Abstract
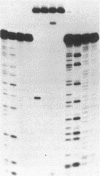
Many carcinogenic as well as chemotherapeutic agents cause covalent linkages between complementary strands of DNA. If unrepaired, DNA crosslinks are blocks to DNA replication and transcription and therefore represent potentially lethal lesions to the cell. Genetic studies of Escherichia coli have demonstrated that the repair enzyme ABC excision nuclease, coded for by the three unlinked genes, uvrA, uvrB, and uvrC, plays a crucial role in DNA crosslink repair. To study the molecular events of ABC excision nuclease-mediated crosslink repair, we have engineered a DNA fragment with a psoralen-DNA interstrand crosslink at a defined position, digested this substrate with pure enzyme, and analyzed the reaction products on DNA sequencing gels. We find that the excision nuclease cuts only one of the two strands involved in the crosslink, incises the crosslink by hydrolyzing the ninth phosphodiester bond 5' and the third phosphodiester bond 3' to the furan-side thymine of the crosslink, and does not produce double-strand breaks at any significant level. Based on these data, we present a model by which ABC excision nuclease initiates crosslink repair in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Elkind M. M. Psoralen plus near ultraviolet light inactivation of cultured Chinese hamster cells and its relation to DNA cross-links. Mutat Res. 1973 Jun;18(3):315–324. doi: 10.1016/0027-5107(73)90216-9. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Inactivation of Escherichia coli by near-ultraviolet light and 8-methoxypsoralen: different responses of strains B/r and K-12. J Bacteriol. 1979 Aug;139(2):454–459. doi: 10.1128/jb.139.2.454-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto E., Gross N., Bardwell E., Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and tri-methylpsoralen or khellin. Biochim Biophys Acta. 1977 Apr 19;475(4):589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Chaw Y. F., Crane L. E., Lange P., Shapiro R. Isolation and identification of cross-links from formaldehyde-treated nucleic acids. Biochemistry. 1980 Nov 25;19(24):5525–5531. doi: 10.1021/bi00565a010. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupido M., Bridges B. A. Uvr-independent repair of 8-methoxypsoralen crosslinks in Escherichia coli: evidence for a recombinational process. Mutat Res. 1985 Sep;146(2):135–141. doi: 10.1016/0167-8817(85)90003-3. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. Inter-strand cross-linkages occurring in the photoreaction between psoralen and DNA. FEBS Lett. 1970 Jul 29;9(2):121–123. doi: 10.1016/0014-5793(70)80330-1. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Giuffrida A. S., Dingman C. W. Repair by human cells of adenovirus-2 damaged by psoralen plus near ultraviolet light treatment. Mutat Res. 1975 Dec;33(2-3):311–320. doi: 10.1016/0027-5107(75)90206-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Cross-link repair in human cells and its possible defect in Fanconi's anemia cells. J Mol Biol. 1977 Jul 15;113(4):635–649. doi: 10.1016/0022-2836(77)90227-3. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. "Reversible" DNA. Proc Natl Acad Sci U S A. 1961 Jul 15;47:950–955. doi: 10.1073/pnas.47.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H., Piette J., Hearst J. E. Efficient formation of a crosslinkable HMT monoadduct at the Kpn I recognition site. Photochem Photobiol. 1984 Jul;40(1):29–34. doi: 10.1111/j.1751-1097.1984.tb04549.x. [DOI] [PubMed] [Google Scholar]
- Hall J. D. Repair of psoralen-induced crosslinks in cells multiply infected with SV40. Mol Gen Genet. 1982;188(1):135–138. doi: 10.1007/BF00333007. [DOI] [PubMed] [Google Scholar]
- Hearst J. E. Psoralen photochemistry. Annu Rev Biophys Bioeng. 1981;10:69–86. doi: 10.1146/annurev.bb.10.060181.000441. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Smith C. A., Hanawalt P. C. DNA repair in human cells containing photoadducts of 8-methoxypsoralen or angelicin. Cancer Res. 1980 Mar;40(3):696–702. [PubMed] [Google Scholar]
- Lin P. F., Bardwell E., Howard-Flanders P. Initiation of genetic exchanges in lambda phage--prophage crosses. Proc Natl Acad Sci U S A. 1977 Jan;74(1):291–295. doi: 10.1073/pnas.74.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Henriques J. A., Chanet R., Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Prakash L., Prakash S. Genetic control of excision of Saccharomyces cerevisiae interstrand DNA cross-links induced by psoralen plus near-UV light. Mol Cell Biol. 1982 Aug;2(8):939–948. doi: 10.1128/mcb.2.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
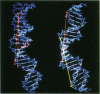
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. Mutagenic SOS repair of site-specific psoralen damage in plasmid pBR322. J Mol Biol. 1984 Sep 25;178(3):595–609. doi: 10.1016/0022-2836(84)90240-7. [DOI] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G. B. Escherichia coli DNA photolyase stimulates uvrABC excision nuclease in vitro. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7397–7401. doi: 10.1073/pnas.81.23.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G., Tang M. S. Repair of psoralen and acetylaminofluorene DNA adducts by ABC excinuclease. J Mol Biol. 1985 Aug 20;184(4):725–734. doi: 10.1016/0022-2836(85)90316-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Seki T., Nozu K., Kondo S. Differential causes of mutation and killing in Escherichia coli after psoralen plus light treatment: monoadducts and cross-links. Photochem Photobiol. 1978 Jan;27(1):19–24. doi: 10.1111/j.1751-1097.1978.tb07559.x. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Cole R. S. Repair of cross-linked DNA and survival of Escherichia coli treated with psoralen and light: effects of mutations influencing genetic recombination and DNA metabolism. J Bacteriol. 1978 Nov;136(2):538–547. doi: 10.1128/jb.136.2.538-547.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Hagerman P. J. Interstrand psoralen cross-links do not introduce appreciable bends in DNA. Biochemistry. 1984 Dec 18;23(26):6299–6303. doi: 10.1021/bi00321a002. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Role of ATP in removal of psoralen cross-links from DNA of Escherichia coli permeabilized by treatment with toluene. J Biol Chem. 1977 Oct 25;252(20):7023–7030. [PubMed] [Google Scholar]