Abstract
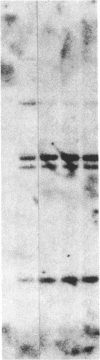
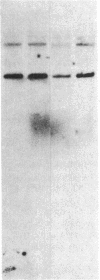
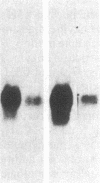
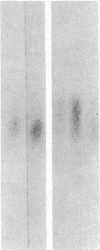
The developmental patterns of rearrangement and expression of the T-cell antigen-receptor genes are precisely regulated during T-cell differentiation and education. The beta- and gamma-subunit RNAs of the T-cell receptor are abundantly expressed in immature thymocytes. In mature thymocytes the alpha- and beta-subunit RNAs are preferentially expressed, whereas there is minimal expression of the gamma RNA. Although aspects of the pattern of known organization and rearrangement of the T-cell receptor gene in the thymus have been studied and the concept of a thymus selection process generally has been accepted, the cellular and molecular basis of thymus education remains obscure. Certain strains of mice with predilections for autoimmunity demonstrate T-cell developmental abnormalities. This is especially true for the lpr/lpr or gld/gld genotypes, in which the major population of peripheral T cells is developmentally disturbed. We have studied the development, expression, and rearrangement of T-cell receptor genes in the C3H/HeJ gld/gld mouse. Our results indicate a high level of expression of the beta and alpha RNAs in C3H/HeJ gld/gld T cells residing in the periphery. In addition, the beta-subunit gene of gld/gld peripheral T cells undergoes more rearrangements than does its normal C3H/HeJ T-cell counterparts. We speculate that this rearrangement pattern and high level of T-cell receptor mRNA reflects an abnormality or deficit in a thymus selection process that permits emigration of T cells with nonfunctionally rearranged T-cell receptor genes to secondary lymphoid organs. However, the normal level of gamma-subunit RNA expression argues that gamma-subunit gene rearrangements are distinct from processes related to alpha- and beta-subunit selection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E., Hashimoto Y., Greene M. I., Maxam A. M. Active T-cell receptor genes have intron deoxyribonuclease hypersensitive sites. Science. 1985 Aug 9;229(4713):528–534. doi: 10.1126/science.3927483. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischbach M. Defective T cell response to presented antigen in autoimmune mice. J Immunol. 1984 Nov;133(5):2365–2368. [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Balderas R. S., Francis S. J., Dixon F. J. The effect of thymectomy on lupus-prone mice. J Immunol. 1984 Apr;132(4):1809–1813. [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Wiedemann L. M., Pittet A. C., Strauss S., Nelson K. J., Davis J., Van Ness B., Perry R. P. Nonproductive kappa immunoglobulin genes: recombinational abnormalities and other lesions affecting transcription, RNA processing, turnover, and translation. Mol Cell Biol. 1985 Jul;5(7):1660–1675. doi: 10.1128/mcb.5.7.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Mountz J. D., Mushinski J. F., Steinberg A. D. Differential gene expression in autoimmune mice. Surv Immunol Res. 1985;4(1):48–64. doi: 10.1007/BF02918586. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Steinberg A. D., Klinman D. M., Smith H. R., Mushinski J. F. Autoimmunity and increased c-myb transcription. Science. 1984 Nov 30;226(4678):1087–1089. doi: 10.1126/science.6494925. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Murphy E. D., Eicher E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984 Jan 1;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Lindsten T., Fowlkes B. J., van den Elsen P., Terhorst C., Davis M. M., Germain R. N., Schwartz R. H. Expression of genes of the T-cell antigen receptor complex in precursor thymocytes. 1985 Jun 27-Jul 3Nature. 315(6022):765–768. doi: 10.1038/315765a0. [DOI] [PubMed] [Google Scholar]
- Santoro T. J., Benjamin W. R., Oppenheim J. J., Steinberg A. D. The cellular basis for immune interferon production in autoimmune MRL-Ipr/Ipr mice. J Immunol. 1983 Jul;131(1):265–268. [PubMed] [Google Scholar]
- Santoro T. J., Luger T. A., Ravache E. S., Smolen J. S., Oppenheim J. J., Steinberg A. D. In vitro correction of the interleukin 2 defect of autoimmune mice. Eur J Immunol. 1983 Jul;13(7):601–604. doi: 10.1002/eji.1830130717. [DOI] [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Etiopathogenesis of murine SLE. Immunol Rev. 1981;55:179–216. doi: 10.1111/j.1600-065x.1981.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Shawler D. L., Katz D. H., Dixon F. J. Patterns of immune reactivity in autoimmune murine strains. I. Cell-mediated immune responses induced by H-2 indentical and H-2 incompatible stimulator cells. J Immunol. 1979 Jun;122(6):2319–2327. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Murphy E. D., Roths J. B., Dauphinée M. J., Kipper S. B., Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981 Nov 1;154(5):1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]