Abstract
Bcl-3 is an atypical member of the family of IκB proteins. Unlike the classic members, Bcl-3 functions as a nuclear transcriptional cofactor that may, depending on context, promote or suppress genes via association with p50/NF-κB1 or p52/NF-κB2 homodimers. Bcl-3 is also an oncogene, since it is a partner in recurrent translocations in B cell tumors, resulting in deregulated expression. Bcl-3’s functions, however, remain poorly understood. We have investigated Bcl-3’s role in B cells and discovered a previously unknown involvement in the splenic development of these cells. Loss of Bcl-3 in B cells resulted in significantly more marginal zone (MZ) and fewer follicular (FO) B cells. Conversely, transgenic expression of Bcl-3 in B cells generated fewer MZ and more FO B cells. Both Bcl-3−/− FO and MZ B cells were more responsive to LPS stimulation compared with their wild-type counterparts, including increased proliferation. By contrast, Bcl-3−/− FO B cells were more prone to apoptosis upon B cell receptor (BCR) stimulation, also limiting their expansion. The data reveal Bcl-3 as a regulator of B cell fate determination, restricting the MZ path and favoring the FO pathway, at least in part via increased signal-specific survival of the latter, a finding of relevance to its tumorigenic activity.
Introduction
Bcl-3 is a member of the IκB family, which is distinguished by shared ankyrin repeat domains capable of interacting with the Rel homology domains present in NF-κB transcription factors. While the classical members IκBα, IκBβ and IκBε primarily retain and thus inhibit p65- and c-Rel containing NF-κB dimers in the cytoplasm, Bcl-3 instead associates with nuclear p50 or p52 homodimers bound to DNA. Depending on the cellular context and target gene, Bcl-3 may promote or suppress transcription of certain NF-κB-regulated genes (13, 44). However, the specific biologic functions and mechanisms of action of Bcl-3 in cells remain poorly understood.
Nevertheless, Bcl-3 can have profound biological impact in vivo. The bcl3 gene was first identified and cloned at the breakpoint of recurring chromosomal translocations t(14;19) in B cell chronic lymphocytic leukemias (33). Subsequently additional translocations of the bcl3 were discovered in other B and some T cell tumors, resulting in increased and deregulated expression of otherwise unchanged Bcl-3 (29, 31, 32, 38). High levels of nuclear Bcl-3 have also been detected in a variety of B cell tumors in the absence of translocations, including classic Hodgkins lymphomas (4, 6, 16). In addition, a number of solid tumors express high levels of Bcl-3 (23).
It has been suggested that Bcl-3 may contribute to the survival and/or proliferation of tumor cells by positively regulating the expression of proteins such as Cyclin D1 and Hdm-2 (17, 41, 47). However these and other reports implicating possible targets of Bcl-3 in tumors remain isolated accounts, and how Bcl-3 actually promotes tumor formation is still an open question. Adding to this uncertainty, Bcl-3 has been suggested to intrinsically slow rather than promote proliferation of non-tumorigenic T cells (3), to contribute to apoptosis in some tumor lines (5, 30) and in distinction to earlier views, may not have a role in survival of activated CD8 T cells in vivo (8).
Apart from its tumorigenic potential, Bcl-3 is critical in host defense against certain pathogens, makes contributions to immune development, and can suppress autoimmunity (12, 34, 40, 42, 43, 49). However, the mechanisms underlying these in vivo roles also remain obscure. In vitro, Bcl-3 negatively regulates LPS-induced expression of some chemokines and cytokines in macrophages and dendritic cells. The proposed mechanism envisions Bcl-3 to aid p50 homodimer-mediated transcriptional inhibition (20, 42, 46) by preventing ubiquitin-mediated degradation of the homodimers (7). However, it remains to be determined whether this is the only or even primary mechanism by which Bcl-3 inhibits, and it leaves open the question when and how Bcl-3 promotes transcription. In this regard it is important to note that the functional activities of Bcl-3 may be controlled not only by its inducible expression (5, 10, 20, 39), but also by post-translational means, including phosphorylation and ubiquitination (15, 18, 19, 25, 45).
Based on such findings Bcl-3 appears to have diverse cell-, signal- and gene-specific effects and how it operates in specific biologic contexts largely remains to be discovered. Despite its oncogenic potential in B cells, Bcl-3’s role in normal B cells has not been addressed. Surprisingly we find here that Bcl-3 delimits the development of marginal zone B cells, favoring development of follicular B cells instead. Bcl-3 promotes survival and thus overall proliferation of cells stimulated via their B cell receptor, while it impairs responses to LPS. We will discuss how these activities may be relevant to the cell fate decision of developing B cells, as well as the tumorigenic potential of Bcl-3.
Materials and Methods
Mice
Bcl-3 KO mice have been described previously (12). Bcl-3 transgenic mice with mb-1-Cre-driven, B cell-specific expression of the transgene are described in Supplemental Materials. These mice were bred and co-housed together with genetically matched C57BL/6 wild-type controls in NIAID Institute facilities. They were analyzed at 6–8 weeks of age and all experiments were done with approval of the NIAID Animal Care and Use Committee and in accordance with all relevant institutional guidelines.
Antibodies
Monoclonal antibodies were purchased from BD Biosciences (San Diego, CA), eBioscience (San Jose, CA), Southern Biotech, and AbD Serotech (Raleigh, NC): FcγRIII/II (2.4G2), IgM (R6–60.20), IgD (11–26), CD1d (1B1), CD9 (M-L13), CD11a (2D7), CD18 (C71/16), CD21 (7G6), CD23 (B3B4), CD24 (M1/69), CD29 (9EG7, HMb1-1), CD40 (3/23), CD43 (S7), CD45R/B220 (RA3-6B2), CD45.2 (104), CD49d (R1-2), CD54 (3E2), CD69 (H1.2F3), CD80 (16-10A1), CD86 (GL1), CD93 (AA4.1), I-A/I-E (2G9), integrin β7 (M293), MOMA (CD169 AF647), MAdCAM (MECA-89).
Cell purification
Total B cells were purified from erythrocyte-lysed single cell suspensions of splenocytes by negative selection with anti-CD43-microbeads on midi MACS columns or by AutoMACS (Miltenyi Biotec), according to the manufacturer’s instructions. For isolation of MZB and FOB cell subsets, purified total B cells pooled from 4–5 mice were incubated with FcR-blocking antibody 2.4G2, stained with APC-anti-CD21, PE-anti-CD23, APC-Cy7-anti-B220, PE-Cy7-anti-AA4.1 and PE-Cy5-CD24, and sorted with a FACSria cell sorter (Becton Dickinson). (MZB cells: AA4.1−B220+CD21hiCD24lowCD23−/low and FOB cells: AA4.1−B220+CD21+ CD24lowCD23+). Alternatively, FOB cells were sorted from total, anti-CD43 negatively selected B cells via positive selection for CD23 with AutoMACS.
Flow cytometry
FACS® analyses were performed on freshly prepared splenocytes (in PBS/0.5% BSA/2 mM EDTA) or on cultured flow- or bead-sorted B cell subsets (see above). For surface marker staining, cells were pre-incubated with anti-FcγRIII/II for 10 min on ice and then stained with conjugated antibodies for 30 min. The LIVE/DEAD Fixable Aqua Dead cellstain kit (Invitrogen) was used for live cell-gating. Staining was analyzed on a FACS Calibur or Canto II (BD Biosciences). Data were analyzed with FlowJo software.
Bone marrow chimeras
Bone marrow cells were harvested by flushing femurs and tibias of mice with cold HBSS. 3–5×106 erythrocyte-lysed bone marrow cells were injected into the tail veins of lethally irradiated (900rads) recipient mice in 0.2 ml HBSS and recipient mice were analyzed 6–8 weeks after transfer.
Cell culture and proliferation and Caspase 3 assays
Flow cytometrically-sorted MZB and FOB cells were stimulated with anti-IgM (Fab’, Jackson Immunoresearch Laboratories, West Grove, PA) or LPS (Sigma, St. Louis, MO) in 96-well-bottomed plates. For proliferation assays, 2×105 cells/well were stimulated for 72h and pulsed with 1μCi per well of [3H] thymidine for the final 4–6 hours before harvest. For analysis of proliferation by CFSE dilution, flow-sorted FOB and MZB cells were labeled with 0.5μM CFSE (Invitrogen), and stimulated for 72h with anti-IgM and LPS, respectively. Incorporation of BrdU (BD Biosciences) was performed on bead-sorted FOB (see above) incubated with anti-IgM for 72hrs, with BrdU present for the last 24hrs. The anti-BrdU antibody (eBioscience) was used according to the manufacturer’s instructions. For caspase 3 activation assays, bead-sorted FOB cells were stimulated for 48h with anti-IgM or LPS and caspase 3 activation was measured using the NucView 488 Caspase 3 Assay Kit for Live Cells (Biotium, Hayward, CA) according to the manufacturer’s instructions. Negatively bead-selected total B cells were also stimulated with LPS or anti-IgM prior to surface staining and analysis. Unless otherwise stated, cultured cells were stimulated with 6.25 μg/ml anti-IgM or 200 ng/ml LPS.
Immunofluorescence
Spleens were embedded in OCT and frozen. Cryostat sections were fixed for 10 min in ice-cold acetone and sequentially blocked with blocking buffer containing 10% rabbit or goat serum. Sections were washed in 50mM TBS, pH 7.6, or PBS between all steps. Sections were incubated sequentially with rat anti-mouse MAdCAM (4C overnight) (detected with Alexa Fluor-568 anti-rat IgG), and then biotin-conjugated anti-CD1d at room temperature for 2h followed with Streptavidin-conjugated Alexa Fluor- 647 Sections were examined using a Leica AF6000X fluorescence microscope and images were acquired with a Hamamatsu video camera and processed with Imaris software.
Statistical analysis
Data were recorded as the mean ±SEM for direct ex vivo experiments and the mean ±SD for in vitro culture experiments. Results were analyzed using Student’s t-tests. p< 0.05 was considered significant.
Results
Bcl-3−/− mice harbor increased numbers of marginal zone B cells
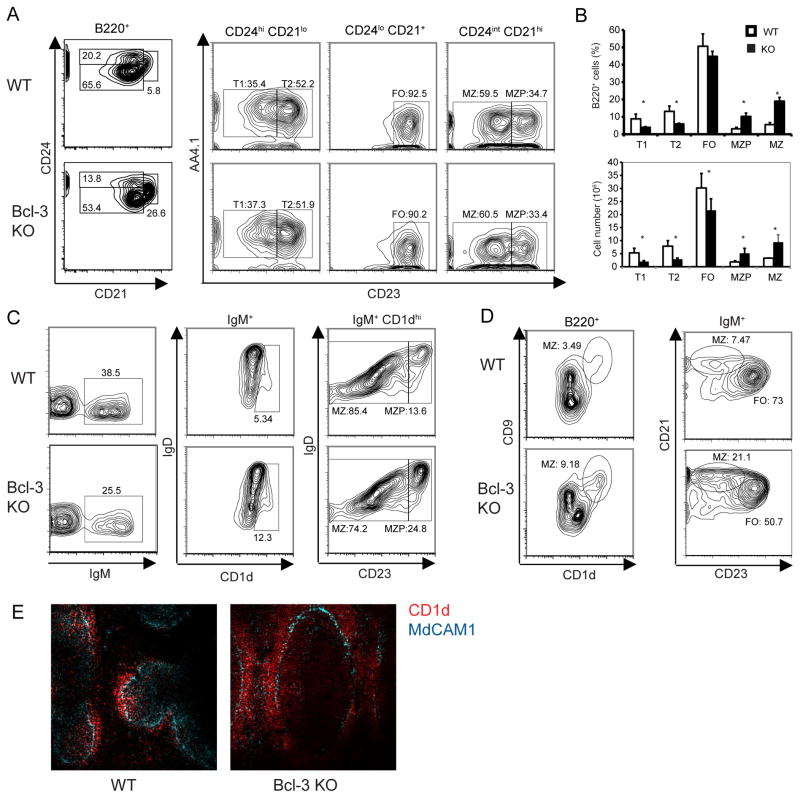
Splenic B cells consist of immature-transitional B cells and two types of mature B cells, follicular (FO) and marginal zone (MZ) B cells; the latter two differ with respect to phenotypic markers, location and function. Previous studies showed a mild overall reduction of total B cells in Bcl-3−/− spleens due to stromal defects (12, 37). To investigate potential roles of Bcl-3 in splenic B cells we performed flow cytometric analyses to enumerate subpopulations, using several different gating strategies (1, 26, 48). These analyses revealed a profound increase in relative and absolute numbers of B220+AA4.1−CD21hiCD24intCD23lo/−MZB cells in spleens of Bcl-3−/− (KO) as compared to Bcl-3+/+ (WT) mice (Fig. 1A; enumerated in 1B). The increase in MZB cells was confirmed in additional flow cytometric analyses (Fig. 1C, D) (MZB cells: CD1dhiCD23lo/−IgM+IgDlo; B220+CD1dhiCD9+ or B220+IgM+CD21hiCD23lo/−). We also detected an increase in relative and absolute numbers of MZB cell precursors (MZP) in Bcl-3−/− mice compared to controls (MZP cells: B220+AA4.1−CD21hiCD23hiCD24int [Fig. 1A, B] and CD1dhiIgDhiCD23hi [Fig. 1C]). These increases were accompanied by compensatory decreases in relative and absolute numbers of FO and transitional B cells (enumerated in Fig. 1B) (FOB cells: B220+AA4.1−CD21+CD24loCD23+; transitional T1: B220+AA4.1+CD21loCD24hiCD23−; transitional T2: B220+AA4.1+CD21+CD24hiCD23+).
Figure 1.
Bcl-3−/− mice exhibit increased numbers of marginal zone B cells. (A) Representative flow cytometric analysis of splenocytes from 6-week-old Bcl-3+/+ (WT) and Bcl-3−/−(KO) mice, analyzed for surface markers with gates shown across top of panels as indicated. Percentages of B cell subpopulations are shown (T, transitional; FO, follicular; MZ, marginal zone; MZP, MZ precursor). Data are representative of two separate experiments, each with at least n=4 independently analyzed mice per group. (B) Percentages (upper panel) and absolute numbers (lower panel) of B cell subpopulations. Data shown are the mean ± SEM based on a flow cytometric analysis experiment from (A) (n≥4). (*= p<0.05). (C, D) Flow cytometric analyses of splenocytes from mice as in (A), analyzed for expression of surface markers with gates as indicated. Percentages of subpopulations are shown; abbreviations as in (A). Data are representative of at least 2 separate experiments, each with at least n=3 independently analyzed mice per group. (E) Representative spleen section from 6-week old KO and WT mice stained for expression of CD1d and MAdCAM1 shown in colors as indicated. Magnification 20x. Data representative of 2 separated experiments with at least n=4 independently processed mice per group.
To assess whether the phenotypically assessed increase in MZB cells was also reflected by an increase in B cells residing in the splenic MZ, we stained frozen sections with antibodies to CD1d and MAdCAM1. We observed more CD1dhi B cells in the MZ (located just outside the ring of MAdCAM1-staining endothelial cells) of Bcl-3−/− than WT mice (Fig. 1E). Collectively, the data indicate that loss of Bcl-3 significantly skewed B cell development towards the mature and precursor MZB cells, and conversely, away from mature FOB cells. Given that FOB cells are more abundant than MZB cells, the relative change in the latter appeared to be more dramatic.
Like MZBs, B1 B cells have innate functions; they contribute to homeostatic serum immunoglobulin levels and respond well to TLR signals, such as LPS. Nevertheless, Bcl-3−/− deficient mice did not exhibit a noticeable change in the number of peritoneal B1 B cells compared to WT mice (not shown). This may not be surprising, since B1 B cells represent a self-renewing and developmentally distinct lineage derived from fetal liver precursors (27).
The increase in MZB cells in Bcl-3−/− mice is intrinsic to B cells and cell-autonomous
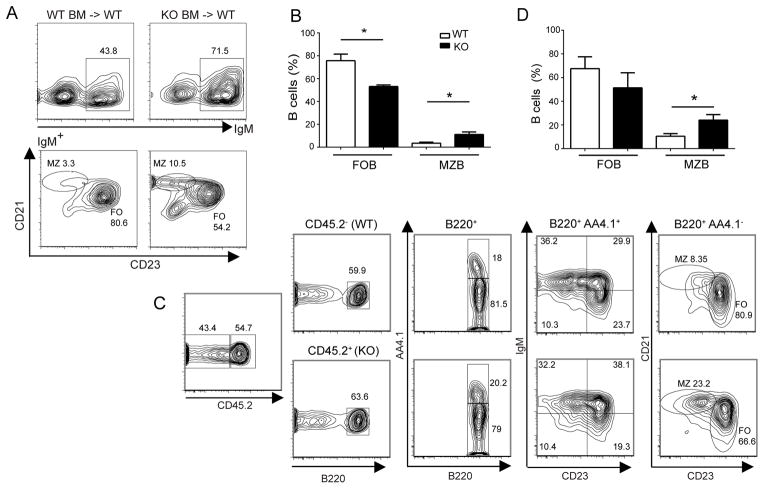
To determine whether the increase in MZB cells and corresponding decrease in FO (and transitional) B cells might result from a defect in hematopoietic (but not stromal) cells, we reconstituted lethally irradiated WT mice with either Bcl-3+/+(WT) or Bcl-3−/− (KO) bone marrow cells. Following full reconstitution, chimeras with Bcl-3−/− bone marrow presented with a marked relative increase in MZ and a relative decrease in FOB cells, while mice reconstituted with WT bone marrow presented with a normal ratio (Fig. 2A; enumerated in 2B). The results indicate that the increase in MZ and loss of FOB cells in Bcl-3−/− mice was due to defects intrinsic to hematopoietic cells.
Figure 2.
Increase in MZ B and decrease in FO B cells in Bcl-3−/− mice is due to a B cell autonomous defect. (A) Lethally irradiated WT mice were reconstituted via transfer of bone marrow cells from WT or KO mice. Representative flow cytometric analysis of splenocytes stained for expression of surface markers with gates across top of panels as indicated. (B) Summary graph of data as in (A) shown as the mean ±SEM from 2 separate experiments with n=5 independently analyzed recipient mice. (C) Irradiated Rag1−/−mice were reconstituted via co-transfer of approximately equal numbers of bone marrow cells from CD45.1+ (CD45.2−) WT and CD45.2+ KO mice. Representative flow cytometric analysis of splenocytes stained for expression of surface markers with gates as indicated. (D) Summary graph of data as in (C) shown as the mean ±SEM from 3 separate experiments with n≥4 independently analyzed recipient mice. Abbreviations as in Fig. 1. (*= p<0.05)
To determine whether the increase in MZB cells was due to a B cell-autonomous process, we carried out mixed bone marrow transfers. CD45.1+ marked WT (Bcl-3+/+) bone marrow cells were co-transferred together with equal numbers of either CD45.2+ Bcl-3+/+ (WT) or CD45.2+ Bcl-3−/− bone marrow cells to serve as donors for lethally irradiated Rag1-deficient recipient mice. We observed a significant relative increase in the numbers of MZB cells and a corresponding reduction in FO (but not transitional AA4.1+) B cells in 45.2+ Bcl-3−/−donor-derived B cells, while the co-transferred 45.1+ (45.2−) Bcl-3+/+ donor-derived B cells showed a normal distribution (Fig. 2C; enumerated in 2D). Together these results indicate that the increase in MZB and reduction in FOB cells in Bcl-3-deficient mice were due to B cell-intrinsic and cell-autonomous defects.
Loss of Bcl-3 accentuates MZB cell phenotypes and responses
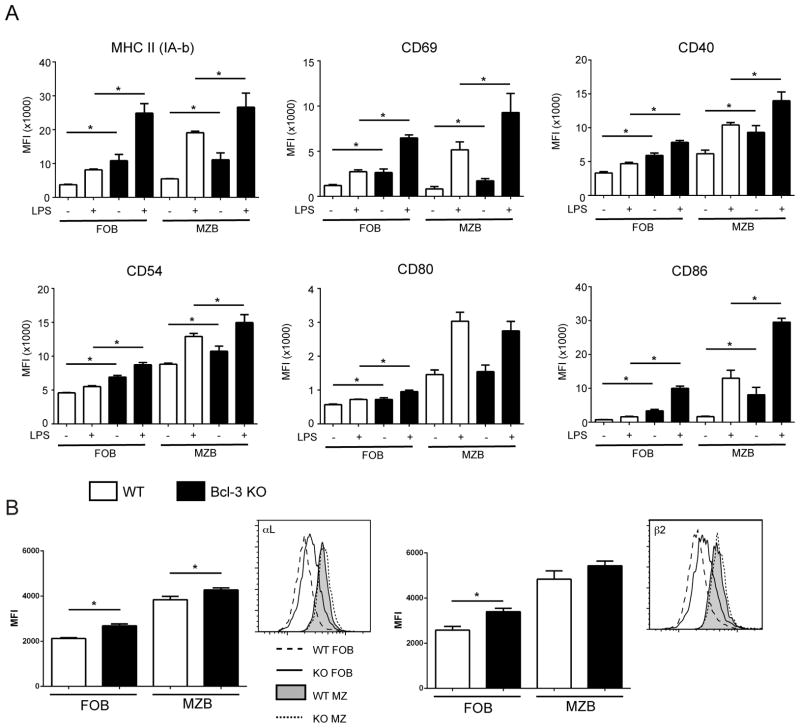
MZB cells are superior to FOB cells in inducing expansion of CD4+ T cells both in vitro and in vivo, consistent with higher basal and LPS-induced levels of MHC class II and co-stimulatory molecules on MZ B cells (2). We stimulated freshly bead-isolated B cells with LPS (or did not stimulate them) and then analyzed these cells for expression of various surface markers after gating on live FO or MZ B cells. Loss of Bcl-3 resulted in increased basal and LPS-induced levels of MHC II, CD86, CD40, CD69 and CD54 (and a smaller increase in basal CD80 levels), in both FO and MZ B cells when compared with their WT counterparts (Fig. 3A). Consistent with this, Bcl-3−/−splenic B cells resulted in better proliferation of CD4+ T cells in co-cultures when compared to WT B cells (not shown). BCR stimulation (αIgM) is known to also induce MHC class II and co-stimulatory markers on FO B cells, albeit to a lesser extent than LPS (2, 21); loss of Bcl-3 resulted in slight increases in αIgM-induced levels of some of these markers in live Bcl-3−/− FO B cells when compared to their WT counterparts (Supplemental Fig. 1A, B).
Figure 3.
Expression of co-stimulatory molecules and integrins on Bcl-3−/− B cells. (A) Naïve CD43− B cells were obtained by MACS separation and were stimulated for 24h with LPS (200 ng/ml) or not stimulated (NS). Cells were then analyzed by flow cytometry for expression of surface makers after gating on MZ B cells (B220+ CD1dhi CD21hi, then CD23−/lo) and FO B cells (B220+ CD1d−/lo CD21int, then CD23hi) as indicated. MFI data are shown as mean ±SD of 3 separate experiments with n=8 independently analyzed mice per group. (B) Splenocytes from 6–8 week-old WT and KO mice were analyzed by flow cytometry after staining for B220, CD23, CD21 and the integrin chains αL and β2, as indicated. After gating on MZ B cells (B220+ CD23−/lo CD21hi) or FO B cells (B220+ CD23+ CD21+), expression of integrin chains was analyzed. Representative plots are displayed next to summary MFI data shown as the mean ±SEM for 3 separate experiments, with n=6–8 independently analyzed mice per group. (*= p<0.05)
Modulation of integrin levels in Bcl-3−/− B cells
Several signals have been proposed to positively impact whether transitional B cells become MZB cells and then are maintained as such (35). This includes Notch2 signals (28) and a preference to respond to S1P over CXCL13, agents which direct the egress and entry of B cells into follicles, respectively (9). We did not find any evidence that Bcl-3 is involved in the Notch2 pathway, as expression of the target gene Deltex1 was not changed by the absence of Bcl-3 in FO or MZB cells; similarly, in initial experiments we failed to observe skewed chemotaxis of Bcl-3 deficient FO or MZ B cells towards S1P over CXCL13 (data not shown). BAFF-induced activation of the alternative NF-κB pathway is another signal critical for MZB cell development (11). Loss of Bcl-3 did not alter activation of this signaling pathway (Supplemental Fig. 2), although this does not rule out possible effects of Bcl-3 on target genes of alternatively activated NF-κB.
MZB cells express somewhat higher levels of the integrins LFA1 (αL/β2) and VLA4 (α4β1) than FOB cells; these integrins are thought to contribute to the long-term retention in the splenic MZ (22). We observed a modest increase in the expression of αL and β2, and a slight increase in β1, though not α4, in both Bcl-3−/− FO and MZ freshly isolated B cells when compared to their WT counterparts (Fig. 3B and Supplemental Fig.1C). Slightly increased levels of in particular LFA1 in Bcl-3−/− B cells may therefore favor retention in the MZ and could contribute modestly to increased levels of MZB cells. However, overall these findings suggest that Bcl-3 likely regulates additional steps to control the process whereby B cells are fated to become either MZ or FO B cells.
Transgenic overexpression of Bcl-3 suppresses development of MZB cells
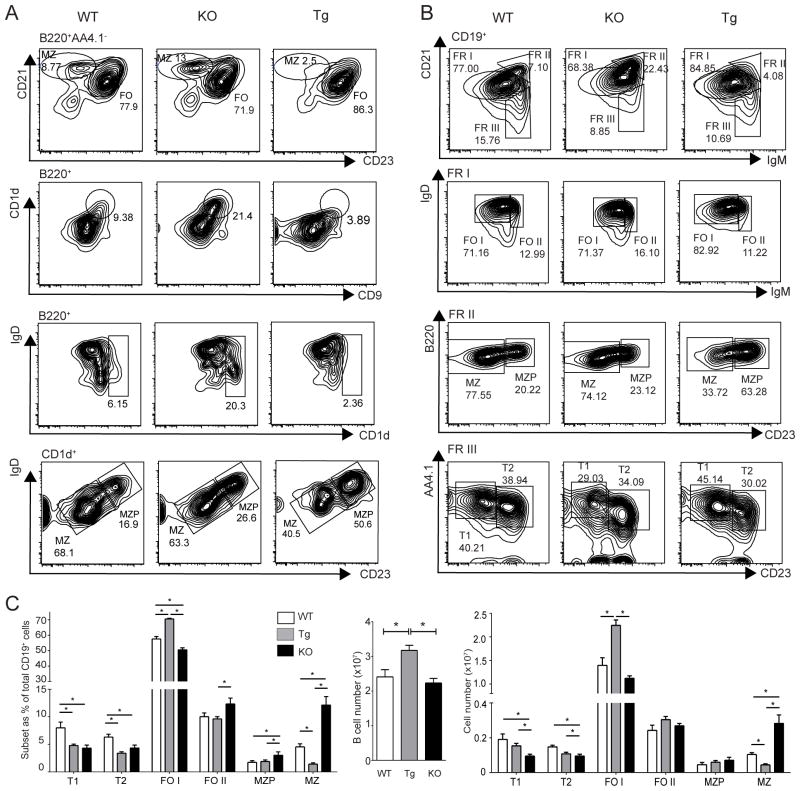
To confirm and further explore the role of Bcl-3 in MZ vs. FO B cell development we generated mice in which expression of a Bcl-3 transgene could be induced specifically in B cells via mb1-cre-mediated (14) removal of a LoxP-flanked Stop casette (Supplemental Fig. 3A–C). B cell-specific expression of the Bcl-3 transgene resulted in reduced relative and absolute numbers of MZB cells when compared to WT, and especially when compared to KO B cells (Fig. 4 A–C) (MZB cells: B220+AA4.1−CD21hiCD23lo/−; B220+CD1dhiCD9+ and B220+CD1dhiCD23lo/−IgDlo [Fig. 4A]; and CD19+CD21hiIgMhiB220+CD23lo/− [Fig. 4B]). Conversely we noted a relative and absolute increase in transgenic FOB cells (CD19+CD21+IgD+IgM+/hiAA4.1−CD23+) when compared to WT and KO B cells (Fig. 4B). FOB cells were further subdivided into the major FO I and the minor FO II fraction, expressing low/intermediate and high levels of IgM, respectively (Fig. 4B). (Data for analyses as in Fig. 4B enumerated in Fig. 4C [relative fractions and cell numbers of B cell subpopulations and total numbers of all B cells in WT, Bcl-3 KO and Bcl-3 transgenic (TG) mice]). Interestingly, among the Bcl-3−/− (KO) B cells the fraction of FO II B cells was increased relative to that of WT (and TG), even though the total fraction of FO, and in particular FO I B cells was decreased. The increase in FO II mutant B cells may be consistent with the increase in MZB cells, since FO II B cells may be a reservoir for MZB cells (see Discussion).
Figure 4.
Transgenic expression of Bcl-3 in B cells reduces MZ and increases FO B cells. (A, B) Representative flow cytometric analyses of splenocytes from 6–8-week-old WT, KO and Bcl-3transgenic (Tg) mice stained for expression of surface markers with gates across top of panels as indicated. Percentages of various subpopulations are shown; fractions (FR) I, II and III in (B) refer to subpopulations shown in first row of panels. (FO I, II = follicular 1, 2; other abbreviations as in Fig.1) Data for (A, B) representative of 3 separate experiments with n=7–9 independently analyzed mice per group. (C) Summary of data as in (B) shown as mean ±SEM (*= p<0.05). Left panel, percentages of B-cell subpopulations; center panel, total number of splenic B cells for each genotype; right panel, total numbers of B-cell subpopulations.
While the fraction of MZB was significantly decreased and that of FO I B cells increased in Bcl-3 transgenic as compared to WT B cells, we did not observe reductions in the fractions of MZP or FO II B cells, indicating that overexpression of Bcl-3 did not result in the complete opposite of what was seen in the absence of Bcl-3 (Fig. 4C). Nevertheless, consistent with the substantial relative decrease of MZB cells in transgenic B cells as determined with surface markers, immunofluorescence analysis showed a relative paucity of B cells in the MZ (Supplemental Fig. 3D). Thus B cell-specific overexpression of Bcl-3 appears to have largely, though not entirely, opposite effects to the loss of Bcl-3. These results further confirm that Bcl-3 negatively impacts MZB cell generation, while promoting generation of FOB cells.
Bcl-3 differentially modulates αIgM- versus LPS-induced B cell proliferation
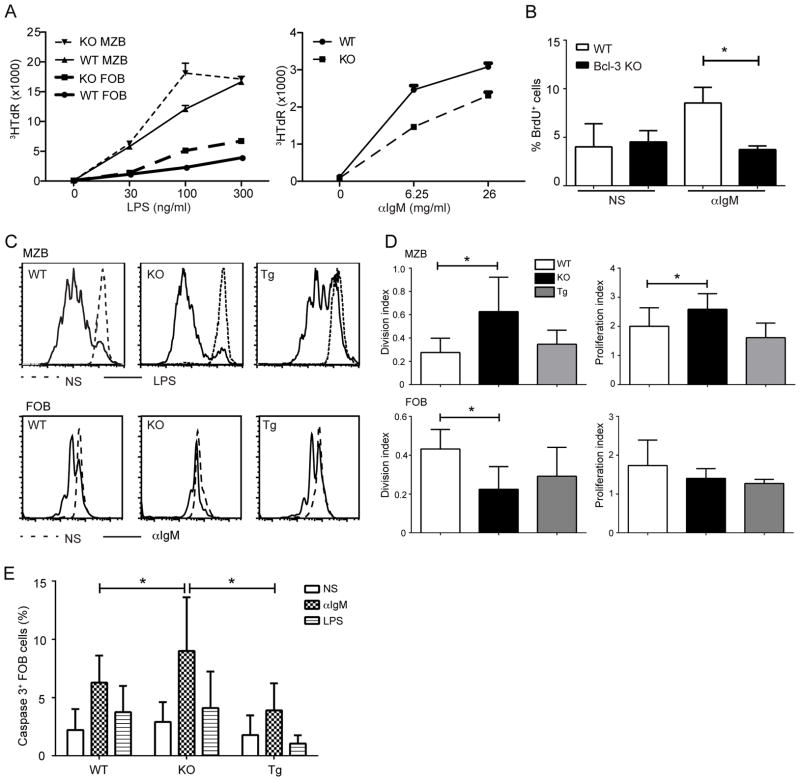
It has been hypothesized that very weak BCR signaling pushes developing B cells towards the MZB cell fate, while relatively stronger BCR signaling (by self-antigens) favors the FOB cell fate (35, 36). We FACS-sorted MZB and FOB cells from B cells pooled from 4–5 mice and examined their proliferative responses to LPS and αIgM stimulation. Both Bcl-3−/−MZB and FOB cells proliferated somewhat better than their WT counterparts when stimulated with LPS, as judged by thymidine incorporation, but the reverse was true when stimulated with αIgM, as now wild-type FOB cells proliferated somewhat better than their Bcl-3−/− counterparts (Fig. 5A), despite similar levels of surface IgM (not shown). We confirmed this by examining BrdU incorporation in αIgM-stimulated, bead-isolated FOB cells, gating on live cells (Fig. 5B). In addition, we examined αIgM- and LPS-driven proliferation of CFSE labeled, FACS-sorted FO and MZ B cells, respectively; in each instance we compared WT, Bcl-3−/− and Bcl-3 transgenic B cells after 72 h of stimulation, gating on live cells (Fig. 5C; summary data shown as proliferation and division indices in Fig.5D). Bcl-3−/− MZB cells again showed significantly increased LPS-induced proliferation over WT B cells, with Bcl-3 transgenic MZB cells proliferating similar to or slightly less well than WT (Fig.5 C, D; upper panels). In contrast, Bcl-3−/− FOB cells proliferated less well upon αIgM stimulation than their WT counterparts, consistent with findings above (Fig.5 C, D; lower panels). Bcl-3 transgenic FOB cells also proliferated slightly less well than WT, albeit not significantly so, which indicated that transgenic overexpression of Bcl-3 did not result in the exact opposite of the Bcl-3 deficient phenotype. Of note, αIgM stimulation of Bcl-3−/−FOB cells showed a significant decrease relative to WT in the division index, but only a trend in that direction in the proliferation index. This suggested the possibility that Bcl-3−/− FOB cells were more susceptible to apoptosis after αIgM stimulation, or else less responsive.
Figure 5.
Bcl-3 differentially modulates BCR- and LPS-induced B cell proliferation. (A) Thymidine incorporation of FO and MZ B cells. Cells were obtained by FACS-sorting from B cells pooled from 4–5 WT and KO mice and stimulated for 72 hours with titrated amounts of LPS (left panel) or anti-IgM (right panel), as indicated, with 3HTdR present during the last 4–6h. Data shown are mean ± SD of triplicate measurements; similar results were obtained in an independent experiment. (B) BrdU incorporation of FO B cells (CD43−CD23+), isolated from WT and KO mice with MACS beads and stimulated with anti-IgM (6.25μg/ml) for 72 hours or not stimulated (NS), with BrdU present during the last 24h. Percentages of BrdU+ cells shown the mean ±SD for n=3 independently analyzed mice. (C) Representative flow cytometric analysis measuring cell division with CFSE dilution. MZ and FO B cells were isolated from WT, KO and Bcl-3 transgenic (Tg) mice as in (A), labeled with CFSE and stimulated for 72 hours with LPS (200 ng/ml) (upper panels) or anti-IgM (6.25 μg/ml) (lower panels), respectively, or cells were not stimulated (NS). (D) Summary of data as in (C) shown as mean± SD for the division and proliferation index (FloJo software analysis) for 3 separate experiments with n=5 independently analyzed pools of mice. (E) FO B cells were isolated as in (B) from WT, KO and Tg mice and stimulated for 48 h with 200ng/ml LPS or 6.25μg/ml anti-IgM or not stimulated (NS), as indicated. Caspase 3 activity was determined by flow cytometry and percentages of cells with activated caspase 3 are shown as mean ±SD from 3–4 separate experiments with n=8 Tg, n=13 WT and n=13 KO independently analyzed mice. (*= p<0.05)
To investigate whether the absence of Bcl-3 in FOB cells might increase their rate of apoptosis upon BCR stimulation, we measured caspase 3 activation in bead-isolated FOB cells from WT, Bcl-3−/− and transgenic mice after 48h of stimulation with αIgM, LPS or no stimulation, gating on live cells. BCR stimulation activated more caspase 3 than LPS across all three genotypes. Importantly, caspase 3 activation was significantly more prominent in BCR-stimulated Bcl-3−/− FOB compared to their WT counterparts, while Bcl-3 transgenic cells exhibited slightly lower levels than WT cells (Fig. 5E). Bcl-3 may thus help protect FOB cells in particular from BCR-induced apoptosis and this likely contributed to the lower level of apparent proliferation observed above in the absence of Bcl-3.
We also assayed αIgM- and LPS-induced expression of select genes in FACS-sorted FOB cells and found a trend towards lower expression of proliferation-related genes in BCR-stimulated Bcl-3−/−FOB cells and a trend towards higher expression of anti-apoptotic genes and IL-10 upon LPS stimulation of these mutant cells, consistent with generally diminished proliferative responses to αIgM and increased responses to LPS (Supplemental Fig. 4).
Discussion
Here we report that Bcl-3 controls the relative proportion of mature MZB and FOB cells that are generated during splenic B cell development. This atypical member of the IκB family acted as a gatekeeper for the MZB cell lineage; it delimited the number of MZB cells, apparently exerting its influence during the time when developing B cells transition into and establish themselves as mature FO or MZ B cells. While many proteins have been found to positively contribute to, or even be required for MZB cell development, including in particular NF-κB factors (24, 35, 36), Bcl-3 is unusual since it negatively impacted this process. Bcl-3 restricted maturation of MZB cells, and conversely, appeared to favor maturation of FOB cells; Bcl-3 may have had similar effects during the maintenance phase of these cells. The findings are based on analyses of mice lacking Bcl-3 or overexpressing Bcl-3 in B cells: Loss of Bcl-3 resulted in a significantly higher proportion of MZB cells and their immediate precursors, while it led to a reduction in the proportion of FOB cells, a phenomenon we showed to be intrinsic and autonomous to B cells. Conversely, transgenic overexpression of Bcl-3 in B cells reduced relative numbers of MZB cells when compared to WT, while it increased those of FOB cells. Analyses of splenic tissue sections furthermore revealed more B cells in the splenic MZ of Bcl-3 deficient mice and fewer in Bcl-3 transgenic mice relative to WT mice, which confirmed the conclusions based on cell surface marker expression. Potential mechanisms for how Bcl-3 so profoundly impacted B cell maturation were suggested by the findings that Bcl-3-deficient B cells exhibited impaired survival and, likely in consequence, impaired proliferation following BCR stimulation, while these cells exhibited increased responses to LPS, including increased proliferation.
MZ and FOB cells are thought to derive primarily from transitional T2 B cells in the spleen. How the decision is made which cell type to develop into is not really understood, but current theory postulates that BCR signaling strength may be the deciding factor (35): In this model B cells carrying BCRs that recognize self-antigens quite well, albeit not too strongly, are destined to become FOB cells, and in the process also become impervious to other signals, such as those delivered via Notch2. Such BCR signals may also help maintain these cells. By contrast, B cells carrying BCRs that recognize self-antigens only quite weakly are postulated to set out along the MZB cell path; these cells remain receptive to and require other signals for full development, localization to and retention in the MZ of the spleen, events that may be linked. Such additional signals include inputs via Notch2, BAFF receptors, integrins, chemokine receptors and likely others (1, 36). How a gradient in BCR signaling strength may be read out is not known, but may involve NF-κB (35, 36).
Our findings may be incorporated into this presently held model of splenic B cell development. We hypothesize that Bcl-3 may promote FOB cell development (and possibly also maintenance) over that of MZB cells by strengthening BCR-mediated proliferation, at least in part by promoting survival of these so-stimulated cells. B cells recognizing self-antigens in vivo may thus be helped by Bcl-3 on their way to become FOB cells. Conversely we hypothesize that Bcl-3 may inhibit MZB development by dampening responses to signals that may promote the development (and possibly also maintenance) of these cells; such signals may include Toll-like receptor signals, which are most likely encountered in the MZ. Loss of Bcl-3 leads to LPS hyper-responsiveness not only in B cells, but also in macrophages, where Bcl-3 has been postulated to aid p50-homodimer-dependent suppression of NF-κB target genes (7, 46). Interestingly, p50/NF-κB1 is required for MZ B cell development, opposite the role of Bcl-3 revealed here (35). However, p50 is also a subunit of several trans-activating NF-κB heterodimers that are necessary for MZ B cell development. The differential in vitro effects of Bcl-3 on BCR-versus LPS-induced proliferation may appear to be modest, but when integrated over time in vivo could well explain the ultimate relative differences in numbers of FO and MZ B cells. Future experiments will be needed to prove this hypothesis.
Bcl-3 supported the survival of B cells especially following BCR stimulation. In the absence of Bcl-3, BCR stimulation of freshly isolated FO B cells led to significantly increased activation of caspase 3, and likely as consequence, reduced proliferation; conversely there was trend towards reduced caspase 3 activation in Bcl-3 transgenic FOB cells. Therefore deregulated expression of Bcl-3 in consequence of gene translocation may contribute to malignant transformation by promoting survival in the face of such stimuli, although proof will have to await future investigations. Nevertheless, transgenic overexpression of Bcl-3 increased not only the proportion of FOBs, but also total numbers of B cells in spleens.
Bcl-3 may have affected the ultimate ratio of MZ to FO B cells by exerting its influence as early as the time when the cell fate decision is made; this is suggested by the observed trend towards increased numbers of MZB precursors (MZP) as well as FO type II cells in the absence of Bcl-3. FO II B cells are a small fraction of FOB cells that is thought to have experienced little/no self-antigen stimulation and that may serve as a reservoir for MZB cells in follicles (35). FO II cells are thus more akin to MZB cells and their precursors. Of note, transgenic expression of Bcl-3 resulted in population shifts that were largely opposite those seen in the absence of Bcl-3: The proportion of FO I B cells was increased and that of MZB cells was reduced. However, relative numbers of MZP and FO II B cells were not significantly changed; the reason for this is not clear, although over-expression of Bcl-3 may not necessarily always accentuate normal functions, especially when these functions may also be regulated by post-translational modifications. Indeed we did not observe significant differences in Bcl-3 mRNA expression in the various splenic subpopulations (data not shown), which suggests that Bcl-3 might also be regulated by means other than just its levels of expression.
MZB cells play important innate roles in host defense; they respond better to LPS and express generally higher levels of co-stimulatory molecules than FOB cells (24). Consistent with this, we observed higher titers of IgM after LPS challenge in the serum of Bcl-3-deficient mice compared to controls (data not shown). LPS-stimulated Bcl-3−/−FO and MZB cells also expressed higher levels of some co-stimulatory molecules and mutant B cells were better able to co-stimulate T cells. Furthermore, mutant FO and MZB cells expressed slightly higher levels of an integrin thought to help retention of MZB cells and they proliferated slightly better to LPS stimulation. These data suggest that loss of Bcl-3 shifts B cells towards MZB-associated phenotypes. Conversely, the presence of Bcl-3 appears to dampen innate and promote adaptive responses to an immune challenge.
We have shown that Bcl-3 skews B cells towards the FOB fate and away from the MZB fate, thus acting as a gatekeeper for MZ B cell development. Bcl-3 limits apoptosis associated in particular with BCR stimulation. We speculate that in this way Bcl-3 may promote cells stimulated by self-antigens on their way to become and be maintained as FOB cells. At the same time Bcl-3 may impair responses to LPS, including their proliferation, signals that could be relevant for MZB development and maintenance.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and with grants from the National Natural Science Foundation of China (30972695 and 31270937).
We thank Dr. Michael Reth for providing Mb1-cre mice. We greatly appreciate the constructive inputs provided by members of the Siebenlist laboratory.
Abbreviations used in this article
- MZ B
marginal zone B cell
- FO B
follicular B cell
- BCR
B cell receptor
- NF-κB
nuclear factor kappa B
- WT
wild-type
- KO
knockout
Footnotes
Disclosures: The authors have no conflicting financial interests.
References
- 1.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172:803–811. doi: 10.4049/jimmunol.172.2.803. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti MF, White J, Kappler JW, Marrack P. Transgenic Bcl-3 slows T cell proliferation. Int Immunol. 2009;21:339–348. doi: 10.1093/intimm/dxp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenne AT, Fagerli UM, Shaughnessy JD, Jr, Vatsveen TK, Ro TB, Hella H, Zhan F, Barlogie B, Sundan A, Borset M, Waage A. High expression of BCL3 in human myeloma cells is associated with increased proliferation and inferior prognosis. Eur J Haematol. 2009;82:354–363. doi: 10.1111/j.1600-0609.2009.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocke-Heidrich K, Ge B, Cvijic H, Pfeifer G, Loffler D, Henze C, McKeithan TW, Horn F. BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene. 2006;25:7297–7304. doi: 10.1038/sj.onc.1209711. [DOI] [PubMed] [Google Scholar]
- 6.Canoz O, Rassidakis GZ, Admirand JH, Medeiros LJ. Immunohistochemical detection of BCL-3 in lymphoid neoplasms: a survey of 353 cases. Mod Pathol. 2004;17:911–917. doi: 10.1038/modpathol.3800140. [DOI] [PubMed] [Google Scholar]
- 7.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 8.Chilton PM, Mitchell TC. CD8 T cells require Bcl-3 for maximal gamma interferon production upon secondary exposure to antigen. Infect Immun. 2006;74:4180–4189. doi: 10.1128/IAI.01749-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 10.Elliott SF, Coon CI, Hays E, Stadheim TA, Vincenti MP. Bcl-3 is an interleukin-1-responsive gene in chondrocytes and synovial fibroblasts that activates transcription of the matrix metalloproteinase 1 gene. Arthritis Rheum. 2002;46:3230–3239. doi: 10.1002/art.10675. [DOI] [PubMed] [Google Scholar]
- 11.Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Franzoso G, Carlson L, Scharton-Kersten T, Shores EW, Epstein S, Grinberg A, Tran T, Shacter E, Leonardi A, Anver M, Love P, Sher A, Siebenlist U. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479–490. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 13.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103:13789–94. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovelmeyer N, Wunderlich FT, Massoumi R, Jakobsen CG, Song J, Worns MA, Merkwirth C, Kovalenko A, Aumailley M, Strand D, Bruning JC, Galle PR, Wallach D, Fassler R, Waisman A. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J Exp Med. 2007;204:2615–2627. doi: 10.1084/jem.20070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim HA, Amen F, Reid AG, Naresh KN. BCL3 rearrangement, amplification and expression in diffuse large B-cell lymphoma. Eur J Haematol. 2011;87:480–485. doi: 10.1111/j.1600-0609.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- 17.Kashatus D, Cogswell P, Baldwin AS. Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev. 2006;20:225–235. doi: 10.1101/gad.1352206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keutgens A, Shostak K, Close P, Zhang X, Hennuy B, Aussems M, Chapelle JP, Viatour P, Gothot A, Fillet M, Chariot A. The repressing function of the oncoprotein BCL-3 requires CtBP, while its polyubiquitination and degradation involve the E3 ligase TBLR1. Mol Cell Biol. 2010;30:4006–4021. doi: 10.1128/MCB.01600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keutgens A, Zhang X, Shostak K, Robert I, Olivier S, Vanderplasschen A, Chapelle JP, Viatour P, Merville MP, Bex F, Gothot A, Chariot A. BCL-3 degradation involves its polyubiquitination through a FBW7-independent pathway and its binding to the proteasome subunit PSMB1. J Biol Chem. 2010;285:25831–25840. doi: 10.1074/jbc.M110.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, Akira S. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- 21.Lenschow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodnow CC, Bluestone JA. Differential up-regulation of the B7–1 and B7–2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- 22.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 23.Maldonado V, Melendez-Zajgla J. Role of Bcl-3 in solid tumors. Mol Cancer. 2011;10:152. doi: 10.1186/1476-4598-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 25.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Bahlburg A, Becker-Herman S, Humblet-Baron S, Khim S, Weber M, Bouma G, Thrasher AJ, Batista FD, Rawlings DJ. Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood. 2008;112:4158–4169. doi: 10.1182/blood-2008-02-140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran ST, Cariappa A, Liu H, Muir B, Sgroi D, Boboila C, Pillai S. Synergism between NF-kappa B1/p50 and Notch2 during the development of marginal zone B lymphocytes. J Immunol. 2007;179:195–200. doi: 10.4049/jimmunol.179.1.195. [DOI] [PubMed] [Google Scholar]
- 29.Nishikori M, Maesako Y, Ueda C, Kurata M, Uchiyama T, Ohno H. High-level expression of BCL3 differentiates t(2;5)(p23; q35)-positive anaplastic large cell lymphoma from Hodgkin disease. Blood. 2003;101:2789–2796. doi: 10.1182/blood-2002-08-2464. [DOI] [PubMed] [Google Scholar]
- 30.Nishikori M, Ohno H, Haga H, Uchiyama T. Stimulation of CD30 in anaplastic large cell lymphoma leads to production of nuclear factor-kappaB p52, which is associated with hyperphosphorylated Bcl-3. Cancer Sci. 2005;96:487–497. doi: 10.1111/j.1349-7006.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohno H, Doi S, Yabumoto K, Fukuhara S, McKeithan TW. Molecular characterization of the t(14;19)(q32; q13) translocation in chronic lymphocytic leukemia. Leukemia. 1993;7:2057–2063. [PubMed] [Google Scholar]
- 32.Ohno H, Nishikori M, Maesako Y, Haga H. Reappraisal of BCL3 as a molecular marker of anaplastic large cell lymphoma. Int J Hematol. 2005;82:397–405. doi: 10.1532/IJH97.05045. [DOI] [PubMed] [Google Scholar]
- 33.Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene Bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- 34.Pene F, Paun A, Sonder SU, Rikhi N, Wang H, Claudio E, Siebenlist U. The IkappaB family member Bcl-3 coordinates the pulmonary defense against Klebsiella pneumoniae infection. J Immunol. 2011;186:2412–2421. doi: 10.4049/jimmunol.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 36.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 37.Poljak L, Carlson L, Cunningham K, Kosco-Vilbois MH, Siebenlist U. Distinct activities of p52/NF-kappa B required for proper secondary lymphoid organ microarchitecture: functions enhanced by Bcl-3. J Immunol. 1999;163:6581–6588. [PubMed] [Google Scholar]
- 38.Rassidakis GZ, Oyarzo MP, Medeiros LJ. BCL-3 overexpression in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Blood. 2003;102:1146–1147. doi: 10.1182/blood-2003-04-1366. [DOI] [PubMed] [Google Scholar]
- 39.Richard M, Louahed J, Demoulin JB, Renauld JC. Interleukin-9 regulates NF-kappaB activity through BCL3 gene induction. Blood. 1999;93:4318–4327. [PubMed] [Google Scholar]
- 40.Riemann M, Endres R, Liptay S, Pfeffer K, Schmid RM. The IkappaB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J Immunol. 2005;175:3560–3568. doi: 10.4049/jimmunol.175.6.3560. [DOI] [PubMed] [Google Scholar]
- 41.Rocha S, Martin AM, Meek DW, Perkins ND. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol. 2003;23:4713–4727. doi: 10.1128/MCB.23.13.4713-4727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan Q, Zheng SJ, Palmer S, Carmody RJ, Chen YH. Roles of Bcl-3 in the pathogenesis of murine type 1 diabetes. Diabetes. 2010;59:2549–2557. doi: 10.2337/db10-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz EM, Krimpenfort P, Berns A, Verma IM. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev. 1997;11:187–197. doi: 10.1101/gad.11.2.187. [DOI] [PubMed] [Google Scholar]
- 44.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 45.Viatour P, Dejardin E, Warnier M, Lair F, Claudio E, Bureau F, Marine JC, Merville MP, Maurer U, Green D, Piette J, Siebenlist U, Bours V, Chariot A. GSK3-mediated BCL-3 phosphorylation modulates its degradation and its oncogenicity. Mol Cell. 2004;16:35–45. doi: 10.1016/j.molcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-kappaB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem. 2004;279:49995–50003. doi: 10.1074/jbc.M404246200. [DOI] [PubMed] [Google Scholar]
- 47.Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS., Jr The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol Cell Biol. 2001;21:8428–8436. doi: 10.1128/MCB.21.24.8428-8436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ, Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Wang H, Claudio E, Brown K, Siebenlist U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity. 2007;27:438–452. doi: 10.1016/j.immuni.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.