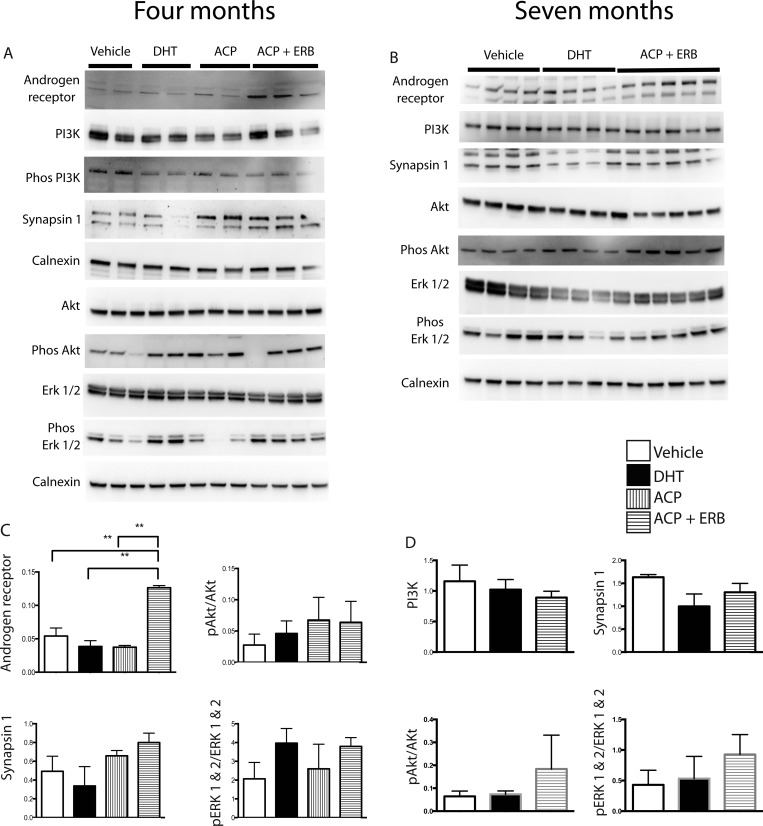
Key proteins associated with AD pathology were additionally assessed by Western blotting in hippocampal lysates in mice treated with the androgen and estrogen receptor modulating drugs for 4 and 7 months. Representative Western blots are shown, and brain protein levels were quantified (Figure 6).
Figure 6.
Androgen and estrogen receptor modulating drugs increase the levels of the androgen receptor in the hippocampus after 4 and 7 months of drug treatment. Gonadectomized 3xTg-AD mice SARM/ERβ agonist treated for 4 and 7 months have increased androgen receptor (AR) levels. Brain protein levels were investigated using Western blotting and quantified using analysis software. Representative Western blot (A, B) and quantification (C, D) of brain protein levels of AR, phosphatidylinositide 3-kinase (PI3K), phosphorylated PI3K, synapsin 1, Akt, phosphorylated Akt (Phos Akt), Erk 1 and 2, and phosphorylated Erk 1 and 2 (phos Erk 1/2) in gonadectomized 3xTg-AD mice treated with DHT or SARM ACP-105 in combination with ERβ agonist AC-186 compared with vehicle control (n = 4 or 5 per group). Calnexin is an ER marker and was utilized as a loading control as the brain homogenates were prepared in RIPA buffer. Data expressed as means ± SEM. Asterisk denotes statistical significance (**p < 0.01, *p < 0.05).
Surprisingly, chronic treatment (4 and 7 months) with the combination of ACP-105 and AC-186 significantly increased levels of the androgen receptor, compared with DHT alone (Figure 6 C,D). The significant increase in AR levels following cotreatment with a ERβ agonist indicates the modulation of androgen receptor levels by estrogens in males.
Androgen and estrogen receptors mediate their functions through the phosphatidylinositide 3-kinase (PI3K) pathway, as well as the mitogen activated protein kinase (MAPK) pathway, investigated by blotting for Erk 1/2.29,47,48 Estrogen-induced changes in IDE levels require ERβ activation of the PI3K pathway.30,47 Components of these pathways were assessed in drug treated mice in this study; however there were no significant changes in the protein levels (Figure 6).
In our hands, the major signaling pathways documented for both AR and ER31,48 remained largely unaltered despite an indication that both phosphorylated Akt/Akt and phosphorylated ERK 1 and 2/ERK 1 and 2 increased with SARM/ERβ agonist treatment after 4 and 7 months (Figure 6C,D). Further studies will target other signaling pathways known to be modulated by E2 and testosterone, including Wnt signaling, dickkopf-1, and the protein kinase A pathway and investigate their possible alterations.
We have demonstrated that 4–7 months treatment with SARM/ERβ agonists improves long-term memory and reduces anxiety-like behavior in gonadectomized 3xTg-AD mice. This combination treatment increased the levels of Aβ-degrading enzymes, reduced Aβ levels (at 7 months), and increased the level of AR in the hippocampus.
ERβ Agonist As a Potential Therapy
Recently it was shown that a metabolite of DHT is a selective ERβ activator.17,32 Thus, the combination of an AR activator and an ERβ activator would potentially mimic DHT activity. Chronic coadministration of a SARM (ACP-105) and an ERβ agonist (AC-186) syngergistically increased AR brain levels, not seen with DHT or ACP-105 treatments alone. Therefore, it can be inferred that an ERβ agonist could impact the anabolic pathway by modulating AR levels. With aging, a reduction of testosterone in men results in lower levels of the potent AR agonist DHT; therefore stimulating ERβ using agonists is a significant potential therapy. Importantly, the ERβ agonist (AC-186) also used in this study exerts gender selective neuroprotection in male animals in a rat model of Parkinson’s disease.33,34,49−51 Taken together, the use of ERβ agonists have beneficial effects in male animals.
Concluding Remarks
Future studies investigating the therapeutic use of both the compounds used in this study to evaluate the ERβ agonist alone or in combination with ACP-105, while using E2 as a reference compound in gonadectomized male 3xTg-AD mice, will be beneficial. Most studies involving estrogens have focused on the female gender. However, a selective ERβ agonist may have a better safety profile and a unique pharmacology in both men and women compared with the unselective E2 that activates both ERα and β. The simultaneous activation of both AR and ER had a synergetic profile in our hands, and based on the current results, treatment of patients with AD using SARMs or ERβ agonists or a combination thereof will potentially interfere with the progression of AD by clearance of Aβ peptides.
Methods
Animals
Colonies of male homozygous 3xTg-AD (APPswe, PS1M146 V, tauP301L) and wild-type (B6129SF2/J; The Jackson Laboratory, Bar Harbor, ME) mice were established in the Department of Experimental Medicine, Lund University, from previously characterized mice.1 Mice were maintained on an ad libitum diet with a 12-h light/dark cycle. Lund University Animal Ethics Committee approved all animal experimental protocols.
Drug Administration
At age 3 months, the mice (n = 9–11 per group) under isoflurane gas anesthesia (1–2%) were gonadectomized to deplete endogenous testosterone and injected intraperitoneally with selective androgen receptor modulating drug (SARM ACP-105, 10 mg/kg, ACADIA Pharmaceuticals) alone or in combination with the selective estrogen receptor β1 agonist (AC-186, 10 mg/kg, ACADIA Pharmaceuticals) or with the equivalent of 0.1 mg/day of DHT or vehicle (sesame oil) 4 days/week for a period of 4 or 7 months. At 4 or 7 months after initiation of hormone treatment, the animals were killed, and brains were collected on dry ice for biochemical analysis and stored at −20 °C until analysis was performed. In a pilot study, 3xTg-AD and wild-type mice underwent sham gonadectomy to compare with gonadectomized 3xTg-AD mice. Pilot mice were analyzed for behavior and biochemical analyses.
Morris Water Maze
The water maze consisted of a circular tank (diameter = 1.8 m) filled with water opacified by the addition of white paint (Beckers, Stockholm, Sweden). Pool temperature was maintained at 22 ± 1 °C. The walls surrounding the water maze were hung with posters and shapes, which served as visual cues and were visible during all stages of training and testing. Movement of the mice within the pool was recorded and analyzed with EthoVision 3.1 video-tracking software (Noldus, Netherlands). For the hidden platform task, the escape platform (15 cm2) was positioned 1.5 cm below water level in the center of one of the pool quadrants. For cued training, transgenic and WT mice were placed in the pool facing the edge at one of four start positions (NE, SE, SW, NW) and were required to locate the submerged platform in the NW quadrant the position of which did not vary across trials. Each mouse was subject to a total of 24 trials (4 trials/block, 1 block/day, 6 days duration). Intertrial intervals averaged 24 min, and maximum trial length was 90 s. If mice failed to find the platform within 90 s, they were guided to its position by the experimenter who was blind to the treatment group of the mice. All mice were allowed to remain on the platform for more than 5 s before being removed and returned to the home cage. Time to reach the platform was recorded for each mouse. The recall task was performed after 6 days of cued trials. The platform was removed on this day. All mice were placed in the pool at the NE quadrant and the time spent in the NW (platform) quadrant was recorded. Working memory was assessed using a reversal learning protocol. Twenty-four hours following the probe trial, mice were placed in the MWM, where the hidden platform had a new location and mice were trained (four trials) to learn the new location of the hidden platform. To test vision, visible platform trials were conducted with the platform visible above the water line. Mice were place in the pool in all four directions and timed to find the platform.
Spontaneous Alternation
Arm choices of mice entering the arm were recorded while animals freely explored the y-maze for 8 min. Mice that made 10 or fewer arm choices were excluded. The score for spontaneous alternations was calculated as the proportion of alternations (an arm choice differing from each of two previous choices) to the total number of alternation opportunities.8,34
Elevated Plus Maze
Mouse anxiety-like behaviors were investigated using an opaque acrylic elevated plus maze, which was raised 30 cm off the floor. The maze had two open and two closed arms, which were 5 cm wide and 28.5 cm long with 20 cm high walls on the closed arms. Mice were placed in the middle of the elevated plus maze. The total session duration was 5 min for each mouse. Number of entries in the open and closed arms were recorded when all four paws of the mouse moved into either the closed or open arms.
Spontaneous Locomotor Activity
Spontaneous locomotor behavior and habituation was assessed using an open field test. Individual mice were placed into an acrylic box (floor 42 cm × 42 cm) and their behavior was recorded on video for a total of 10 min. Behavior was analyzed using the EthoVision 3.1 video-tracking software (Noldus, Netherlands). The analysis included distinguishing activity within an inner zone area (740 cm2), intermediate zone area (284 cm2), and an outer zone area 740 cm2 of the open field to assess thigmotaxis.
Rotarod
The rotarod (Rotamex 4/8, Columbus Instruments, USA) consists of a horizontal rod (3.8 cm in diameter). Mice were placed on the 4.5 cm rod facing the direction opposite to that of rotation. Pretraining consisted of walking on the rod turning at a constant speed of 5 rotations per min for 30 s. Four test trials were conducted in which the speed of rotation increased gradually from 4 to 40 rpm over 5 min. An average intertrial time of 20–30 min was kept. The time spent on the rotarod was recorded and averaged for the four trials.
Tissue Preparation and Western Blotting
Brains were removed after cervical dislocation, and the hemispheres were dissected in half. Tissue was separated for Western blot and ELISA analysis. Tissue for Western blotting was sonicated (Branson Sonifier, CT, USA) for 30 cycles with 2× 15-s bursts at 1:10 (w/v) in RIPA buffer (50 mM Tris–HCl, pH 7.4, containing 0.15 M NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate protease, and phosphatase inhibitor cocktail; Thermoscientific, Rockford, IL, USA). Protein levels were determined using the bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). Homogenates (20 μg protein) were mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer (to final concentration of 2% SDS, 25 mM Tris–HCl, pH 6.8, 1% β-mercaptoethanol). Samples were heated at 95 °C for 5 min then electrophoresed on 4–20% Tris–HCL SDS–polyacrylamide gels (BioRad Laboratories, Hercules, CA, USA) and electrotransferred to poly(vinylidene fluoride) membranes using Trans-blot Turbo system (BioRad Laboratories, Hercules, CA, USA). Membranes were incubated with primary antibodies, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell signaling, Danvers, MA, USA). Signals were detected by chemiluminescence (BioRad Laboratories, Hercules, CA, USA) using a Biorad Chemi Doc molecular Imager and quantified with Image Laboratories analysis software (BioRad Laboratories, Hercules, CA, USA).
Western Blotting Antibodies
Rat antibody recognizing human and mouse neprilysin was obtained from R&D Systems, MN, USA. Mouse monoclonal anti-tau antibody recognizes the N-terminal region of human tau (Millipore, MA, USA). Monoclonal antibody to PHF-tau (clone AT8) was purchased from Thermo Scientific (Rockford, IL, USA). Rabbit antibodies against Akt, phosphorylated Akt, PI3K, Erk 1 and 2, phosphorylated Erk 1 and 2, GSK3β, and phosphorylated GSK3β were purchased from Cell Signaling (Danvers, MA, USA). Mouse monoclonal cathepsin D antibody was purchased from Abcam (Cambridge, UK). Rabbit anti-synapsin 1 antibody was purchased from Merck Millipore (Darmstadt, Germany). Rabbit anti-IDE antibody was purchased from Abcam (Cambridge, UK). Rabbit anti-androgen receptor was purchased from Merck Millipore (Darmstadt, Germany).
Aβ ELISA
To determine the levels of Aβ levels by enzyme-linked immunosorbent assay (ELISA), samples of mouse brain hemispheres were homogenized in ice-cold PBS containing 5 M guanidine HCl and 1× protease inhibitor mixture (pH 8.0) (Calbiochem, Merck Millipore, Darmstadt, Germany). Homogenates were centrifuged at 16 000g for 20 min at 4 °C. Aβ1–40 and Aβ1–42 levels in the brain homogenates were quantitated with a sandwich ELISA (Invitrogen, Camarillo, CA, USA) according to the manufacturer’s instructions.
Immunohistochemistry and Microscopy
3xTg-AD mice following 4 and 7 months of treatment were anesthetized with sodium pentobarbitone and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde (PFA) in phosphate buffer. Brains were removed and postfixed in PFA for 4 h before placing them in 30% sucrose until sectioning. Thirty micrometer thick free-floating sections were cut on a freezing microtome and immunostained with primary antibody recognizing C99 and Aβ, but not full-length βAPP1,35,52−55 (antibody 82E1, IBL, Japan). Sections were counter stained with hemotoxylin. We analyzed the sections with a conventional light microscope (Eclipse 80i microscope; Nikon).
Statistical Analyses
Data are presented as mean ± standard error of the mean (SEM). Differences between groups were examined using a one-way ANOVA followed by Bonferroni-corrected posthoc comparison, or in the case of the MWM training and open field data, a two-way repeated measures ANOVA was conducted for normally distributed and equal variance data; Kruskal–Wallis followed by a Dunn’s posthoc test were used for non-normal distributions (Prism, GraphPad, La Jolla, CA USA).
Acknowledgments
The authors thank F. M. LaFerla for the transgenic mice and D. Tampellini for consultation and advice and are also grateful to Alicja Flasch, B. Haraldsson, and M. Persson-Vejgården for their excellent technical assistance.
Glossary
Abbreviations
- E2
17β-estradiol
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- Aβ
amyloid-β
- AR
androgen receptor
- DHT
dihydrotestosterone
- ELISA
enzyme-linked immunosorbent assay
- ERβ
estrogen receptor β
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- GSK3β
glycogen synthase kinase 3β
- IDE
insulin-degrading enzyme
- MAPK
mitogen activated protein kinase
- MWM
Morris water maze
- NEP
neprilysin
- PI3K
phosphatidylinositide 3-kinase
- Phos Akt
phosphorylated Akt
- phos Erk 1/2
phosphorylated Erk 1 and 2
- SARMs
selective androgen receptor modulators
- 3xTg-AD
triple transgenic AD
Supporting Information Available
Exposure including brain/plasma ratios for ACP-105 and AC-186, in vitro safety for ACP-105 and AC-186 and selectivity and safety evaluations, assessment of cognitive deficits and anxiety-like behavior motor ability in gonadectomized 3xTg-AD mice, protein levels of APP, Aβ, neprilysin, and AR in gonadectomized 3xTg-AD mice, and levels of Aβ and the C99 fragment of APP in the subiculum of drug-treated gonadectomized 3xTg-AD mice. This information is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
S.G. designed, managed, and performed all the experiments, analyzed the data, and wrote the paper. G.H.P. helped design behavioral in vivo experiments and provided input to the manuscript. G.K.G. assisted with analyzing data, consultation, and advice and provided manuscript input. P.B. contributed to the initiation of the project and in vivo experiments and wrote the manuscript. R.O. conceived the initiation of the project, conceived in vivo experiments, analyzed data, and wrote the paper.
This work was supported by grants from the Alzheimer’s Drug Discovery Foundation and Kungliga Fysiografiska Sällskapet i Lund.
The authors declare the following competing financial interest(s): R.O. is a former employee of ACADIA Pharmaceuticals Inc. and hold stocks in the company.
Supplementary Material
References
- Oddo S.; Caccamo A.; Shepherd J. D.; Murphy M. P.; Golde T. E.; Kayed R.; Metherate R.; Mattson M. P.; Akbari Y.; Laferla F. M. (2003) Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles. Neuron 39, 409–421. [DOI] [PubMed] [Google Scholar]
- Gouras G. K.; Xu H.; Gross R. S.; Greenfield J. P.; Hai B.; Wang R.; Greengard P. (2000) Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc. Natl. Acad. Sci. U.S.A. 97, 1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E.; Williams J.; Budge M.; Barnetson L. (2001) Serum total testosterone is lower in men with Alzheimer’s disease. Neuroendocrinol. Lett. 22, 163–168. [PubMed] [Google Scholar]
- Moffat S. D.; Zonderman A. B.; Metter E. J.; Kawas C.; Blackman M. R.; Harman S. M.; Resnick S. M. (2004) Free testosterone and risk for Alzheimer disease in older men. Neurology 62, 188–193. [DOI] [PubMed] [Google Scholar]
- Billings L. M.; Oddo S.; Green K. N.; McGaugh J. L.; Laferla F. M. (2005) Intraneuronal Aβ Causes the Onset of Early Alzheimer’s Disease-Related Cognitive Deficits in Transgenic Mice. Neuron 45, 675–688. [DOI] [PubMed] [Google Scholar]
- Moffat S. D. (2006) Does testosterone mediate cognitive decline in elderly men?. J. Gerontol., Ser. A 61, 521. [DOI] [PubMed] [Google Scholar]
- Clinton L. K.; Billings L. M.; Green K. N.; Caccamo A.; Ngo J.; Oddo S.; McGaugh J. L.; Laferla F. M. (2007) Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol. Dis. 28, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario E. R.; Carroll J. C.; Oddo S.; Laferla F. M.; Pike C. J. (2006) Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J. Neurosci. 26, 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M.; Nyborg A. C.; Murphy M. P.; Chang L.; Stanczyk F. Z.; Golde T. E.; Pike C. J. (2004) Androgens modulate β-amyloid levels in male rat brain. J. Neurochem. 87, 1052–1055. [DOI] [PubMed] [Google Scholar]
- Gao W.; Dalton J. T. (2007) Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discovery Today 12, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler M. L.; Bohl C. E.; Jones A.; Coss C. C.; Narayanan R.; He Y.; Hwang D. J.; Dalton J. T.; Miller D. D. (2009) Nonsteroidal selective androgen receptor modulators (SARMs): Dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem. 52, 3597–3617. [DOI] [PubMed] [Google Scholar]
- Schlienger N.; Lund B. W.; Pawlas J.; Badalassi F.; Bertozzi F.; Lewinsky R.; Fejzic A.; Thygesen M. B.; Tabatabaei A.; Bradley S. R.; Gardell L. R.; Piu F.; Olsson R. (2009) Synthesis, Structure–Activity Relationships, and Characterization of Novel Nonsteroidal and Selective Androgen Receptor Modulators. J. Med. Chem. 52, 7186–7191. [DOI] [PubMed] [Google Scholar]
- Acevedo S.; Gardell L.; Bradley S.; Piu F.; Raber J. (2008) Selective Androgen Receptor Modulators Antagonize Apolipoprotein E4-Induced Cognitive Impairments. Lett. Drug Des. Discovery 5, 271–276. [Google Scholar]
- Osborne D. M.; Edinger K.; Frye C. A. (2009) Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age 31, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piu F.; Gardell L. R.; Son T.; Schlienger N.; Lund B. W.; Schiffer H. H.; Vanover K. E.; Davis R. E.; Olsson R.; Bradley S. R. (2008) Pharmacological characterization of AC-262536, a novel selective androgen receptor modulator. J. Steroid Biochem. Mol. Biol. 109, 129–137. [DOI] [PubMed] [Google Scholar]
- Frye C. A.; Edinger K.; Sumida K. (2007) Androgen Administration to Aged Male Mice Increases Anti-Anxiety Behavior and Enhances Cognitive Performance. Neuropsychopharmacology 33, 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T. D.; Hinds L. R.; Handa R. J. (2006) The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J. Neurosci. 26, 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. J.; Lee J. S.; Gamboa M. C.; Roth A. J. (2008) Cognitive effects of hormone therapy in men with prostate cancer. Cancer 113, 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Brinton R. D. (2007) WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev. Neurother. 7, 1549–1564. [DOI] [PubMed] [Google Scholar]
- Benice T. S.; Raber J. (2009) Dihydrotestosterone modulates spatial working-memory performance in male mice. J. Neurochem. 110, 902–911. [DOI] [PubMed] [Google Scholar]
- Carroll J. C.; Rosario E. R.; Chang L.; Stanczyk F. Z.; Oddo S.; Laferla F. M.; Pike C. J. (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 27, 13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer M. D.; Gill M.; Weinberg A.; Galea L. A. M. (2008) Castration differentially affects spatial working and reference memory in male rats. Arch. Sex. Behav. 37, 19–29. [DOI] [PubMed] [Google Scholar]
- Petanceska S. S.; Nagy V.; Frail D.; Gandy S. (2000) Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp. Gerontol. 35, 1317–1325. [DOI] [PubMed] [Google Scholar]
- Zheng H.; Xu H.; Uljon S. N.; Gross R.; Hardy K.; Gaynor J.; LaFrancois J.; Simpkins J.; Refolo L. M.; Petanceska S.; Wang R.; Duff K. (2002) Modulation of A(beta) peptides by estrogen in mouse models. J. Neurochem. 80, 191–196. [DOI] [PubMed] [Google Scholar]
- Pfankuch T.; Rizk A.; Olsen R.; Poage C.; Raber J. (2005) Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Res. 1053, 88–96. [DOI] [PubMed] [Google Scholar]
- Heikkinen T.; Kalesnykas G.; Rissanen A.; Tapiola T.; Iivonen S.; Wang J.; Chaudhuri J.; Tanila H.; Miettinen R.; Puoliväli J. (2004) Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp. Neurol. 187, 105–117. [DOI] [PubMed] [Google Scholar]
- Heldring N.; Pike A.; Andersson S.; Matthews J.; Cheng G.; Hartman J.; Tujague M.; Ström A.; Treuter E.; Warner M.; Gustafsson J.-Å. (2007) Estrogen Receptors: How Do They Signal and What Are Their Targets. Physiol. Rev. 87, 905–931. [DOI] [PubMed] [Google Scholar]
- Liu F.; Day M.; Muñiz L. C.; Bitran D.; Arias R.; Revilla-Sanchez R.; Grauer S.; Zhang G.; Kelley C.; Pulito V.; Sung A.; Mervis R. F.; Navarra R.; Hirst W. D.; Reinhart P. H.; Marquis K. L.; Moss S. J.; Pangalos M. N.; Brandon N. J. (2008) Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 11, 334–343. [DOI] [PubMed] [Google Scholar]
- Jacome L. F.; Gautreaux C.; Inagaki T.; Mohan G.; Alves S.; Lubbers L. S.; Luine V. (2010) Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol. Learn. Mem. 94, 488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S.; Koehler K. F.; Gustafsson J. Å. (2011) Development of subtype-selective oestrogen receptor-based therapeutics. Nat. Rev. Drug Discovery 10, 778–792. [DOI] [PubMed] [Google Scholar]
- Udrisar D. P.; Wanderley M. I.; Porto R. C.; Cardoso C. L.; Barbosa M. C.; Camberos M. C.; Cresto J. C. (2005) Androgen-and estrogen-dependent regulation of insulin-degrading enzyme in subcellular fractions of rat prostate and uterus. Exp. Biol. 230, 479–486. [DOI] [PubMed] [Google Scholar]
- Iwata N.; Tsubuki S.; Takaki Y.; Shirotani K.; Lu B. (2001) Metabolic regulation of brain Aβ by neprilysin. Science 292, 1550–1552. [DOI] [PubMed] [Google Scholar]
- Farris W.; Schütz S. G.; Cirrito J. R.; Shankar G. M.; Sun X.; George A.; Leissring M. A.; Walsh D. M.; Qiu W. Q.; Holtzman D. M.; Selkoe D. J. (2007) Loss of Neprilysin Function Promotes Amyloid Plaque Formation and Causes Cerebral Amyloid Angiopathy. Am. J. Pathol. 171, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring M. A.; Farris W.; Chang A. Y.; Walsh D. M.; Wu X.; Sun X.; Frosch M. P.; Selkoe D. J. (2003) Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093. [DOI] [PubMed] [Google Scholar]
- Bittner T.; Fuhrmann M.; Burgold S.; Ochs S. M.; Hoffmann N.; Mitteregger G.; Kretzschmar H.; Laferla F. M.; Herms J. (2010) Multiple Events Lead to Dendritic Spine Loss in Triple Transgenic Alzheimer’s Disease Mice. PLoS One 5, e15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega M. S.; Cummings J. L.; Fiorello T.; Gornbein J. (1996) The spectrum of behavioral changes in Alzheimer’s disease. Neurology 46, 130–135. [DOI] [PubMed] [Google Scholar]
- Teri L.; Ferretti L. E.; Gibbons L. E.; Logsdon R. G.; McCurry S. M.; Kukull W. A.; McCormick W. C.; Bowen J. D.; Larson E. B. (1999) Anxiety in Alzheimer’s disease: Prevalence and comorbidity. J. Gerontol., Ser. A 54, M348–M352. [DOI] [PubMed] [Google Scholar]
- Rosario E. R.; Pike C. J. (2008) Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res. Rev. 57, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.; Logge W.; Low J. K.; Garner B.; Karl T. (2013) Novel behavioural characteristics of the APPSwe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. Behav. Brain Res. 245, 120–127. [DOI] [PubMed] [Google Scholar]
- Walf A. A.; Frye C. A. (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M.; Nguyen T.-V. V.; Rosario E. R.; Ramsden M.; Pike C. J. (2008) Androgens regulate neprilysin expression: role in reducing β-amyloid levels. J. Neurochem. 105, 2477–2488. [DOI] [PubMed] [Google Scholar]
- Guan H.; Liu Y.; Daily A.; Police S.; Kim M.-H.; Oddo S.; Laferla F. M.; Pauly J. R.; Murphy M. P.; Hersh L. B. (2009) Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer’s disease. J. Neurosci. Res. 87, 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D. M.; Gharkholonarehe N.; Van Nostrand W. E.; Davis J.; Vitek M. P.; Colton C. A. (2009) Amyloid reduction by amyloid-beta vaccination also reduces mouse tau pathology and protects from neuron loss in two mouse models of Alzheimer’s disease. J. Neurosci. 29, 7957–7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario E. R.; Carroll J.; Pike C. J. (2010) Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 1359, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R. (2003) Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 86, 225–230. [DOI] [PubMed] [Google Scholar]
- Carroll J. C.; Pike C. J. (2008) Selective Estrogen Receptor Modulators Differentially Regulate Alzheimer-Like Changes in Female 3xTg-AD Mice. Endocrinology 149, 2607–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Yao J.; Mao Z.; Chen S.; Wang Y.; Brinton R. D. (2010) 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ /PI3-K pathway in hippocampus: Relevance to Alzheimer’s prevention. Neurobiol. Aging 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J.; Nguyen T.; Ramsden M.; Yao M. (2008) Androgen cell signaling pathways involved in neuroprotective actions. Horm. Behav. 53, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K.; Price D. L.; Davis C. N.; Ma J.-N.; Bonhaus D. W.; Burstein E. S.; Olsson R. (2013) AC-186, a Selective Nonsteroidal Estrogen Receptor β Agonist, Shows Gender Specific Neuroprotection in a Parkinson’s Disease Rat Model. ACS Chem Neurosci. 4, 1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R. A.; Guan H.; Rockenstein E.; Kindy M.; Gage F. H.; Verma I.; Masliah E.; Hersh L. B. (2004) Neprilysin Regulates Amyloid β Peptide Levels. J. Mol. Neurosci. 22, 5–12. [DOI] [PubMed] [Google Scholar]
- Saido T. C., and Nakahara H. (2003) Proteolytic degradation of Aβ by neprilysin and other peptidases, in Aβ Metabolism and Alzheimer’s Disease (Saido T. C., Ed.) pp 61–74, Landes Bioscience: Austin, TX. [Google Scholar]
- Horikoshi Y.; Mori T.; Maeda M.; Kinoshita N.; Sato K.; Yamaguchi H. (2004) Abeta N-terminal-end specific antibody reduced beta-amyloid in Alzheimer-model mice. Biochem. Biophys. Res. Commun. 325, 384–387. [DOI] [PubMed] [Google Scholar]
- Lauritzen I.; Pardossi-Piquard R.; Bauer C.; Brigham E.; Abraham J. D.; Ranaldi S.; Fraser P.; St George-Hyslop P.; Le Thuc O.; Espin V.; Chami L.; Dunys J.; Checler F. (2012) The β-Secretase-Derived C-Terminal Fragment of βAPP, C99, But Not Aβ, Is a Key Contributor to Early Intraneuronal Lesions in Triple-Transgenic Mouse Hippocampus. J. Neurosci. 32, 16243–16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Wirths O.; Theil S.; Gerth J.; Bayer T. A.; Walter J. (2013) Early intraneuronal accumulation and increased aggregation of phosphorylated Abeta in a mouse model of Alzheimer’s disease. Acta Neuropathol. 125, 699–709. [DOI] [PubMed] [Google Scholar]
- Wirths O.; Dins A.; Bayer T. A. (2012) AβPP accumulation and/or intraneuronal amyloid-β accumulation? The 3xTg-AD mouse model revisited. J. Alzheimer’s Dis. 28, 897–904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.