Abstract
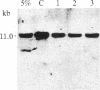
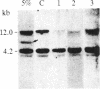
The lineage and clonality of Hodgkin's disease (HD) were investigated by analyzing the organization of the immunoglobulin and T-cell receptor beta-chain (T beta) gene loci in 18 cases of HD, and for comparison, in a panel of 103 cases of B- and T-cell non-Hodgkin's lymphomas (NHLs) and lymphoid leukemias (LLs). Sizable clonal B- or T-cell populations, representing greater than or equal to 10% of the pathologic sample, were readily detectable by immunogenotypic analysis in all 103 NHLs and LLs but not in any of the 18 cases of HD. However, extremely minor clonal populations (less than or equal to 1%) were detectable in 3 of 18 cases of HD. We demonstrated that these minor clonal populations do not correspond to Reed-Sternberg (RS) cells since clonal immunoglobulin or T beta gene rearrangements are not detectable in cases of HD containing greater than 25% RS cells. The number of RS cells present in these samples appeared to correlate directly with the pattern of gene rearrangements characteristic of polyclonal T cells. These studies demonstrate that Southern blot hybridization analysis for clonal immunoglobulin and T beta gene rearrangements represents an accurate, objective tool in the differential diagnosis between HD and NHL; that HD is predominantly composed of polyclonal B and T cells; that minor clonal B- or T-cell populations unrelated to RS cells occasionally can be found in HD; and that RS cells do not represent clonal B- or T-cell expansions. Finally, our data preliminarily suggest that RS cells may represent polyclonal T-cell populations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Chao J., Warnke R., Sklar J. Immunoglobulin gene rearrangement as a diagnostic criterion of B-cell lymphoma. Proc Natl Acad Sci U S A. 1984 Jan;81(2):593–597. doi: 10.1073/pnas.81.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. C., Jones E. L. Hodgkin's disease: an immunohistochemical and histological study. J Pathol. 1978 May;125(1):39–51. doi: 10.1002/path.1711250107. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flug F., Pelicci P. G., Bonetti F., Knowles D. M., 2nd, Dalla-Favera R. T-cell receptor gene rearrangements as markers of lineage and clonality in T-cell neoplasms. Proc Natl Acad Sci U S A. 1985 May;82(10):3460–3464. doi: 10.1073/pnas.82.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa R., Pelicci P. G., Migone N., Lauria F., Pizzolo G., Flug F., Knowles D. M., 2nd, Dalla-Favera R. Analysis of T-cell receptor beta chain (T beta) gene rearrangements demonstrates the monoclonal nature of T-cell chronic lymphoproliferative disorders. Blood. 1986 Jan;67(1):247–250. [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Cossman J., Bolen J. B., Hsu S. M., Israel M., Fisher R. I. Production and characterization of a monoclonal antibody that binds Reed-Sternberg cells. J Immunol. 1985 Jun;134(6):4231–4236. [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E. Possible origin of the Reed-Sternberg cell from an interdigitating reticulum cell. Cancer Treat Rep. 1982 Apr;66(4):601–608. [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Dodson L., Burke J. S., Wang J. M., Bonetti F., Pelicci P. G., Flug F., Dalla-Favera R., Wang C. Y. SIg-E- ("null-cell") non-Hodgkin's lymphomas. Multiparametric determination of their B- or T-cell lineage. Am J Pathol. 1985 Sep;120(3):356–370. [PMC free article] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Halper J. P., Jakobiec F. A. T-lymphocyte subpopulations in B-cell-derived non-Hodgkin's lymphomas and Hodgkin's disease. Cancer. 1984 Aug 15;54(4):644–651. doi: 10.1002/1097-0142(1984)54:4<644::aid-cncr2820540410>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C. C., Mittler R. S., Talle M. A., Goldstein G. Distribution of antigens defined by OKB monoclonal antibodies on benign and malignant lymphoid cells and on nonlymphoid tissues. Blood. 1984 Apr;63(4):886–896. [PubMed] [Google Scholar]
- Miller T. P., Byrne G. E., Jones S. E. Mistaken clinical and pathologic diagnoses of Hodgkin's disease: a Southwest oncology group study. Cancer Treat Rep. 1982 Apr;66(4):645–651. [PubMed] [Google Scholar]
- O'Connor N. T., Wainscoat J. S., Weatherall D. J., Gatter K. C., Feller A. C., Isaacson P., Jones D., Lennert K., Pallesen G., Ramsey A. Rearrangement of the T-cell-receptor beta-chain gene in the diagnosis of lymphoproliferative disorders. Lancet. 1985 Jun 8;1(8441):1295–1297. doi: 10.1016/s0140-6736(85)92791-6. [DOI] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G., Chilosi M., Semenzato G., Caligaris-Cappio F., Fiore-Donati L., Perona G., Janossy G. Immunohistological analysis of Tac antigen expression in tissues involved by Hodgkin's disease. Br J Cancer. 1984 Sep;50(3):415–417. doi: 10.1038/bjc.1984.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S. The diversity of the immunohistological staining pattern of Sternberg-Reed cells. J Histochem Cytochem. 1980 Aug;28(8):788–791. doi: 10.1177/28.8.6777426. [DOI] [PubMed] [Google Scholar]
- Rambaldi A., Pelicci P. G., Allavena P., Knowles D. M., 2nd, Rossini S., Bassan R., Barbui T., Dalla-Favera R., Mantovani A. T cell receptor beta chain gene rearrangements in lymphoproliferative disorders of large granular lymphocytes/natural killer cells. J Exp Med. 1985 Dec 1;162(6):2156–2162. doi: 10.1084/jem.162.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Battifora H., Burke J. S., Rappaport H. Leu-M1 antigen in human neoplasms. An immunohistologic study of 400 cases. Am J Surg Pathol. 1986 Apr;10(4):227–236. doi: 10.1097/00000478-198604000-00001. [DOI] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Taylor C. R. An immunohistological study of follicular lymphoma, reticulum cell sarcoma and Hodgkin's disease. Eur J Cancer. 1976 Jan;12(1):61–75. doi: 10.1016/0014-2964(76)90125-0. [DOI] [PubMed] [Google Scholar]
- Tindle B. H. Malignant lymphomas. Am J Pathol. 1984 Jul;116(1):119–174. [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- Wieczorek R., Burke J. S., Knowles D. M., 2nd Leu-M1 antigen expression in T-cell neoplasia. Am J Pathol. 1985 Dec;121(3):374–380. [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]