Abstract
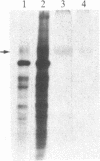
An overexpression of the plasma membrane glycoprotein of relative molecular size 170-180 kDa is consistently found in different multidrug-resistant human and animal cell lines, although the functional role of the protein in multidrug resistance is not known. Two monoclonal antibodies that interfere with biochemical functions were generated against the human myelogenous leukemia K-562 cells resistant to adriamycin (K-562/ADM). These antibodies, designated MRK16 and MRK17, are specifically reactive to K-562/ADM and a human ovarian cancer cell line resistant to adriamycin (2780AD). MRK16 modulated vincristine and actinomycin D transport in the resistant cells, while MRK17 specifically inhibited the growth of the resistant cells. Both antibodies recognized the 170- to 180-kDa glycoprotein. These data indicate that the 170- to 180-kDa glycoprotein is involved, directly or indirectly, in the drug transport mechanisms and the proliferation of multidrug-resistant tumor cell lines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Bell D. R., Gerlach J. H., Kartner N., Buick R. N., Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985 Mar;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- Danks M. K., Metzger D. W., Ashmun R. A., Beck W. T. Monoclonal antibodies to glycoproteins of Vinca alkaloid-resistant human leukemic cells. Cancer Res. 1985 Jul;45(7):3220–3224. [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Häyry P., Andersson L. C. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976 Mar;68(3):642–653. doi: 10.1083/jcb.68.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Garman D., Center M. S. Alterations in cell surface membranes in Chinese hamster lung cell resistant to adriamycin. Biochem Biophys Res Commun. 1982 Mar 15;105(1):157–163. doi: 10.1016/s0006-291x(82)80025-9. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., Ozols R. F. Experimental model systems of ovarian cancer: applications to the design and evaluation of new treatment approaches. Semin Oncol. 1984 Sep;11(3):285–298. [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Tsuruo T., Iida-Saito H., Kawabata H., Oh-hara T., Hamada H., Utakoji T. Characteristics of resistance to adriamycin in human myelogenous leukemia K562 resistant to adriamycin and in isolated clones. Jpn J Cancer Res. 1986 Jul;77(7):682–692. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Naganuma K., Tsukagoshi S., Sakurai Y. Promotion by verapamil of vincristine responsiveness in tumor cell lines inherently resistant to the drug. Cancer Res. 1983 Feb;43(2):808–813. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Ohkochi E., Tsukagoshi S., Sakurai Y. Establishment and properties of vincristine-resistant human myelogenous leukemia K562. Gan. 1983 Oct;74(5):751–758. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]