Abstract
Endothelial cells (ECs) provide inductive signals for cell differentiation in vivo. However, it is unknown if these cells promote such differentiation in vitro and the signals involved. We investigated whether ECs are able to enhance the differentiation of the three germ layers and the underlying mechanisms. We established a coculture system of mouse embryoid bodies (EBs) and ECs. Then, we analyzed the expression of markers representative of the three germ layers, such as PDX-1, proinsulin, insulin1 (endoderm), nestin, neurofilament light (ectoderm), CD31, cardiotin, and cardiac troponin I (mesoderm) in EBs cultured alone (controls) or with ECs. A significant increase of these markers was observed in EBs cocultured with ECs compared to controls. The cocultured EBs also exhibited more robust vascular networks similar to those EBs treated with bone morphogenetic protein-2 or -4 (BMP-2 or -4). Therefore, the role of these peptides in the differentiation was investigated. We found a significant upregulation of BMP-2/-4 and BMP receptor 1A in EBs treated with EC conditioned medium (EC-CM) at early or middle stages of EB development. Recombinant human BMP-2 and BMP-4 exerted similar effects than EC-CM in the expression of BMPs or in the upregulation of the three germ layer specific markers. BMP-2/-4 antagonists, such as noggin and chordin-like-1, respectively inhibited the EC-CM inductive effects. These results demonstrate that ECs enhance the differentiation in vitro of cells that derived from the three germ layers and that BMP-2/-4 play a central role in this process.
Introduction
Endothelial cells (ECs) play an important role in organogenesis [1]. For instance, the development of liver and pancreas depends on the presence and interaction with endothelium [2,3]. Therefore, ECs are not only necessary for tissue nourishment but they provide inductive signals for tissue differentiation and development [4]. Other in vitro analyses have demonstrated that ECs provide extracellular matrix molecules important to maintain the development and function of endocrine cells, such as beta cells from pancreatic islets [5,6]. With the emergence of embryonic stem cells (ESCs), studies to investigate the role of ECs in organogenesis can now be performed in vitro. We previously described an approach to investigate the inductive effects of ECs in cell differentiation by implanting embryoid bodies (EBs) into surrogate vascular beds, such as quail chorioallantoic membranes [7]. Recently, we also reported the enhancement of pancreatic progenitors and insulin-producing cells in EBs cocultured with human microvascular endothelial cells (HMECs) [8]. EBs are composed of ectodermal, mesodermal, and endodermal cells [9]. Although many in vivo studies have demonstrated the critical role of ECs in differentiation, the EC-derived factors involved are still under investigation [2]. It is known that ECs express factors involved in differentiation, such as fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs) that belong to transforming growth factor β (TGF-β) superfamily, and jagged 1 that belongs to Notch family [10–12]. However, the role of other factors that might be involved is still unknown. In this work, we studied the inductive effects of ECs on EBs. We found that ECs cocultured with EBs enhanced the expression of markers, such as PDX-1, proinsulin, insulin1, nestin, neurofilament light (NF-L), CD31, cardiac troponin I (cTnI), and cardiotin as representatives of the three germ layers. Further, the effects of EC conditioned medium (EC-CM) were similar to combinatorial effects of BMP-2 and BMP-4 on EBs alone. Most of these effects were inhibited by noggin (NOG) or chordin-like-1 (CHRDL1), respectively suggesting a role of endothelial BMPs in the enhancement of such differentiation.
Materials and Methods
Cells and reagents
Mouse ESC (mESC) line R1 (from [strains 129/Sv×129/Sv-CP] F1 3.5-day blastocyst; Samuel Lunenfeld Research Institute, ON, Canada) passage 15–20 were plated on Mitomycin C (Sigma, St. Louis, MO) -inactivated mouse embryonic fibroblasts (MEFs) (ATCC, Manassas, VA) as feeder layers. Culture medium for maintenance of these cells in undifferentiated stage consisted of Dulbecco modified Eagle medium (DMEM) with high glucose, supplemented with 15% heat-inactivated fetal bovine serum (FBS; Omega Scientific Inc., Tarzana, CA), 1 mM sodium pyruvate, 0.1 mM nonessential amino-acids, 200 μM l-glutamine (Invitrogen, Grand Island, NY), 1,000 U/mL leukemia inhibitor factor (Chemicon, Temecula, CA), and 100 μM β-mercaptoethanol (Sigma). MEFs were grown at 37°C under 5% CO2 in DMEM high glucose (Invitrogen, Carlsbad, CA) supplemented with 15% FBS (Omega Scientific Inc.). To induce formation of EBs, R1 cells were cultured in hanging drops after disaggregating with accutase (Innovative Cell Technologies Inc., San Diego, CA). Six hundred cells were plated in each drop of 20 μL hanging on the lid of a Petri dish for 2 days. The medium used was the same as described above but supplemented with 20% heat-inactivated FBS (Omega Scientific Inc.). After this time, complete media was added to the cells to keep them in suspension for additional 3 days for EB formation. The HMEC line was donated by E.W. Ades and F.J. Candal from the CDC (Atlanta, GA) and T.J. Lawley (Emory University, Atlanta, GA). These cells retain specific markers for microvascular ECs and EC primary cultures [13,14]. Confluent monolayers were grown at 37°C under 5% CO2 in MCDB131 medium (Invitrogen, Carlsbad, CA) supplemented with 1% l-glutamine (Invitrogen, Carlsbad, CA), 10% FBS (Omega Scientific Inc.), and 100 μg/mL endothelial cell growth supplement (Upstate, Temecula, CA). These cells were used at passages 20–25. To test the effects of HMEC conditioned medium (HMEC-CM) on EBs, the normal media of these cells were replaced every 2–3 days by the medium described above in which knock-out serum replacer (KOSR; Invitrogen, Carlsbad, CA) was substituted for FBS. The HMEC-CM was then used to treat growing EBs for additional 15 days. For coculturing experiments, EBs were plated on glass coverslips precoated with 0.1% gelatin type A (Sigma). After 24 h, HMECs were disassociated with accutase (Innovative Cell Technologies, Inc.) and 75×103 cells/mL were plated together with already attached EBs. The media were changed after 24 h to medium for EBs with KOSR. The coculture continued for 15 days. At this time, the EBs were 20 days of age (EBd20). EBd10 and EBd30 were also analyzed. Some of these EBs were fixed and analyzed by immunocytochemistry (ICC). Another group of EBs were analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Confluent monolayers of mouse hemangioendothelioma cell line (EOMA) were grown at 37°C under 5% CO2 in DMEM-H medium (Invitrogen, Carlsbad, CA) supplemented with 1% l-glutamine (Invitrogen, Carlsbad, CA) and 15% FBS (Omega Scientific Inc.).
Immunocytochemistry
The EBs plated on coverslips cocultured with HMECs and those treated with HMEC-CM for 15 days were fixed with paraformaldehyde 4% (Polysciences, Inc., Warrington, PA) and permeabilized with 0.3% triton X-100 in PBS for 5 min. After rinsing with phosphate buffered saline, cells were blocked with PBS/5% BSA for 1 h and exposed overnight using primary antibodies to NF-L, nestin, cardiotin, PDX-1, proinsulin C-peptide, PARP (AB3565), Ki-67 (Millipore, Billerica, MA), CD31 (BD Biosciences Pharmingen, San Diego, CA), mouse IgG1, rabbit IgG, and rat IgG2a (isotype controls; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The secondary antibodies used were the following: Alexa Fluor 555 goat anti-rabbit IgG, Alexa Fluor 555 goat anti-mouse IgG, Alexa Fluor 488 goat anti-rat IgG (Molecular Probes, Eugene, OR). Images were acquired with a multipurpose zoom microscope (Nikon AZ 100; www.nikon.com/) attached to a DS-Qi1 high-sensitivity charge-coupled device camera (www.nikon.com/) and analyzed using an imaging software NIS-Elements AR 3.10 (Nikon Instruments, Melville, NY) and the image tools of ImageJ 1.30v software (Wayne Rasband National Institutes of Health). Another group of images were acquired with a true confocal scanner SP5×confocal microscope (Leica Microsystems, Mannheim, Germany).
Cytokine treatment
100 ng/mL of recombinant human BMP-2 (rhBMP-2), 100 ng/mL of rhBMP-4 (R&D Systems, Inc., Minneapolis, MN), or a combination were added directly to growing EBs. To inhibit the effects of these factors, 100 or 500 ng/mL NOG or 3 μg/mL CHRDL1 (R&D Systems, Inc.) were added to growing EBs treated or untreated with BMPs. Other groups of EBs cocultured with HMECs were treated with these inhibitors (NOG or CHRDL1). The media were replaced with fresh media with cytokines every 3 days.
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated from 100 EBs cultured alone, cocultured with HMECs or treated either with BMPs or EC-CM, using RNAeasy mini kit (Qiagen, Valencia, CA). After cDNA synthesis, using a QuantiTect Reverse Transcription kit (Qiagen), quantitative real-time PCR analysis was performed using SYBR Green RT-PCR kit (Qiagen) and the LightCycler instrument (AB Applied Biosystems, Foster City, CA; www3.appliedbiosystems.com/AB_Home/index.htm). PCR cycle conditions included a first step for initial polymerase activation for 10 min at 95°C and 45 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 20 s, and elongation at 72°C for 30 s. The forward and reverse primers used (all sequences are 5′–3′) were as follows:
CD31, GCTTGGCAGCGAAACACT, and TGGGAGGTGATGAATGGG;
cTnI, CCCTTCTCCCCTCTGCTGAT, and CCACAGCATTAAGCTGGGATCT;
Nestin, TGGAAGGTGGGCAGCAA, and AGCAGAGTCCTGTATGTAGCCACTT;
NF-L, CATGCAGAACGCCGAAGA, and CGGCGCTCTCGGTTAGC;
PDX-1, ATGAAATCCACCAAAGCTC, and GATGTGTCTCTCGGTCAAGT;
Insulin1, AACAGCATCTTTGTGGTCCC, and CACTTGTGGGTCCTCCACTT.
BMP-2, CTGCCTGCACCCTGTTCTCT, and GTTCAAACACATATCCCTGGAAAGA;
BMP-4, GGTCCAGGAAGAAGAATAA, and GGTACAACATGGAAATGG;
BMP receptor 1A (BMPR1A), GAAGTTGCTGTATTGCTGA, and GTAATACAACGACGAGCC;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ATTGACCACTACCTGGGCAA, and GAGATACACTTCAACACTTGACCT.
Negative controls were included in each analysis. In this case, the RNA was not treated with reverse transcriptase (No RT). All samples were run in triplicate and PCR products were observed by gel electrophoresis on 2% agarose ethidium bromide-stained gels. Analysis was performed using 7300 Sequence Detection Software (SDS) Version 1.3 (Software Core Application; AB Applied Biosystems; www3.appliedbiosystems.com/AB_Home/index.htm). Following the real-time PCR, a dissociation curve was run to detect primer dimmers, contaminating DNA, and PCR products from misannealed primers. We used a standard curve obtained by running a GAPDH-plasmid with a known copy number value based on its molecular weight. Automatic baseline and threshold feature (Ct) of the SDS software (auto Ct) was performed and the system considered Ct values established in the geometric phase of the amplification curve for each marker with minimal standard deviation. The standard curve was then used as a reference for extrapolating quantitative information for mRNA targets of unknown concentrations. In this way, the absolute number of copies was determined for each marker. The absolute number of copies of the specific marker was then divided by the absolute number of copies of GAPDH of the same sample for normalization (mouse housekeeping gene).
Cytokine determination
BMP-2 and BMP-4 were measured by MicroELISA (Quantikine Immunoassay; R&D Systems) in EC-CM from HMEC confluent monolayers. According to manufacturer information, the minimum detectable dose (MDD) for BMP-2 evaluated in 35 assays ranged from 4.3 to 29 pg/mL with a mean of 11 pg/mL. For BMP-4 evaluated in 36 assays the MDD ranged from 0.43 to 3.68 pg/mL with a mean of 1.04 pg/mL.
Videos
The beating clusters were recorded using a Spot camera RT-KE Slider 7.4.2 attached to a Nikon Eclipse TE2000-S microscope (Diagnostic Instruments, Inc., Sterling Heights, MI). The SPOT v4.6 software (Diagnostic Instruments, Inc.) was used to obtained sequential images that were transformed to audio video interleave files using the image tools of imageJ software 1.37v (Wayne Rasband National Institutes of Health).
Statistics
Data are expressed as mean±standard error of absolute quantification of gene expression values from three independent experiments. To find significant differences in the tested cell and EB groups, the values were assessed by Student's t-test using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
Results
EB-EC interface
Mouse EBs developed in hanging drops for 2 days, and then in suspension for three more days (Supplementary Fig. S1a; Supplementary Data are available online at www.liebertpub.com/scd). They were placed on coverslips alone (Supplementary Fig. S1b) or together with HMECs (Supplementary Fig. S1c). After attaching, EB cells spread out (Supplementary Fig. S1b). Those EBs cocultured with HMECs also spread out and contacted HMECs. After 15 days in culture, a well-defined cell interface between EBs and HMECs (EB-EC) was observed and close cell–cell interaction took place (black arrows in Supplementary Fig. S1c). EB cells at the interface and in the center were positive for Ki-67 and negative for PARP antibody (not shown) indicating cell proliferation and no apoptosis. This fact indicates that EB cells and HMECs can survive together in this condition.
HMECs promoted increase of three germ layer marker expression in cocultured EBs
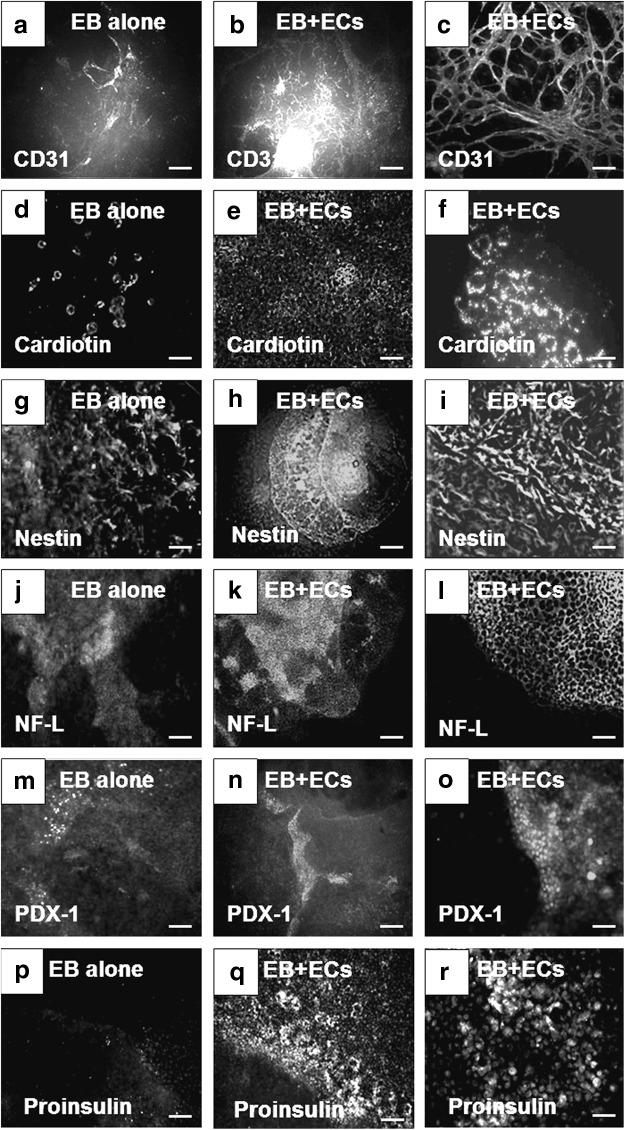
After 15 days in culture, enhancement in the expression of cellular markers from the three germ layers was observed in EBs cocultured with HMECs compared to controls (Fig. 1). Higher expression of CD31 (Fig. 1b, c) and cardiotin (Fig. 1e, f) was observed in EBs cocultured with HMECs in comparison to EBs cultured alone (controls) (Fig. 1a and 1d, respectively). Enhancement of cardiomyocyte differentiation was also evident by increase in the number and complexity of beating structures found in cocultured EBs (Supplementary Video SV1) in comparison to controls (Supplementary Video SV2). For ectoderm, we analyzed the expression of nestin and NF-L. Both markers were found in EB cultured alone (Fig. 1g and 1j, respectively). However, higher expression of nestin (Fig. 1h, i) and NF-L (Fig. 1k, l) was observed in EBs cocultured with ECs. Finally, expression of PDX-1 and proinsulin was evaluated for endoderm markers. Similarly, scarce positive cells were found in EBs cultured alone (Fig. 1m and 1p, respectively) in contrast with abundant cells that expressed PDX-1 (Fig. 1n, o) at cell–cell interface and proinsulin (Fig. 1q, r). These data suggested that HMECs provide inductive signals on EB cells to enhance the expression of markers representative of the three germ layers.
FIG. 1.
Enhancement in the expression of markers representative of the three germ layers in EBs cocultured with endothelial cells (HMECs). (a) Expression of CD31 in EBs alone. (b) Lower and (c) higher magnification of the expression of CD31 in EBs cocultured with HMECs. (d) Expression of cardiotin in EBs alone. (e) Lower and (f) higher magnification of the expression of cardiotin in EBs cocultured with HMECs. (g) Expression of nestin in EBs alone. (h) Lower and (i) higher magnification of the expression of nestin in EBs cocultured with HMECs. (j) Expression of NF-L in EBs alone. (k) Lower and (l) higher magnification of the expression of NF-L in EBs cocultured with HMECs. (m) Expression of PDX-1 in EBs alone. (n) Lower and (o) higher magnification of the expression of PDX-1 in EBs cocultured with HMECs. (p) Expression of proinsulin in EBs cultured alone. (q) Lower and (r) higher magnification of proinsulin expression in EBs cocultured with HMECs. (a, b, d, e, g, h, j, k, n, p, q) Scale bars=250 μm. (c, i, l, m, o, r) Scale bars=100 μm. (f) Scale bar=25 μm. EBs, embryoid bodies; HMECs, human microvascular endothelial cells; NF-L, neurofilament light.
BMP-2/-4 mimicked the effects of EC-CM on the enhancement of EB vascular networks
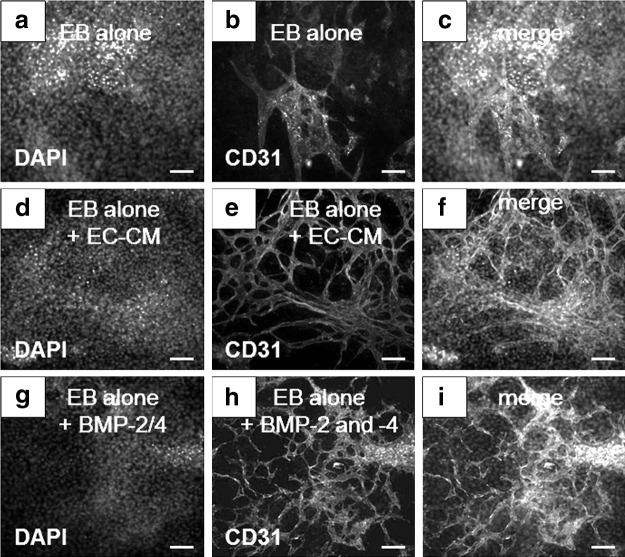
The dramatic effects observed in EB vasculature induced by HMECs prompted us explore effects of endothelial-derived factors involved in the enhancement of EB differentiation. We analyzed the expression of CD31 that was easily monitored by ICC. An identical angiogenic effect observed previously in EBs cocultured with HMECs (see Fig. 1b, c) was induced in EBs treated with EC-CM (Fig. 2d–f) in contrast to EBs not treated with this medium (Fig. 2a–c). Therefore, we tested factors involved in organogenesis that also promotes angiogenesis, such as BMPs [15]. We recently described the role of BMPs in the differentiation of mouse EBs to pancreatic endocrine progenitors and insulin producing cells [8]. We also reported that BMPs are expressed by mouse dermal microvascular ECs [11]. In the present work, we observed that EBs treated with a combination of rhBMP-2 and rhBMP-4 recapitulated the effects induced by EC-CM (Fig. 2g–i). In addition, we found a concentration of 210±0.035 pg/mL of BMP-2 and 130±0.80 pg/mL of BMP-4 in the conditioned media from confluent monolayers of HMECs. These results indicated that BMPs, possibly released by HMECs, played a central role in the enhancement of differentiation observed in EB-EC interactions.
FIG. 2.
Enhancement of EB blood vessel formation induced by EC-CM. Untreated EBs stained to (a) DAPI, and (b) CD31. (c) Merged image. EBs treated with EC-CM stained to (d) DAPI and (e) CD31. (f) Merged image. EBs treated with a combination of rhBMP-2 and rhBMP-4 stained to (g) DAPI, and (h) CD31. (i) Merged image. Scale bars=100 μm. EC-CM, endothelial-cell conditioned medium; DAPI, 4′,6-diamidino-2-phenylindole; rhBMP-2, recombinant human bone morphogenetic protein-2.
Combinatorial effects of rhBMP-2 and rhBMP-4 are similar to EC-CM effects on BMPs or BMPR1A upregulation in EBs at different stages of development
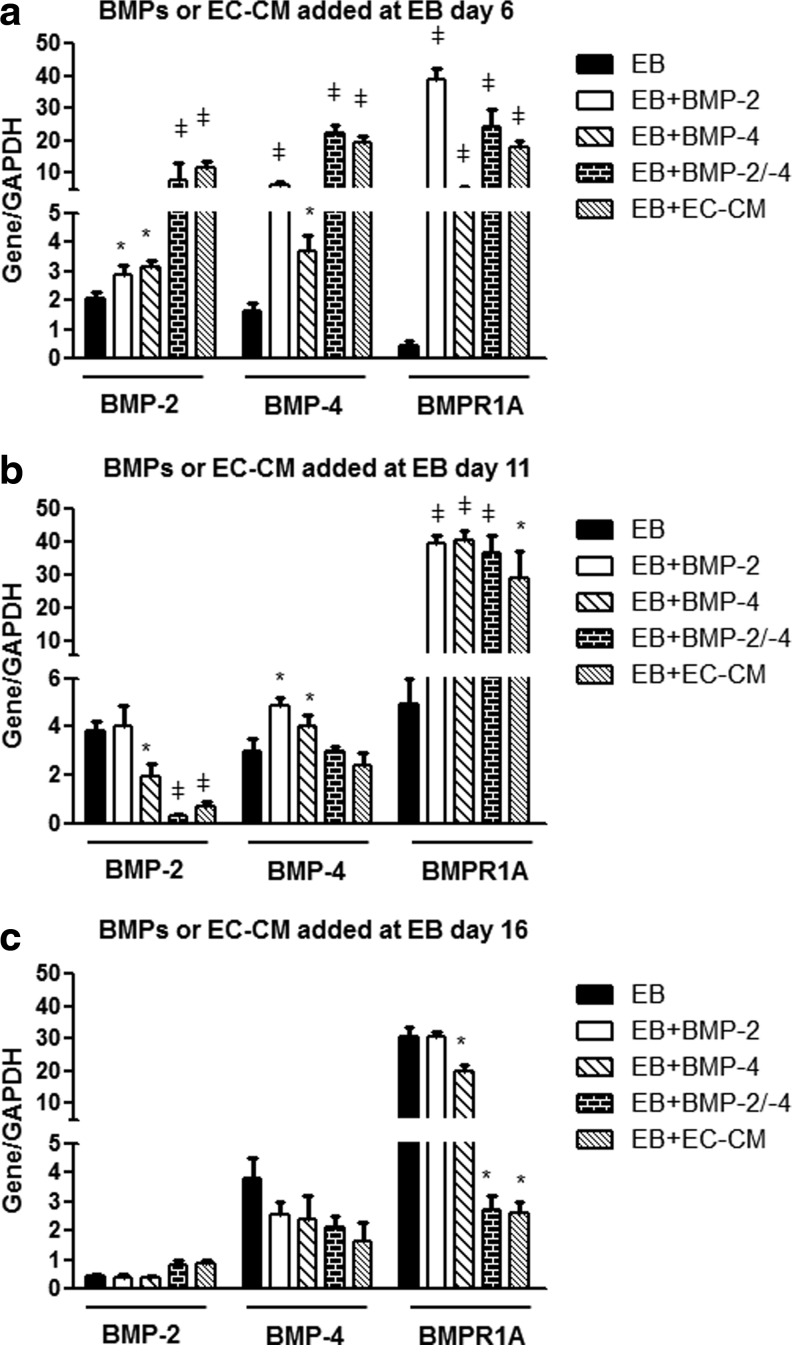
We hypothesized that HMECs enhanced differentiation of three germ layer marker expression in EBs by secreting endothelial BMPs. To confirm this hypothesis, we first analyzed the expression of BMP-2, BMP-4, or BMPR1A in EBs treated with EC-CM and compared these effects with those exerted by rhBMP-2, rhBMP-4, or a combination (BMP-2+BMP-4). These effects were analyzed in EBs treated at early (EB day 6), middle (EB day 11), or late (EB day 16) stages of development. Significant upregulation of BMP-2, BMP-4, and BMPR1A was found in EBs treated at early stages of development (Fig. 3a). At middle stages development, upregulation of BMP-2, BMP-4, or BMPR1A was observed only after adding BMP-4. In contrast, BMP combination or EC-CM induced downregulation of BMP-2. No upregulation of BMP-2/-4 was observed in EBs treated at later stages of development. Downregulation of BMPR1A was found in these EBs treated with BMPs or EC-CM. In the three groups, the upregulation or downregulation effects were similar between the combined BMPs and EC-CM. Interestingly, expression of BMP-2, BMP-4, and BMPR1A changed as function of EB age without any stimuli (Fig. 3a–c). These data suggested that EC soluble factors promote the expression of BMP-2, BMP-4, and BMPR1A at early stages of EB development and that these effects tend to be reduced at later stages of development (eg, EB day 16).
FIG. 3.
Quantification of BMP-2/-4 and BMPR1A by qRT-PCR in mouse EBs treated with BMPs or EC-CM at early, middle, or late stages of EB development. All of these EBs were harvested after 20 days of development. (a) BMP-2, BMP-4, and BMPR1A expression in EBs treated at early stages of development (EB day 6). (b) BMP-2, BMP-4, and BMPR1A expression in EBs treated at middle stages of development (EB day 11). (c) BMP-2, BMP-4, and BMPR1A expression in EBs treated at late stages of development (EB day 16). *p≤0.05,  p≤0.01. BMPR1A, BMP receptor 1A; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
p≤0.01. BMPR1A, BMP receptor 1A; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
BMP-2/-4 antagonists inhibit the effects of either EC-CM or rhBMP-2/-4
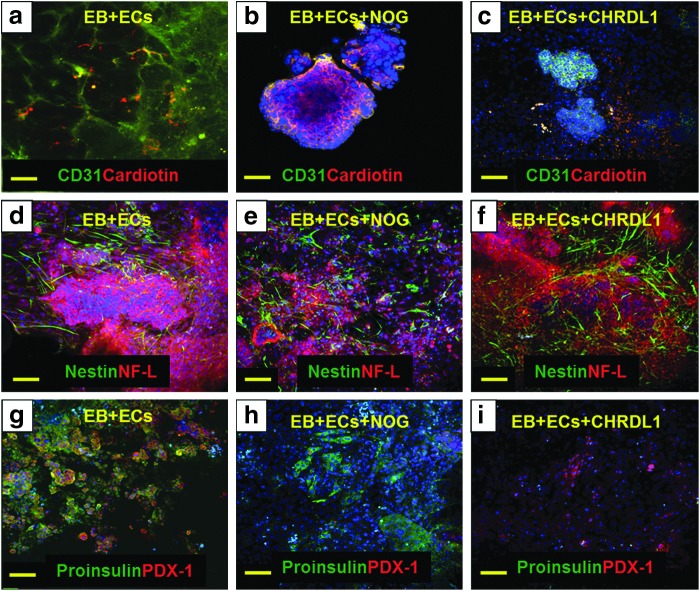
To corroborate the role of BMP-2/-4 in the enhancement of EB differentiation, we used NOG and CHRDL1. These molecules are known to inhibit BMP-2 and BMP-4 bioactivities, respectively [16,17]. Very low expression of CD31 and cardiotin was observed in a coculture system treated with NOG (Fig. 4b) or CHRDL1 (Fig. 4c), in comparison to untreated and cocultured EBs used as controls (Fig. 4a). NOG did not affect the expression of nestin but decreased the expression of NF-L (Fig. 4e). Expression of both markers was not affected by CHRDL1 (Fig. 4f) when compared to untreated cocultured EBs (Fig. 4d). Finally, NOG inhibited the expression of PDX-1 but not proinsulin; whereas, CHRDL1 effects were opposite with inhibition of proinsulin expression and preservation of PDX-1 (Fig. 4h, i).
FIG. 4.
Expression of markers from three germ layers in EBs cocultured with HMECs and treated with BMP inhibitors. Expression of CD31 (green) and cardiotin (red) in (a) EBs cocultured with HMECs, (b) EBs cocultured with HMECs and treated with NOG, and (c) EBs cocultured with HMECs and treated with CHRDL1. Expression of nestin (green) and NF-L (red) in (d) EBs cocultured with HMECs, (e) EBs cocultured with HMECs and treated with NOG, and (f) EBs cocultured with HMECs and treated with CHRDL1. Expression of proinsulin (green) and PDX-1 (red) in (g) EBs cocultured with HMECs, (h) EBs cocultured with HMECs and treated with NOG, and (i) EBs cocultured with HMECs and treated with CHRDL1. Scale bars=100 μm.
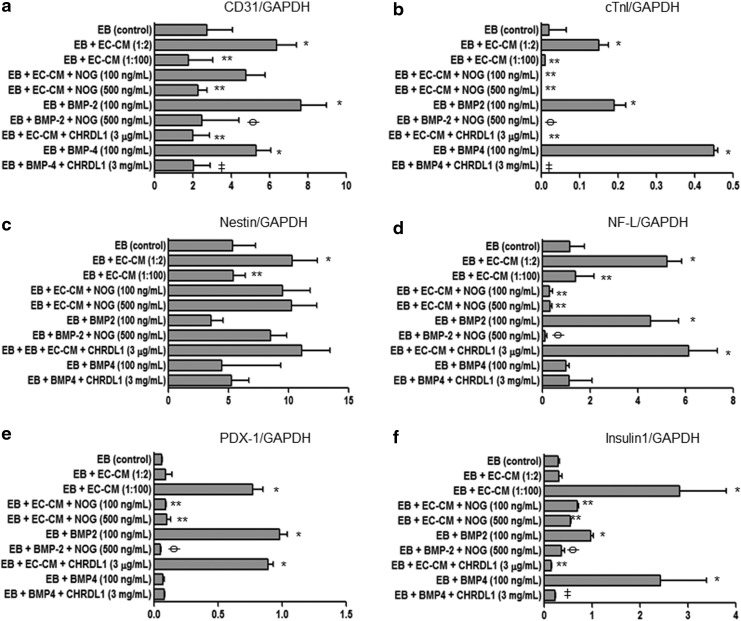
To quantify these observations, we performed qRT-PCR on the same samples. Figure 5 shows the three germ layer marker expression after treatment with EC-CM or BMPs with or without BMP antagonists. Upregulation of all the markers examined was found after treating the EBs with EC-CM. Dilution of the medium decreased these effects except for PDX-1 and insulin1 in which the EC-CM diluted 1:100 enhanced the effects. NOG inhibited EC-CM effects except in the case of nestin expression. Recombinant BMP-2 mimicked the upregulation observed in all of the markers except for nestin. CHRDL1 inhibited EC-CM effects only for CD31, cTn1, and insulin1. Recombinant BMP-4 induced upregulation of CD31, cTn1, and insulin1. Upregulation of nestin induced by EC-CM was neither inhibited by rhBMP-2 nor by rhBMP-4 and the respective antagonists have no significant effects. These data corroborated the ICC observations and confirm the role of BMP-2/-4 in the enhancement of differentiation observed in EBs cocultured with HMECs for most of the markers. The data suggested that CD31, cTnI, and insulin1 expression may be regulated by both BMPs (BMP-2 and BMP-4), while NF-L and PDX-1 expression may be regulated mainly by BMP-2. These results also suggest that nestin expression is not regulated by BMPs but by other EC factors.
FIG. 5.
Quantification of three germ layer marker expression by qRT-PCR in EBs treated with EC-CM, rhBMP-2, rhBMP-4, NOG, CHRDL1, or combinations. (a) CD31. (b) cTnI. (c) Nestin. (d) NF-L. (e) PDX-1. (f) Insulin1. Increases in relation to control (eg, EB control vs. EB+EC-CM) are represented by “*.” Decreases in relation to the conditioned media (eg, EB+EC-CM vs. EB+EC-CM+NOG) are represented by “**.” Decreases in relation to BMP-2 (eg, EB+BMP-2 vs. EB+BMP-2+NOG) effects are represented by “ .” Decreases in relation to BMP-4 (eg, EB+BMP-4 vs. EB+BMP-4+CHRDL1) effects are represented by “
.” Decreases in relation to BMP-4 (eg, EB+BMP-4 vs. EB+BMP-4+CHRDL1) effects are represented by “ .” *p≤0.05, **p≤0.05,
.” *p≤0.05, **p≤0.05,  p≤0.01,
p≤0.01,  p≤0.01. NOG, noggin; CHRDL1, chordin-like-1; cTnI, cardiac troponin 1.
p≤0.01. NOG, noggin; CHRDL1, chordin-like-1; cTnI, cardiac troponin 1.
Discussion
A cocultured system with enriched EC was used to stimulate the differentiation of the three germ layers within EBs. The EBs are considering structures composed of heterogenic cell populations derived from endoderm, mesoderm, and ectoderm [9]. We used this model to analyze the effects of ECs and EC-derived factors on the differentiation process. An interface between EBs and ECs (EB-EC) can be generated in vitro and in this way, the cell–cell interaction is established and effective. We previously used these coculture approach to induce differentiation of mESCs toward pancreatic cells [8]. In the present work, we found that ECs from human dermis (HMECs) can enhance the differentiation of mouse EBs in vitro. Other types of ECs that we tested, such as mouse ECs from EOMA failed to induce such differentiation (not shown). This fact indicates that not all the ECs produce the same EC-derived factors. These differences between ECs from different regions have been described before [18–20]. In the present work, HMECs enhanced the blood vessel networks within EBs. The blood vessels are one of the first structures formed during embryogenesis [12]. Hemangioblasts develop from mesodermal cells in the E7.5 mouse embryo and interact with other developing tissues and organs even before blood formation and nourishment function [3,12]. These facts suggest that hemangioblasts have to “cross-talk” to the surrounding cells to become mature ECs and induce maturation of the surrounding tissues or organs. Our results support this hypothesis since scarce blood circulation was observed in the blood vessels yet the differentiation enhancement took place. In addition, we reported enhancement of differentiation using mouse EBs implanted in surrogate vasculature from quail embryos [7]. These findings also support the idea of EC-derived factors as mediators of the differentiation process. We tested the EC-CM to evaluate the presence of soluble factors and found that the CM reproduced most of the effects observed in the coculture system. For instance, the robust vasculature was observed after treatment with EC-CM. As a first approach to determine the factors involved, we tested factors reported to be essential for organogenesis and angiogenesis [15,21]. The first candidates were BMP-2 and BMP-4 [15]. We tested this differentiation factors in previous experiments [8]. The effects induced by EC-CM were similar to those effects observed in EB vasculature after treatment with a combination of rhBMP-2 and rhBMP-4. We recently reported that these factors are produced by mouse dermal microvascular ECs (mDMECs) but not by EC from mouse EOMA indicated a differential marker expression between ECs [11]. In another work, it has been reported that BMPs play a crucial role in vascular development and pathophysiological processes [21]. Other BMPs also play an important role in embryo developing through interrelation of their intracellular pathways [1,12]. BMPs are multifunctional differentiation factors that belong to TGF-β superfamily [15]. Smad1, 5, and 8 are immediate downstream molecules of BMP receptors and play a crucial role in BMP signal transduction during embryo development for heart, neural, cartilage, and bone formation [15]. We found that EC-CM enhances the expression of BMP-2, BMP-4, and BMPR1A in our system. It is possible that ECs within EBs can produce these BMPs, but we are in a way to test this hypothesis. In the present work, we demonstrated that upregulation of these BMPs takes place at early stages of EB development in which parallel upregulation of BMPR1A suggest more sensitivity and BMP pathway activity at this early stage. When the BMPs or EC-CM were added at middle EB development, only BMP-4 promoted BMP-2/-4 upregulation and the combination of BMPs induced upregulation of the receptor. These facts suggest that the effects at the middle stage of development can be induced mainly by BMP-4. Further, downregulation of BMP-2 by EC-CM can also enhance BMP-4 activity at this EB stage. At late stages of EB development, BMPs have very poor effect in BMP upregulation and the combination of BMPs, as well as EC-CM induced downregulation of the BMPR1A. Taken together, these facts suggest that enhancement of the three germ layer marker expression is an effect of EC derived factors that affect EB cells at early and middle stages of EB development. This increase can be result of more BMP producing cells or BMP expression promoted by BMPs [16,21]. When we inhibit these BMPs with specific antagonists, the formation of blood vessels and cardiomyocytes was almost abolished. These data suggested that both developing factors are essential for endothelial and cardiomyocyte differentiation. Although this concept has been described previously, in the work herein we have shown that the culture enrichment with ECs is able to upregulates these developing factors in vitro and enhance the differentiation of ESCs toward different cell lineages [22–25]. BMP-2 inhibition by NOG reduced expression of NF-L without affecting nestin expression. In addition, nestin distribution and expression was almost unaffected by CHRDL1. These results suggest that nestin expression does not depend of endothelial BMPs, while NF-L expression indeed depends on BMP-2. These data is consistent with some reports in which BMP-2 exerts trophic effects in cultured sympathetic neurons with regulation of NF-L expression [26]. Additionally, it has been demonstrated that BMP-2 has an important role in the differentiation of catecholaminergic enteric neurons through SMAD-1 phosphorylation [27]. This fact is consistent with our results in which inhibition of BMP-2 by NOG decreases NF-L expression evaluated by ICC and qRT-PCR, while inhibition of BMP-4 does not affect NF-L expression. It is known that neural inducers bind to BMP-4, present in the ectoderm, and prevent BMP-4 interaction with its receptors allowing the acquisition of the neural fate of the ectoderm [27]. Therefore, downregulation of BMP-4 is essential for neural phenotype which is consistent with the data presented herein. We found that nestin expression was neither affected by NOG nor by CHRDL1. It is known that Notch signaling pathways affect nestin expression in brain tumors [28]. In another research work, nestin positive cells have been selected from a serum free medium to become insulin-producing cells after treatment with bFGF, B27, and nicotinamide [29]. These facts suggest that nestin expression can be regulated by other factors unrelated to BMPs. Finally, we observed that NOG inhibits the effects of EC-CM on PDX-1 and insulin1 expression, while CHRDL1 only inhibits insulin expression. These data suggest that BMP-2 but not BMP-4 is essential in the regulation of PDX-1 expression, while BMP-2 and BMP-4 are essential for insulin expression. However, apparently synergistic effects are important to maintain expression of PDX-1 and proinsulin in beta cells for suitable endocrine function not only during development but in adult beta cells [8]. In accordance with these ideas, an interaction between BMP-2 and BMP-4 to support organogenesis in mouse has been described [30]. We previously demonstrated that upregulation of BMP-2, BMP-4, and BMP1A appeared after treatment of EBs with EC-CM and that these EBs expressed more insulin-1/-2 and PDX-1 than controls [8]. Some studies have demonstrated that BMP-4 and its receptor are essential to induce expression of glucose sensor proteins (GLUT2, GKS, SUR1, and Kir6.2) in pancreatic beta cells [31]. Other studies indicate that BMP-2 is essential for the induction of insulin-positive cells in AR 42J cells [32]. Taken together with the present results, the data suggest that both developing factors are essential for beta cell differentiation and function. In the present work we described the effects of ECs in the in vitro enhancement in differentiation of ESCs toward cells derived from the three germ layers. Our work supports the idea that ECs play a central role in the differentiation and maturation of tissues and organs during embryogenesis. The “cross-talk” between ECs and developing organs has not been totally characterized and can be crucial for adequate function in the adult organism. We focused our analysis in the effects of EC-soluble factors, in particular BMPs. However, other EC-derived factors can be involved in three germ layer marker upregulation, for example, in the case of nestin expression. Our in vitro model will allow more efficient study of the mechanisms underlying organogenesis after this “cross-talk” takes place effectively. This model will also allow enhancing adequate differentiation of mouse or human EBs to obtain mature cells that can be suitable for regenerative medicine purposes.
Supplementary Material
Acknowledgments
We acknowledge Eris M. Field Endowment for Diabetes Research. We also acknowledge Kolja A. Wawrowsky for his help at the Cedars-Sinai confocal core facility.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lammert E, Cleaver O. and Melton D. (2003). Role of endothelial cells in early pancreas and liver development. Mech Dev 120:59–64 [DOI] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O. and Melton D. (2001). Induction of pancreatic differentiation by signals from blood vessels. Science (New York, NY) 294:564–567 [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Yoshitomi H, Rossant J. and Zaret KS. (2001). Liver organogenesis promoted by endothelial cells prior to vascular function. Science (New York, NY) 294:559–563 [DOI] [PubMed] [Google Scholar]
- 4.Cleaver O. and Melton DA. (2003). Endothelial signaling during development. Nat Med 9:661–668 [DOI] [PubMed] [Google Scholar]
- 5.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber H-P, et al. (2006). The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell 10:397–405 [DOI] [PubMed] [Google Scholar]
- 6.Nikolova G, Strilic B. and Lammert E. (2007). The vascular niche and its basement membrane. Trends Cell Biol 17:19–25 [DOI] [PubMed] [Google Scholar]
- 7.Talavera-Adame D, Dafoe DC, Ng TT, Wachsmann-Hogiu S, Castillo-Henkel C. and Farkas DL. (2009). Enhancement of embryonic stem cell differentiation promoted by avian chorioallantoic membranes. Tissue Eng Part A 15:3193–3200 [DOI] [PubMed] [Google Scholar]
- 8.Talavera-Adame D, Wu G, He Y, Ng TT, Gupta A, Kurtovic S, Hwang JY, Farkas DL. and Dafoe DC. (2011). Endothelial cells in co-culture enhance embryonic stem cell differentiation to pancreatic progenitors and insulin-producing cells through BMP signaling. Stem Cell Rev 7:532–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karbanová J. and Mokrý J. (2002). Histological and histochemical analysis of embryoid bodies. Acta Histochem 104:361–365 [DOI] [PubMed] [Google Scholar]
- 10.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB. and Mignatti P. (1998). Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 141:1659–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talavera-Adame D, Ng TT, Gupta A, Kurtovic S, Wu GD. and Dafoe DC. (2011). Characterization of microvascular endothelial cells isolated from the dermis of adult mouse tails. Microvasc Res 82:97–104 [DOI] [PubMed] [Google Scholar]
- 12.Nikolova G. and Lammert E. (2003). Interdependent development of blood vessels and organs. Cell Tissue Res 314:33–42 [DOI] [PubMed] [Google Scholar]
- 13.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC. and Lawley TJ. (1992). HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99:683–690 [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Swerlick RA, Sepp N, Bosse D, Ades EW. and Lawley TJ. (1994). Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1). J Invest Dermatol 102:833–837 [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Zhao M. and Mundy GR. (2004). Bone morphogenetic proteins. Growth Factors 22:233–241 [DOI] [PubMed] [Google Scholar]
- 16.Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D. and Mummery C. (2004). Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci 117:1269–1280 [DOI] [PubMed] [Google Scholar]
- 17.Piccolo S.Sasai Y.Lu B. and De Robertis EM. (2011). Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86:589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glienke J, Schmitt AO, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A. and Thierauch KH. (2000). Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem 267:2820–2830 [DOI] [PubMed] [Google Scholar]
- 19.Viemann D, Goebeler M, Schmid S, Nordhues U, Klimmek K, Sorg C. and Roth J. (2006). TNF induces distinct gene expression programs in microvascular and macrovascular human endothelial cells. J Leukoc Biol 80:174–185 [DOI] [PubMed] [Google Scholar]
- 20.Ho M, Yang E, Matcuk G, Deng D, Sampas N, Tsalenko A, Tabibiazar R, Zhang Y, Chen M, et al. (2003). Identification of endothelial cell genes by combined database mining and microarray analysis. Physiol Genomics 13:249–262 [DOI] [PubMed] [Google Scholar]
- 21.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG. and Ungvari Z. (2005). Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111:2364–2372 [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, et al. (2008). Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453:524–528 [DOI] [PubMed] [Google Scholar]
- 23.Van Wijk B.Moorman AFM. and van den Hoff MJB. (2007). Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res 74:244–255 [DOI] [PubMed] [Google Scholar]
- 24.DeGeorge BR, Jr, Rosenberg M, Eckstein V, Gao E, Herzog N, Katus HA, Koch WJ, Frey N. and Most P. (2010). BMP-2 and FGF-2 synergistically facilitate adoption of a cardiac phenotype in somatic bone marrow c-kit+/Sca-1+ stem cells. Clin Transl Sci 1:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji M. and Birren SJ. (2012). Target-dependent inhibition of sympathetic neuron growth via modulation of a BMP signaling pathway. Dev Biol 315:404–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anitha M, Shahnavaz N, Qayed E, Joseph I, Gossrau G, Mwangi S, Sitaraman SV, Greene JG. and Srinivasan S. (2010). BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 298:G375–G383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gómez-Skarmeta J, de La Calle-Mustienes E. and Modolell J. (2001). The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development (Cambridge, England) 128:551–560 [DOI] [PubMed] [Google Scholar]
- 28.Shih AH. and Holland EC. (2006). Notch signaling enhances nestin expression in gliomas. Neoplasia (New York, NY) 8:1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R. and McKay R. (2001). Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science (New York, NY) 292:1389–1394 [DOI] [PubMed] [Google Scholar]
- 30.Uchimura T, Komatsu Y, Tanaka M, McCann KL. and Mishina Y. (2010). Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis 47:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goulley J, Dahl U, Baeza N, Mishina Y. and Edlund H. (2007). BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab 5:207–219 [DOI] [PubMed] [Google Scholar]
- 32.Yew K-H, Hembree M, Prasadan K, Preuett B, McFall C, Benjes C, Crowley A, Sharp S, Tulachan S, et al. (2005). Cross-talk between bone morphogenetic protein and transforming growth factor-beta signaling is essential for exendin-4-induced insulin-positive differentiation of AR42J cells. J Biol Chem 280:32209–32217 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.