Abstract
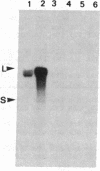
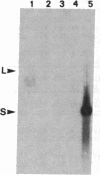
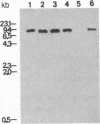
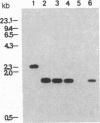
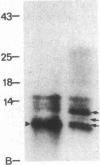
Membranes of erythrocytes infected with the human malaria parasite Plasmodium falciparum develop protrusions called knobs. These structures are essential for the survival of the parasite in the host, and their induction requires the synthesis of the knob protein by the parasite. We describe the isolation of a cDNA clone encoding the amino-terminal half of the knob protein. A cDNA library was constructed from RNA prepared from ring stages of a P. falciparum isolate that has retained its ability to induce knobs (knob+ phenotype). A synthetic oligonucleotide probe encoding polyhistidine was used to isolate the cDNA clone, which encodes the amino-terminal half of a polypeptide with all the known attributes of the knob protein. The gene is not transcribed in variants that do not synthesize the knob protein and thereby cannot induce knobs (knob- phenotype). The apparent lack of transcription in knob- variants is due to different mechanisms: although the gene is present in one knob- isolate, it has been deleted in a cloned knob- variant. The primary structure of the polypeptide deduced from a partial sequence of the cDNA is distinctly different from other malarial histidine-rich polypeptides. The amino-terminal sequence shows the characteristic features of a signal peptide. This is followed by a histidine-rich domain and a subsequent region which contains one histidine. Peptide map analysis of the knob protein is consistent with the structural features deduced from the sequence analysis of the cDNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhasin V. K., Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Jul;33(4):534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Corcoran L. M., Forsyth K. P., Bianco A. E., Brown G. V., Kemp D. J. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986 Jan 17;44(1):87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. J., Gadsden G., Seed T., Jacobs R., Morhardt M., Brackett R. Cloning and characterization of Plasmodium falciparum FCR-3/FMG strain. Am J Trop Med Hyg. 1985 Jan;34(1):24–30. doi: 10.4269/ajtmh.1985.34.24. [DOI] [PubMed] [Google Scholar]
- Gritzmacher C. A., Reese R. T. Reversal of knob formation on Plasmodium falciparum-infected erythrocytes. Science. 1984 Oct 5;226(4670):65–67. doi: 10.1126/science.6382613. [DOI] [PubMed] [Google Scholar]
- Irving D. O., Cross G. A., Feder R., Wallach M. Structure and organization of the histidine-rich protein gene of Plasmodium lophurae. Mol Biochem Parasitol. 1986 Feb;18(2):223–234. doi: 10.1016/0166-6851(86)90040-x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. A unique histidine-rich polypeptide from the malaria parasite, Plasmodium lophurae. J Biol Chem. 1974 Jul 25;249(14):4650–4655. [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Chen S., Sloma A. The biosynthesis of the histidine-rich protein of Plasmodium lophurae and the cloning of its gene in Escherichia coli. Mol Biochem Parasitol. 1985 Jan;14(1):1–10. doi: 10.1016/0166-6851(85)90100-8. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Homology between a histidine-rich protein from Plasmodium lophurae and a protein associated with the knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. J Exp Med. 1980 Jun 1;151(6):1534–1538. doi: 10.1084/jem.151.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Immunological cross-reactivity of the histidine-rich protein of Plasmodium lophurae and the knob protein of Plasmodium falciparum. J Parasitol. 1983 Apr;69(2):257–261. [PubMed] [Google Scholar]
- Kilejian A., Rosenbaum S. Reactivity of a monoclonal antibody produced to the histidine-rich protein of Plasmodium lophurae with Plasmodium falciparum. Mol Biochem Parasitol. 1985 Nov;17(2):155–162. doi: 10.1016/0166-6851(85)90014-3. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Stage-specific proteins and glycoproteins of plasmodium falciparum: identification of antigens unique to schizonts and merozoites. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3695–3699. doi: 10.1073/pnas.77.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. The biosynthesis of the knob protein and a 65 000 dalton histidine-rich polypeptide of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jun;12(2):185–194. doi: 10.1016/0166-6851(84)90134-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect Immun. 1985 Mar;47(3):760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T., Motyl M. R., Trager W. Plasmodium falciparum: loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol. 1979 Oct;48(2):213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- Ravetch J. V., Feder R., Pavlovec A., Blobel G. Primary structure and genomic organization of the histidine-rich protein of the malaria parasite Plasmodium lophurae. Nature. 1984 Dec 13;312(5995):616–620. doi: 10.1038/312616a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl H. D., Kemp D. J., Crewther P. E., Scanlon D. B., Woodrow G., Brown G. V., Bianco A. E., Anders R. F., Coppel R. L. Sequence of a cDNA encoding a small polymorphic histidine- and alanine-rich protein from Plasmodium falciparum. Nucleic Acids Res. 1985 Nov 11;13(21):7837–7846. doi: 10.1093/nar/13.21.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Trager W., Tershakovec M., Lyandvert L., Stanley H., Lanners N., Gubert E. Clones of the malaria parasite Plasmodium falciparum obtained by microscopic selection: their characterization with regard to knobs, chloroquine sensitivity, and formation of gametocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6527–6530. doi: 10.1073/pnas.78.10.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Graves P. M., Carter R., Aikawa M., Miller L. H. Plasmodium falciparum: effect of time in continuous culture on binding to human endothelial cells and amelanotic melanoma cells. Exp Parasitol. 1983 Oct;56(2):207–214. doi: 10.1016/0014-4894(83)90064-4. [DOI] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. Time-course of synthesis, transport and incorporation of a protein identified in purified membranes of host erythrocytes infected with a knob-forming strain of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jul;12(3):337–350. doi: 10.1016/0166-6851(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Wallach M., Cully D. F., Haas L. O., Trager W., Cross G. A. Histidine-rich protein genes and their transcripts in Plasmodium falciparum and P. lophurae. Mol Biochem Parasitol. 1984 May;12(1):85–94. doi: 10.1016/0166-6851(84)90046-x. [DOI] [PubMed] [Google Scholar]